Introduction
Diffuse large B-cell lymphoma (DLBCL) is defined by
the World Health Organization (WHO) classification as a
heterogeneous entity, encompassing morphologic and genetic variants
with variable clinical presentations and outcomes, while
constituting the most common type of non-Hodgkin’s lymphoma (NHL)
in adults. More than one-third of the world’s population has been
infected with the hepatitis B virus (HBV), with the vast majority
living in developing regions (1–3).
China is a highly endemic HBV area with ∼170 million carriers
(4) and previous studies have
revealed a high prevalence of HBV infections in NHLs, particularly
in DLBCLs (5,6). Several studies have suggested that
HBV may even act as an etiological factor for NHL (5,7).
However, few studies have focused on the prognosis of hepatitis B
surface antigen (HBsAg)-positive DLBCL patients, and the clinical
features, prognostic factors and the efficacy of rituximab
treatment remain unclear, with no relevant studies comparing the
risks and benefits of rituximab application in HBsAg-positive DLBCL
patients. Thus, we performed this retrospective study to focus on
HBsAg-positive DLBCL patients.
HBV-carrying DLBCL patients are at a higher risk of
reactivating hepatitis B with reactivation rates of 20–50%
(8) and related mortality rates of
10–40% (9), when receiving
cytotoxic chemotherapies, which may be associated with the
immunosuppressive effects of the treatments (10). There is a broad range of clinical
manifestations of HBV infections, ranging from asymptomatic
self-limiting anicteric hepatitis to potentially fatal, severe,
progressive liver failure, while two different clinical scenarios
contribute to HBV reactivation. Firstly, viral reactivation occurs
in patients who suffer from a chronic HBV infection
(HBsAg-positive), in whom the diagnosis of HBV reactivation is
based on detectable serum HBV-DNA loads in the presence of
biochemical or clinical evidence of hepatitis. In the second
scenario, viral reactivation occurs in patients who have resolved a
HBV infection as shown by the clearance of circulating HBsAg and
appearance of antibodies to hepatitis B core antigens (HBcAg) with
or without antibodies to HBsAg. In these patients, a low level of
HBV replication has been shown to persist in the liver and in
peripheral-blood mononuclear cells for decades (11,12).
HBV reactivation with the reappearance of HBsAg (HBsAg
seroreversion) has been reported following transplantation and
immunosuppressive therapy, as well as allogeneic and autologous
hematopoietic stem-cell transplantations (13–15).
The cyclophosphamide, doxorubicin, vincristine, and
prednisone (CHOP) regimen was once the classical frontline
treatment for DLBCL; however, the development of monoclonal
antibodies has led to further improvement of DLBCL treatment
outcomes. Currently, patients with DLBCL are medicated with an
additional immunochemotherapy, usually the rituximab plus CHOP
(RCHOP) regimen. Rituximab is a chimeric mouse human monoclonal
antibody against CD20+, which is an antigen expressed in
the majority of B lymphocytes, including malignant lymphomatous B
cells and normal B lymphocytes. The incorporation of rituximab into
standard chemotherapies has been shown to improve the clinical
outcomes of CD20+ DLBCL (16,17).
However, rituximab, when used alone or in combination with
chemotherapies, has been associated with HBV reactivation in
patients with DLBCL (18,19). Lamivudine (a reverse-transcriptase
inhibitor of the HBV-DNA polymerase) prophylaxis has demonstrated
excellent efficacy in the prevention of HBV reactivation for
HBsAg-positive patients with DLBCL undergoing conventional
chemotherapy, with an excellent safety and tolerability profile
(20).
In this retrospective study, we compared the
clinical features of DLBCL patients with or without HBV infections
and evaluated the potential prognostic factors in HBsAg-positive
DLBCL patients. We also compared the treatment outcomes and HBV
reactivation rates of patients who received CHOP or RCHOP
regimens.
Materials and methods
Patients and measurements
We reviewed 536 consecutive newly diagnosed patients
with DLBCL who were hospitalized in the First and Second Affiliated
Hospitals of the Medical School of Zhejiang University between
January 2006 and April 2011. All diagnoses were confirmed by
histopathological staining with hematoxylin and eosin (H&E), as
well as determination of the immunophenotypes according to the WHO
classification. Complete clinical profiles were obtained from 451
patients who received chemotherapy and completed the follow-up.
Clinical staging and diagnostic methods included clinical history
and physical examinations, chest, abdominal and pelvic computed
tomography (CT) scans, digital full-body color Doppler ultrasound
diagnosis, marrow aspirate and biopsy, as well as serum lactate
dehydrogenase (LDH) and serum β2-microglobulin (β2-M) level
measurements (21). Enzyme-linked
immunosorbent assays (ELISA) were used to detect HBsAg, hepatitis B
surface antibodies (HBsAb), hepatitis B e antigens (HBeAg),
hepatitis B e antibodies (HBeAb) and hepatitis B core antibodies
(HBcAb).
Routine liver function tests included determinations
of alanine aminotransferase (ALT), aspartate aminotransferase
(AST), galactosylhydroxylysyl glucosyltransferase (GGT) and
bilirubin (direct and indirect forms) levels. These assays were
first performed within one week prior to the start of each
chemotherapy cycle.
The HBV-DNA loads of all 96 HBsAg-positive patients
were determined and an HBV-DNA load >103 cps/ml,
which is the upper normal limit in the First Affiliated Hospital of
Medical School of Zhejiang University, was regarded as an elevated
value. The research was approved by the ethics committee of The
First Affiliated Hospital of the Medical School of Zhejiang
University and informed consent was obtained from all
participants.
Treatments
Of the 451 patients, for economical reasons, 156
patients were treated only with a CHOP regimen (median number of
courses, 6; range, 4–8) and 295 cases were treated with an RCHOP
regimen (median number of courses, 6; range, 4–8). Lamivudine
prophylaxis was prescribed for all HBsAg-positive patients prior to
chemotherapy. Once patients presented an abnormal liver function,
they were treated with hepatinica (diammonium glycyrrhizinate,
compound glycyrrhizin, reduced glutathione, or polyene
phosphatidylcholine) and all chemotherapies were restarted only in
cases where the liver function had recovered.
Follow-up
Following treatment, routine clinical follow-up was
conducted every 3 months for the first 2 years and then every 6
months for the additional 3 years. Ultrasound or CT scans were
performed every six months during the first two years and then
afterwards annually during the follow-up period. The median
follow-up time was 27 months (range, 1–76 months).
Statistical analyses
Significant differences in the baseline clinical
parameters and treatment characteristics between the HBsAg-positive
patients and HBsAg-negative patients were evaluated by either
Chi-square test or Fisher’s exact test for categorical parameters.
Overall survival (OS) rates and survival curves were calculated by
the Kaplan-Meier method. The OS rate calculation was performed from
the date of diagnosis to the date of mortality or the last
follow-up. The multivariate analysis of outcome in terms of OS was
performed by Cox regression, which included the variables that were
significant in a univariate analysis. Two-tailed P-values <0.05
were considered to indicate a statistically significant difference.
All statistical analyses were performed with the SPSS software for
Windows v.19.0 (SPSS, Inc., Chicago, IL, USA).
Results
Clinical characteristics and OS rates of
HBsAg-positive and -negative patients
Of the 451 patients, 90 were HBsAg-positive and 361
were HBsAg-negative. The clinical characteristics of these two
groups are summarized in Table I.
The HBsAg-positive group was associated with an earlier onset age
(45 years for HBsAg-positive vs. 56 years for HBsAg-negative,
P<0.001), and lower international prognostic index (IPI, 0–2;
63.3% HBsAg-positive vs. 51.2% HBsAg-negative, P=0.040). There were
no significant differences between the two groups in gender, Ann
Arbor stage, LDH levels, β2-M levels and incidence of B symptoms.
In addition, in the HBsAg-positive patient group, rituximab was
less applied than in the HBsAg-negative patient group (54.4%
HBsAg-positive vs. 68.1% HBsAg-negative, P= 0.015), which may
reflect physicians’ concerns about HBV reactivation.
 | Table I.Clinical characteristics of
HBsAg-positive and negative patients. |
Table I.
Clinical characteristics of
HBsAg-positive and negative patients.
| HBsAg-positive
patients (n=90, 20%) | HBsAg-negative
patients (n=361, 80%) | P-value |
|---|
| Median age
(years) | 45 | 56 | <0.001 |
| Age range | 18–69 | 18–83 | |
| Gender | | | |
| Male | 58 (64.4%) | 197 (54.6%) | 0.091 |
| Female | 32 (35.6%) | 164 (45.4%) | |
| Stage | | | |
| I/II | 16 (17.8%) | 69 (19.1%) | 0.772 |
| III/IV | 74 (82.2%) | 292 (80.9%) | |
| IPI | | | |
| 0–2 | 57 (63.3%) | 185 (51.2%) | 0.040 |
| 3–5 | 33 (36.7%) | 176 (48.8%) | |
| LDH | | | |
| >ULN (225
U/l) | 44 (48.9%) | 159 (44.0%) | 0.409 |
| Normal | 46 (51.1%) | 202 (56.0%) | |
| β2-M | | | |
| >ULN (2200
μg/l) | 32 (35.6%) | 161 (44.6%) | 0.121 |
| Normal | 58 (64.4%) | 200 (55.4%) | |
| B symptoms | | | |
| Yes | 28 (31.1%) | 121 (33.5%) | 0.664 |
| No | 62 (68.9%) | 240 (66.5%) | |
| Regimen | | | |
| CHOP | 41 (45.6%) | 115 (31.9%) | 0.015 |
| RCHOP | 49 (54.4%) | 246 (68.1%) | |
At a median follow-up time of 27 months, we compared
the OS rates between HBsAg-positive and HBsAg-negative patients
with Kaplan-Meier analyses. HBsAg-negative patients demonstrated a
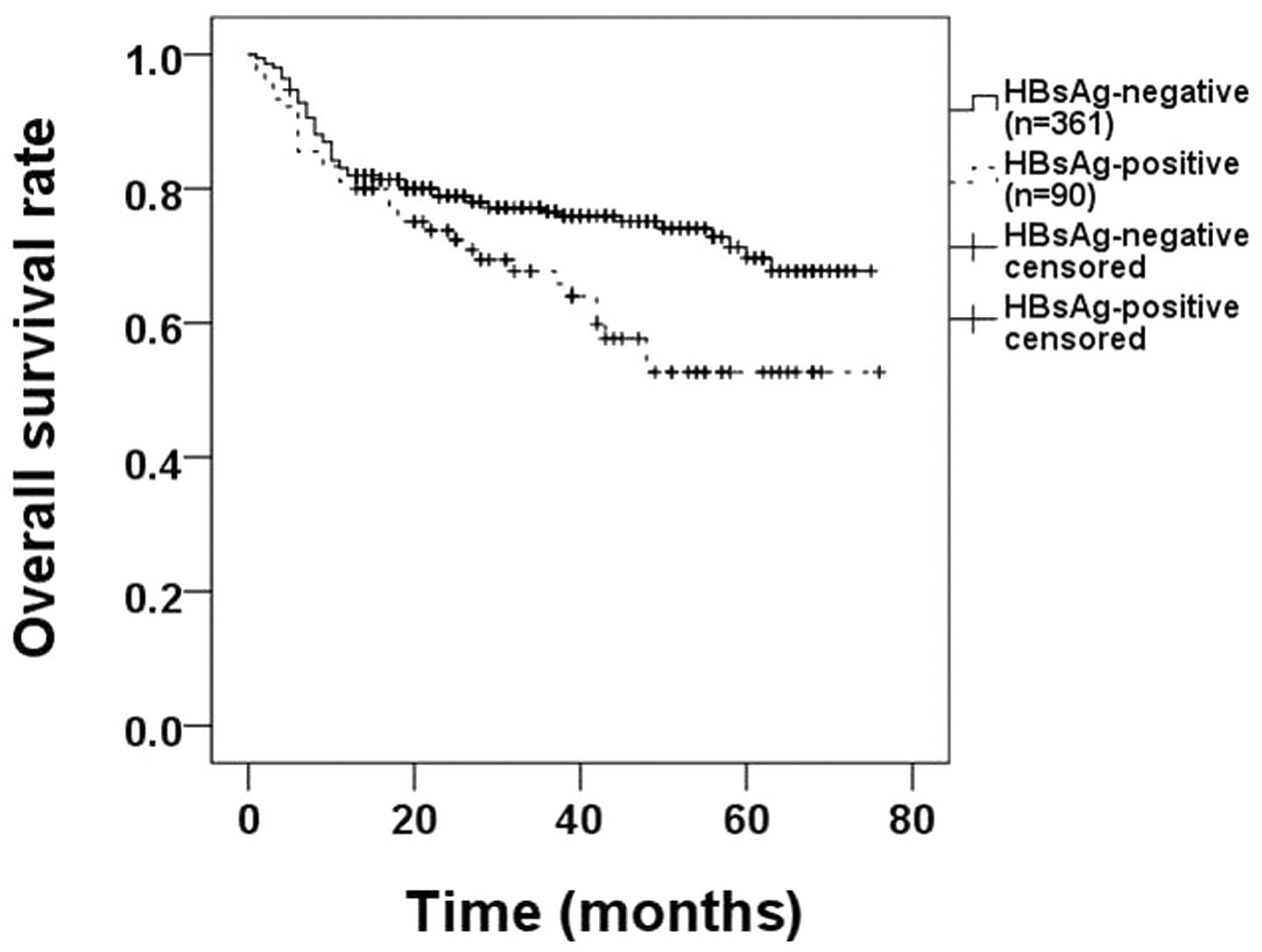
higher OS rate (62.2% HBsAg-positive vs. 76.2% HBsAg-negative,
P=0.018; Fig. 1).
Comparison of OS rates between
HBsAg-positive patients with different HBV-DNA loads during
chemotherapy
As previously mentioned, the prognosis of
HBsAg-positive patients was poorer than that of HBsAg-negative
patients. We further investigated whether prognosis was related to
the level of HBV activity. We divided patients into two groups
according to their HBV-DNA status. In the first group, the HBV-DNA
load of the patients was constantly <103 cps/ml
during chemo-therapy, while in the second subgroup the HBV-DNA load
was >103 cps/ml at least once during chemotherapy;
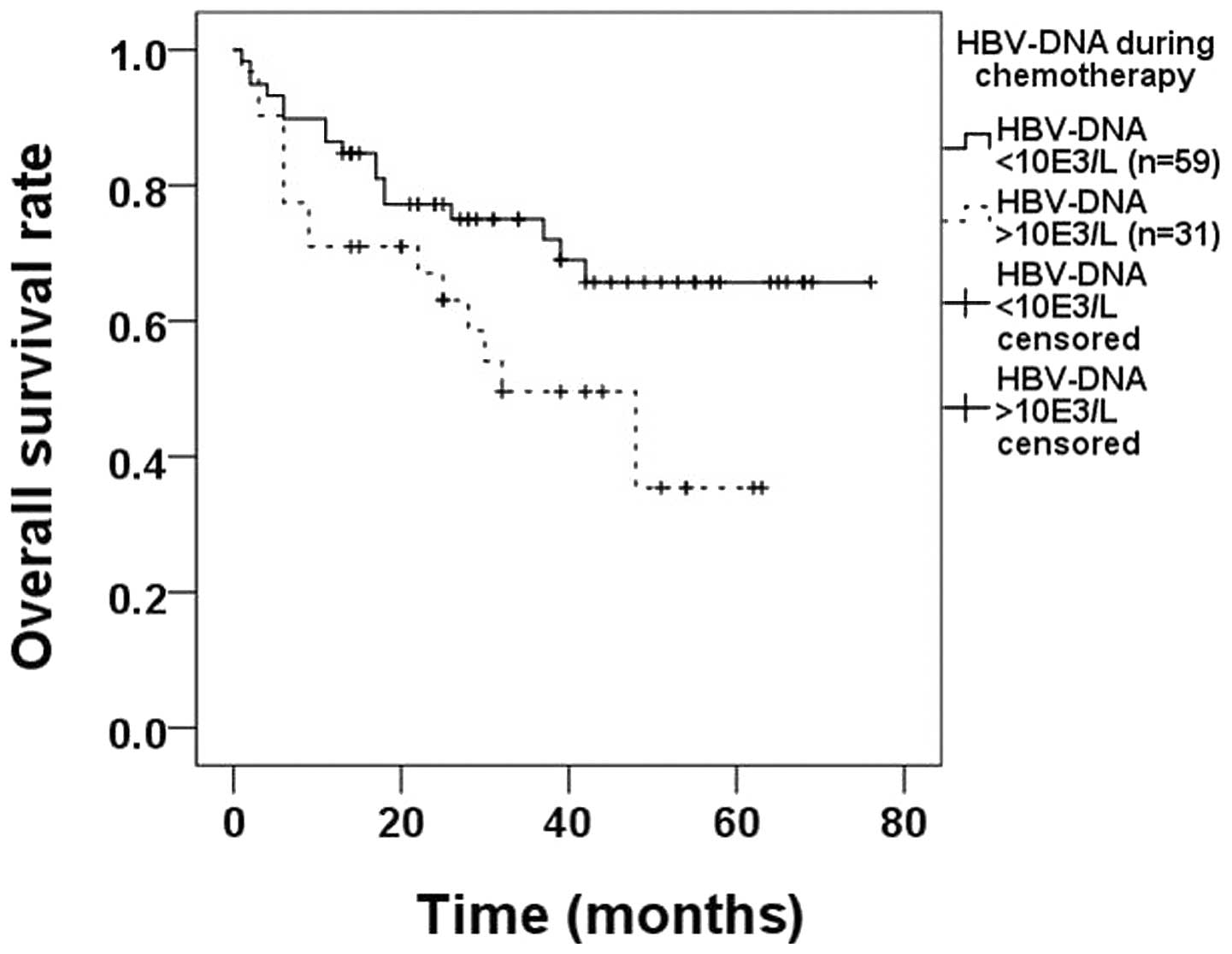
there were 59 and 31 patients in each group, respectively. The OS
rate in the first group (71.2%) was significantly higher compared
with that in the second subgroup (48.4%, P=0.037; Fig. 2).
Analysis of prognostic factors for
HBsAg-positive DLBCL patients
A univariate analysis revealed that positive B
symptoms (P=0.001), Ann Arbor stages III/IV (P=0.002) and elevated
LDH levels (P=0.001) were poor prognostic factors for
HBsAg-positive DLBCL patients. A multivariate analysis revealed
that positive B symptoms [hazard ratio (HR), 14.434; 95% confidence
interval (CI), 3.063–68.013; P<0.001), elevated LDH levels (HR,
8.369; 95% CI, 2.059–34.026; P=0.003) and male gender (HR, 0.160;
95% CI, 0.031–0.817; P=0.028) were poor prognostic factors for OS
rates (Table II).
 | Table II.Univariate and multivariate analyses
of clinical factors for OS rates of HBsAg-positive DLBCL
patients. |
Table II.
Univariate and multivariate analyses
of clinical factors for OS rates of HBsAg-positive DLBCL
patients.
| Variables | OS
|
|---|
Univariate analysis
| Multivariate analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age >60 years | 0.889 | 0.203–3.899 | 0.876 | 0.705 | 0.144–3.441 | 0.665 |
| Gender, male | 0.662 | 0.308–1.427 | 0.293 | 0.160 | 0.031–0.817 | 0.028 |
| Ann Arbor stage
III/IV | 9.071 | 1.240–66.340 | 0.002 | 9.729 | 0.991–95.528 | 0.051 |
| Positive B
symptoms | 3.256 | 1.657–6.399 | 0.001 | 14.434 | 3.063–68.013 | <0.001 |
| LDH level >ULN
(225 U/l) | 3.863 | 1.745–8.549 | 0.001 | 8.369 | 2.059–34.026 | 0.003 |
| β2-M >ULN (2200
μg/l) | 1.019 | 0.504–2.059 | 0.959 | 0.353 | 0.108–1.151 | 0.084 |
Comparison of therapeutic efficacies and
HBV reactivation rates between CHOP and RCHOP treatments
In the 90 HBsAg-positive patients, 41 patients were
treated with CHOP (median course, 6; range, 4–8) and 49 patients
with RCHOP (median course, 6; range, 4–8). We compared the
therapeutic efficacies and the reactivation rates of HBV between
the DLBCL patients were treated with CHOP and those who were
treated with RCHOP.
For a prognosis evaluation, we used the Kaplan-Meier
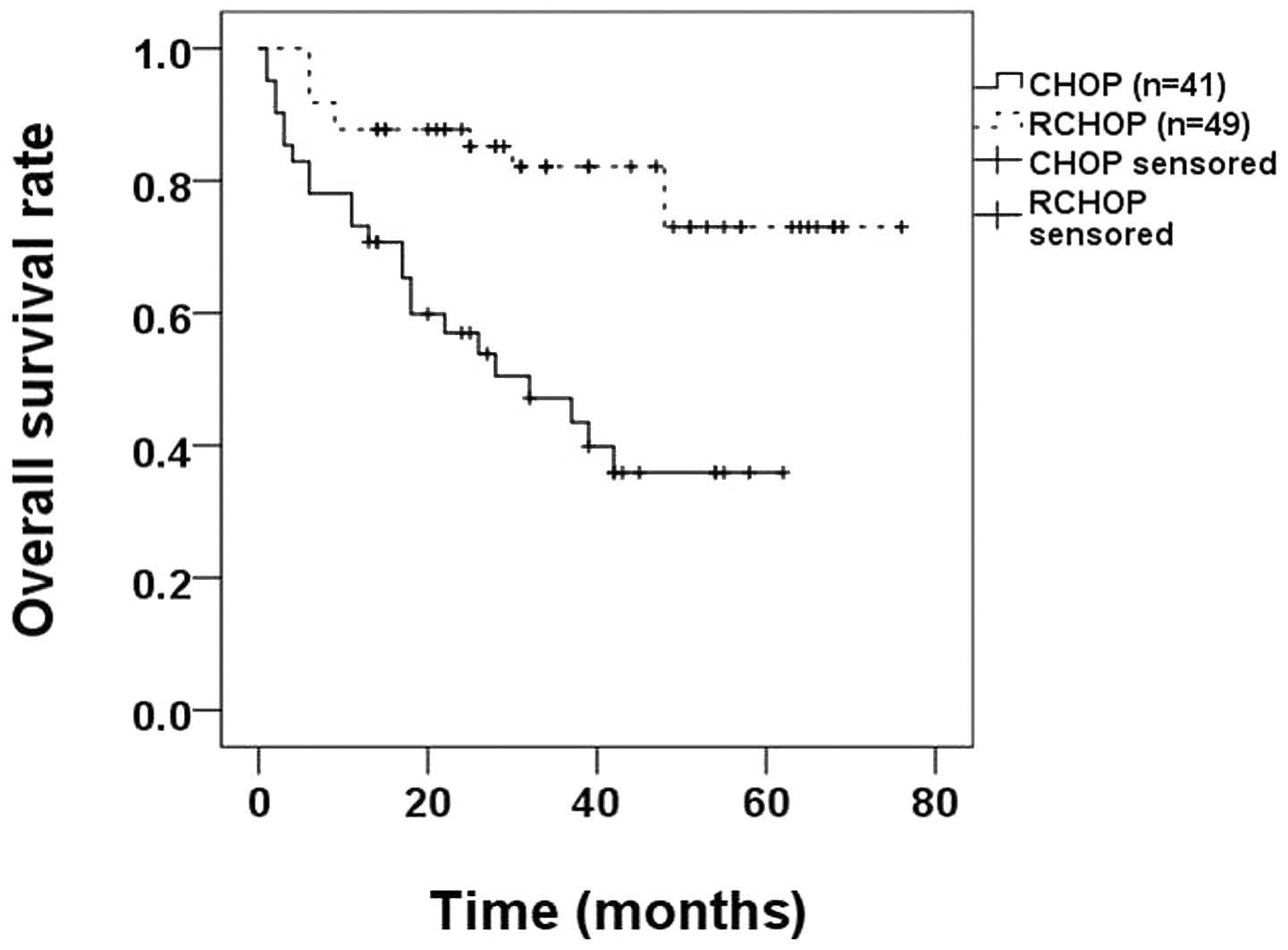
method to compare the OS rates between the CHOP and RCHOP groups
and identified that the OS rate of the RCHOP group (79.6%) was
higher compared with that of the CHOP group (43.9%; P<0.0010;
Fig. 3).
Regarding HBV-reactivation, we did not identify any
patient whose HBsAg status changed from negative to positive
following chemotherapy. Therefore, we compared the HBV-DNA loads of
the CHOP and RCHOP groups of HBsAg-positive DLBCL patients prior to
and during chemotherapy.
Prior to chemotherapy the HBV-DNA level was
>103 cps/ml in 22 CHOP (53.7%) and 31 RCHOP (63.3%)
patients, without statistical difference between the two groups
(P=0.356; Table III).
 | Table III.HBV reactivation comparisons of DLBCL
patients treated with CHOP or RCHOP. |
Table III.
HBV reactivation comparisons of DLBCL
patients treated with CHOP or RCHOP.
| CHOP group (n=41,
20.3%) | RCHOP group (n=49,
79.7%) | P-value |
|---|
| HBV-DNA
>103 cps/ml before chemotherapy | 22 (53.7%) | 31 (63.3%) | 0.356 |
| HBV-DNA
>103 cps/ml during chemotherapy | 16 (39.0%) | 15 (30.6%) | 0.403 |
During chemotherapy, there were 16 patients (39.0%)
whose HBV-DNA level was >103 cps/ml in the CHOP group
and 15 (30.6%) in the RCHOP group. Due to the anti-viral efficacy
of lamivudine, the percentages of patients with HBV-DNA levels
>103 cps/ml declined in the two groups when compared
with their status before chemotherapy; however, there was no
significant difference between the two groups (P=0.403; Table III). Therefore a combined
lamivudine application did not bear a greater risk of CHOP-induced
HBV reactivation.
Discussion
HBV infection is a common co-morbidity of DLBCL
patients in China. In our study, of the 451 patients, there were 90
HBsAg-positive DLBCL patients and the percentage of HBV carriers
was as high as 20.0%, which is higher than the result of a 2006
population census showing that 7.18% of the Chinese population were
carrying HBV (22). The present
study demonstrated an earlier age of disease onset in the
HBsAg-positive DLBCL patient group. These two facts suggest that
HBV acts as an etiologic factor for DLBCL development, which is in
agreement with the results of an earlier relevant study; however,
an apparent correlation between HBsAg and prognosis was not
revealed in the earlier study (23). The current study demonstrated that
HBsAg-positive DLBCL patients had poorer OS rates compared with
HBsAg-negative patients (62.2% HBsAg-positive vs. 76.2%
HBsAg-negative, P=0.018; Fig. 1).
One possible reason for the different conclusion of former scholars
is that in our study the percentage of patients receiving rituximab
in the HBsAg-negative group was higher compared with that in
HBsAg-positive group. To address whether HBV infection is another
cause for a worse prognosis in HBsAg-positive DLBCL patients, we
further divided the HBsAg-positive DLBCL patients into two groups
according to the HBV-DNA loads during chemotherapy. We identified
that patients with HBV-DNA loads >103 cps/ml during
chemotherapy had poorer prognoses compared with patients with
HBV-DNA loads <103 cps/ml (71.2% HBV-DNA normal vs.
48.4% HBV-DNA elevated, P=0.037; Fig.
2) and we suggest that in HBsAg-positive DLBCL patients, the
poor management of HBV was related to poor prognosis. Therefore, we
suggest that a more rigorous anti-viral management should be
administered to patients whose HBV-DNA titer is >103
cps/ml, despite a standard anti-viral treatment during
chemotherapy.
The results of the current study suggest that HBsAg
and HBV-DNA loads during chemotherapy may be used as prognostic
indicators for patients with DLBCL. A univariate analysis revealed
that positive B symptoms (P=0.001), Ann Arbor stages III/IV
(P=0.002) and elevated LDH levels (P=0.001) were poor prognostic
factors for HBsAg-positive DLBCL patients and a multivariate
analysis revealed that positive B symptoms (P<0.001), elevated
LDH levels (P=0.003) and male gender (P=0.028) were poor prognostic
factors for the OS rates in HBsAg-positive DLBCL patients.
We compared therapeutic efficacies and HBV
reactivation rates between CHOP and RCHOP treatments in the present
study. Regarding HBV-reactivation, we did not identify any patient
whose HBsAg status changed from negative to positive following
chemotherapy. We also compared the CHOP and RCHOP groups’ serum
HBV-DNA values of HBsAg-positive DLBCL patients before and during
chemotherapy and identified that there was no significant
difference between the two groups (Table III), indicating that an RCHOP
regimen did not bear a greater risk of HBV reactivation compared
with a CHOP regimen when combined with a lamivudine anti-viral
treatment. In addition, for HBsAg-positive DLBCL patients, the OS
rate of patients receiving RCHOP was 79.6%, which is higher than
than the 43.9% for patients receiving CHOP (P<0.001; Fig. 3), suggesting that HBsAg-positive
DLBCL patients benefit from the additional rituximab
application.
Since RCHOP has an advantage over CHOP on the OS
rate and since the management of lamivudine with the RCHOP regime
did not increase the risk of HBV reactivation, our study
demonstrated that a RCHOP treatment was appropriate for
HBsAg-positive patients and resulted in a better prognosis.
References
|
1.
|
Ganem D and Prince AM: Hepatitis B virus
infection - natural history and clinical consequences. N Engl J
Med. 350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lok AS and McMahon BJ: Chronic hepatitis
B. Hepatology. 45:507–539. 2007. View Article : Google Scholar
|
|
3.
|
Lok AS, Lai CL, Wu PC, Wong VC, Yeoh EK
and Lin HJ: Hepatitis B virus infection in Chinese families in Hong
Kong. Am J Epidemiol. 126:492–499. 1987.PubMed/NCBI
|
|
4.
|
Sun Z, Ming L, Zhu X and Lu J: Prevention
and control of hepatitis B in China. J Med Virol. 67:447–450. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kuniyoshi M, Nakamuta M, Sakai H, et al:
Prevalence of hepatitis B or C virus infections in patients with
non-Hodgkin’s lymphoma. J Gastroenterol Hepatol. 16:215–219.
2001.
|
|
6.
|
Marcucci F, Mele A, Spada E, et al: High
prevalence of hepatitis B virus infection in B-cell non-Hodgkin’s
lymphoma. Haematologica. 91:554–557. 2006.
|
|
7.
|
Cucuianu A, Patiu M, Duma M, et al:
Hepatitis B and C virus infection in Romanian non-Hodgkin’s
lymphoma patients. Br J Haematol. 107:353–356. 1999.PubMed/NCBI
|
|
8.
|
Vento S, Cainelli F and Longhi MS:
Reactivation of replication of hepatitis B and C viruses after
immunosuppressive therapy: an unresolved issue. Lancet Oncol.
3:333–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Skrabs C, Müller C, Agis H, Mannhalter C
and Jäger U: Treatment of HBV-carrying lymphoma patients with
rituximab and CHOP: a diagnostic and therapeutic challenge.
Leukemia. 16:1884–1886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Liang RH, Lok AS, Lai CL, Chan TK, Todd D
and Chiu EK: Hepatitis B infection in patients with lymphomas.
Hematol Oncol. 8:261–270. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Rehermann B, Ferrari C, Pasquinelli C and
Chisari FV: The hepatitis B virus persists for decades after
patients’ recovery from acute viral hepatitis despite active
maintenance of a cytotoxic T-lymphocyte response. Nat Med.
2:1104–1108. 1996.
|
|
12.
|
Yuki N, Nagaoka T, Yamashiro M, et al:
Long-term histologic and virologic outcomes of acute self-limited
hepatitis B. Hepatology. 37:1172–1179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yeo W and Johnson PJ: Diagnosis,
prevention and management of hepatitis B virus reactivation during
anticancer therapy. Hepatology. 43:209–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Xunrong L, Yan AW, Liang R and Lau GK:
Hepatitis B virus (HBV) reactivation after cytotoxic or
immunosuppressive therapy - pathogenesis and management. Rev Med
Virol. 11:287–299. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kitano K, Kobayashi H, Hanamura M, et al:
Fulminant hepatitis after allogenic bone marrow transplantation
caused by reactivation of hepatitis B virus with gene mutations in
the core promotor region. Eur J Haematol. 77:255–258. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Coiffier B, Lepage E, Briere J, et al:
CHOP chemotherapy plus rituximab compared with CHOP alone in
elderly patients with diffuse large-B-cell lymphoma. N Engl J Med.
346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Habermann TM, Weller EA, Morrison VA, et
al: Rituximab-CHOP versus CHOP alone or with maintenance rituximab
in older patients with diffuse large B-cell lymphoma. J Clin Oncol.
24:3121–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Dai MS, Chao TY, Kao WY, Shyu RY and Liu
TM: Delayed hepatitis B virus reactivation after cessation of
preemptive lamivudine in lymphoma patients treated with rituximab
plus CHOP. Ann Hematol. 83:769–774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Westhoff TH, Jochimsen F, Schmittel A, et
al: Fatal hepatitis B virus reactivation by an escape mutant
following rituximab therapy. Blood. 102:19302003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hsu C, Hsiung CA, Su IJ, et al: A revisit
of prophylactic lamivudine for chemotherapy-associated hepatitis B
reactivation in non-Hodgkin’s lymphoma: a randomized trial.
Hepatology. 47:844–853. 2008.PubMed/NCBI
|
|
21.
|
Conconi A, Zucca E, Roggero E, et al:
Prognostic models for diffuse large B-cell lymphoma. Hematol Oncol.
18:61–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chinese Society of Hepatology and Chinese
Society of Infectious Diseases, Chinese Medical Association: The
guideline of prevention and treatment for chronic hepatitis B (2010
version). Zhonghua Liu Xing Bing Xue Za Zhi. 32:405–415. 2011.(In
Chinese).
|
|
23.
|
Wang F, Xu RH, Luo HY, et al: Clinical and
prognostic analysis of hepatitis B virus infection in diffuse large
B-cell lymphoma. BMC Cancer. 8:1152008. View Article : Google Scholar : PubMed/NCBI
|