Introduction
Telomeres are specialized deoxyribonucleic acid
(DNA)-protein structures that contain non-coding TTAGGG repeats and
telomere-associated proteins, and are essential for chromosome
stability. Telomere length is an indicator of replicative history,
and has been considered a biomarker of aging (1). The maintenance of telomeres is
primarily achieved by telomerase, a ribonucleoprotein with reverse
transcriptase activity that uses its internal ribonucleic acid
component as a template for the synthesis of telomeric DNA.
Telomerase activity is present during early development and in
adult germline and stem cells of self-renewing tissues; however, it
is absent or functionally insufficient in adult somatic cells.
Human telomerase reverse transcriptase (hTERT) is a subunit of
telomerase, and is essential for telomerase activity (2,3). In
the absence of hTERT, telomeres undergo shortening with cell
division, a process that may act as a mitotic clock and trigger
entry into senescence. The shortening of telomeres is thus
considered to be responsible for the limited lifespan of somatic
cells in culture, and has also been correlated with organismal
aging (4–6). The mechanisms by which telomerase
controls these processes are beginning to be understood, and
include effects on the signaling cascades that regulate apoptosis.
The inhibition of telomerase and the ensuing shortening of
telomeres below a critical length may result in apoptosis in
various cell types, whereas the induction of telomerase activity is
correlated with a resistance to apoptosis (7,8). In
particular, polymorphonuclear neutrophils (PMNs) have a finite
lifespan, and typically die by undergoing apoptosis. Thus, PMN
apoptosis represents a control mechanism limiting the toxic
potential of these short-lived, terminally differentiated cells
(9,10). Post-mortem studies have revealed
the infiltration of PMNs in unstable atherosclerotic plaques,
suggesting that PMNs may be involved in plaque destabilization
(11). Classical non-steroidal
anti-inflammatory drugs (NSAIDs), including aspirin, exert
chemopreventive effects on atherosclerosis. Animal, epidemiological
and clinical studies have indicated that aspirin may significantly
reduce the risk of atherosclerosis, and predict the risk of
cerebrovascular diseases, due to its inhibition of cell
proliferation and angiogenesis (12,13).
Although the anti-atherosclerotic mechanisms of aspirin have been
extensively studied, little is known with regard to the
interrelation between aspirin and the telomerase activity in PMNs.
Therefore, the aim of this study was to determine whether aspirin
inhibited hTERT and telomerase activity in unstable carotid
plaques.
Materials and methods
Design and subjects
Ten patients with lipid-rich plaques and severe
(>70%) internal carotid artery (ICA) stenosis underwent carotid
angioplasty and stenting procedures in the Department of Neurology,
Jiangmen Central Hospital (Jiangmen, China). Patients with chronic
or acute infections, or an inflammatory condition, as defined
elsewhere (14), were excluded
from the study.
There study included seven males (70%) and three
females (30%), with a mean age of 73±10 years (range, 47–75 years).
Concomitant risk factors, such as hypertension, coronary artery
disease, diabetes and a history of tobacco use, were present in 80,
50, 40 and 30% of patients, respectively. The protocol was approved
by the Ethics Committee of Jinan University (Shenzhen, China), and
written informed consent was obtained from all patients.
Isolation of PMNs from ICA plaques, cell
culture and aspirin treatment
PMNs were isolated from carotid atherosclerotic
plaques with a novel approach, as described in a previous study by
Narducci et al (15).
Following carotid angiography, all patients underwent carotid
artery balloon angioplasty and stenting (CBAS) in the culprit
stenosis. Heparin (3,000 IU) was administered to all patients. The
stent deployment was preceded by predilation with Maverick
angioplasty balloons (Boston Scientific, Natick, MA, USA). In
brief, the PMNs were collected as follows: the predilation balloon
was inflated for 20 sec at a mean of 10 atmospheres (range, 6–14
atmospheres). The balloon was then deflated and the guiding
catheter was immediately retracted. The washing medium was
collected into tubes, and was then supplemented with 5 ml
Cytolyt® solution (Cytyc Corporation, Boxborough, MA,
USA). The balloon remained inside the guiding catheter for <5
sec, in order to minimize the contamination by blood. The washing
medium was centrifuged at 1,200 × g for 10 min, and the cell pellet
was then collected and added to 20 ml Preservcyt®
solution (Cytyc Corporation). The cells were maintained at 37°C in
an atmosphere with 5% CO2 and 95% air, in RPMI-1640
medium containing 10% fetal bovine serum (FBS; Gibco®,
Invitrogen Life Technologies, Carlsbad, CA, USA), 100 U/ml
penicillin and 100 μg/ml streptomycin. Following treatment
with aspirin (0.5 mM) (16) for 48
h, the cell lysate was collected for the detection of telomerase
activity. Cells treated with dimethyl sulfoxide (DMSO) served as a
control, and the final concentration of DMSO was ≤0.1%. Data are
expressed as the mean ± standard deviation from three independent
experiments.
Peripheral cell isolation
During the carotid angiography, blood was sampled
from the right femoral artery. The PMNs were isolated with
Polymorphprep™ separation medium (Nycomed Pharma AS, Oslo, Norway)
and, following centrifugation at 500 × g for 30 min at 20°C, the
PMNs were collected and washed twice in phosphate-buffered saline
(PBS). Contaminating erythrocytes were removed by hypotonic lysis.
The PMNs were stored at −80°C.
Detection of telomerase activity
The activity of telomerase was analyzed with a
TRAPeze® Telomerase Detection kit (Intergen Co., Oxford,
UK), in accordance with the manufacturer's instructions. In brief,
50 ng cell extract was used in a polymerase chain reaction (PCR)
assay, prior to 5 μl PCR product being used in an
enzyme-linked immunosorbent assay (ELISA). The absorbance was
measured at 450 and 690 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Reverse transcription (RT)-PCR
Total RNA was extracted from the PMN cells using
TRIzol® Reagent (Invitrogen Life Technologies), and the
cDNA was synthesized using a Thermoscript RT-PCR System kit
(Invitrogen Life Technologies). The PCR primers were as follows:
hTERT, forward: 5′-CGG AAG AGT GTC TGG AGC AA-3′ and reverse:
5′-GGA TGA AGC GGA GTC TGG A-3′; and GAPDH, forward: 5′-GAC CAC ACG
CCA TGC CAT CAC-3′ and reverse: 5′-GTC CAC CAC CC TG TTG CTG TA-3′.
A total of 30 cycles of PCR were completed for hTERT and 28 cycles
for GAPDH (94°C for 1 min, 55°C for 1 min and 72°C for 1 min), and
the expression of hTERT was normalized to that of GAPDH.
Western blot analysis
The extracted proteins (60 μg) were subjected
to sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), and were then transferred to a nitrocellular membrane.
hTERT and actin were detected with hTERT and actin polyclonal
antibodies, respectively (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). Actin served as an internal control.
Statistical analysis
Statistical analysis was performed using Excel
software (Microsoft Corporation, Redmond, WA, USA). The Student's
t-test was used to compare data between two groups. Data are
expressed as the mean ± standard deviation (SD). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of aspirin on telomerase
activity
The activity of telomerase, measured by a telomeric
repeat amplification protocol (TRAP) assay, in the PMNs isolated
from the arterial blood and from the washing medium of the
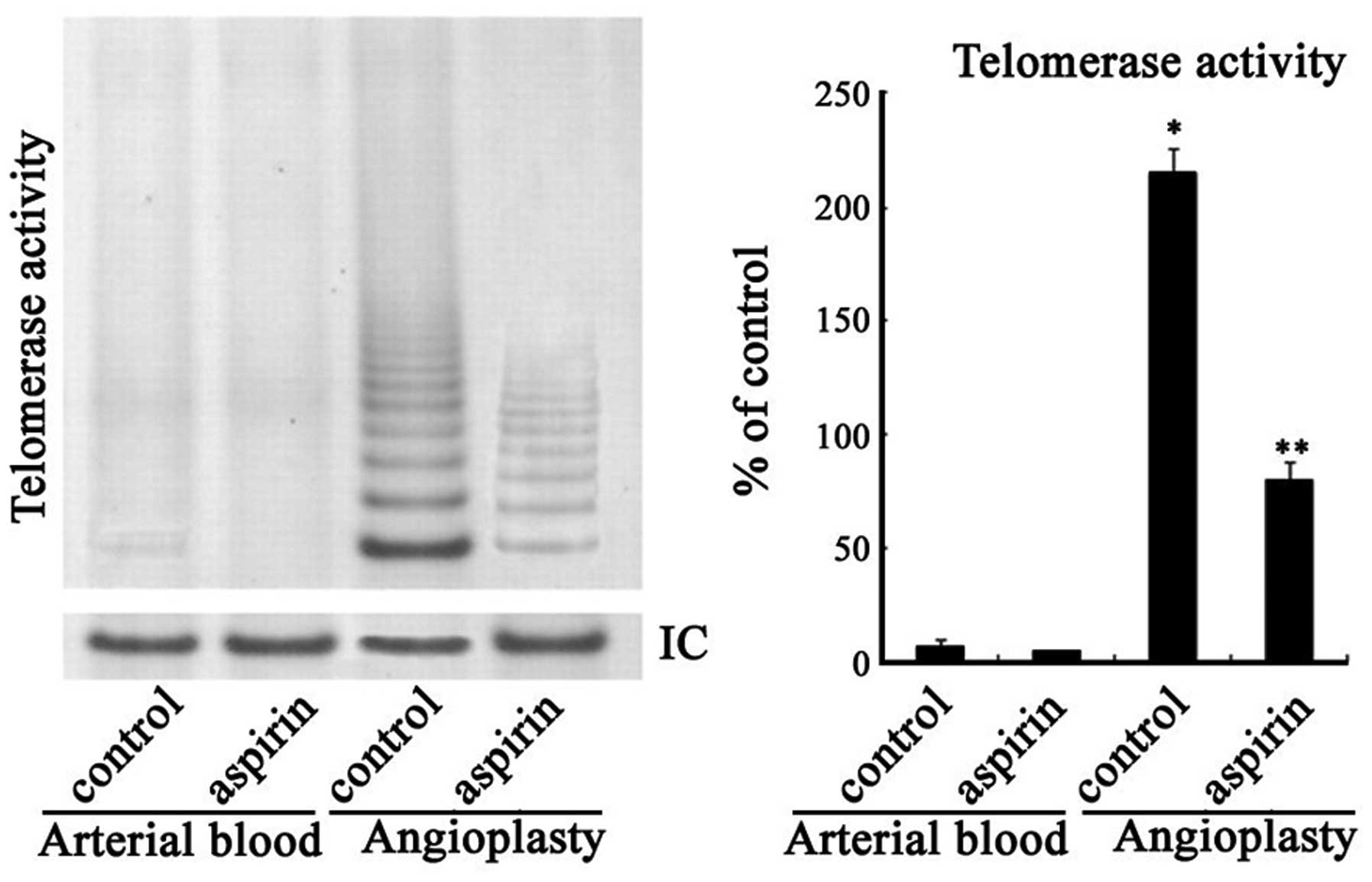
angioplasty balloons, is displayed in Fig. 1. Telomerase activity was observed
to be significantly increased in the PMNs isolated from the
angioplasty washing medium compared with the arterial blood
control. Telomerase activity was undetectable in the PMNs derived
from the arterial blood of patients with CBAS.
Telomerase activity was higher in the PMNs from the
unstable carotid plaques than in the circulating PMNs. Aspirin (0.5
mM) inhibited the telomerase activity of the plaque-derived PMNs by
∼62.8% compared with that of the PMNs treated with DMSO (79.5
versus 213.8% activity, for aspirin and DMSO-treated cells,
respectively). However, aspirin did not exert any inhibitory effect
on the telomerase activity in the circulating PMNs.
Effect of aspirin on the mRNA and protein
expression of hTERT in unstable carotid plaques
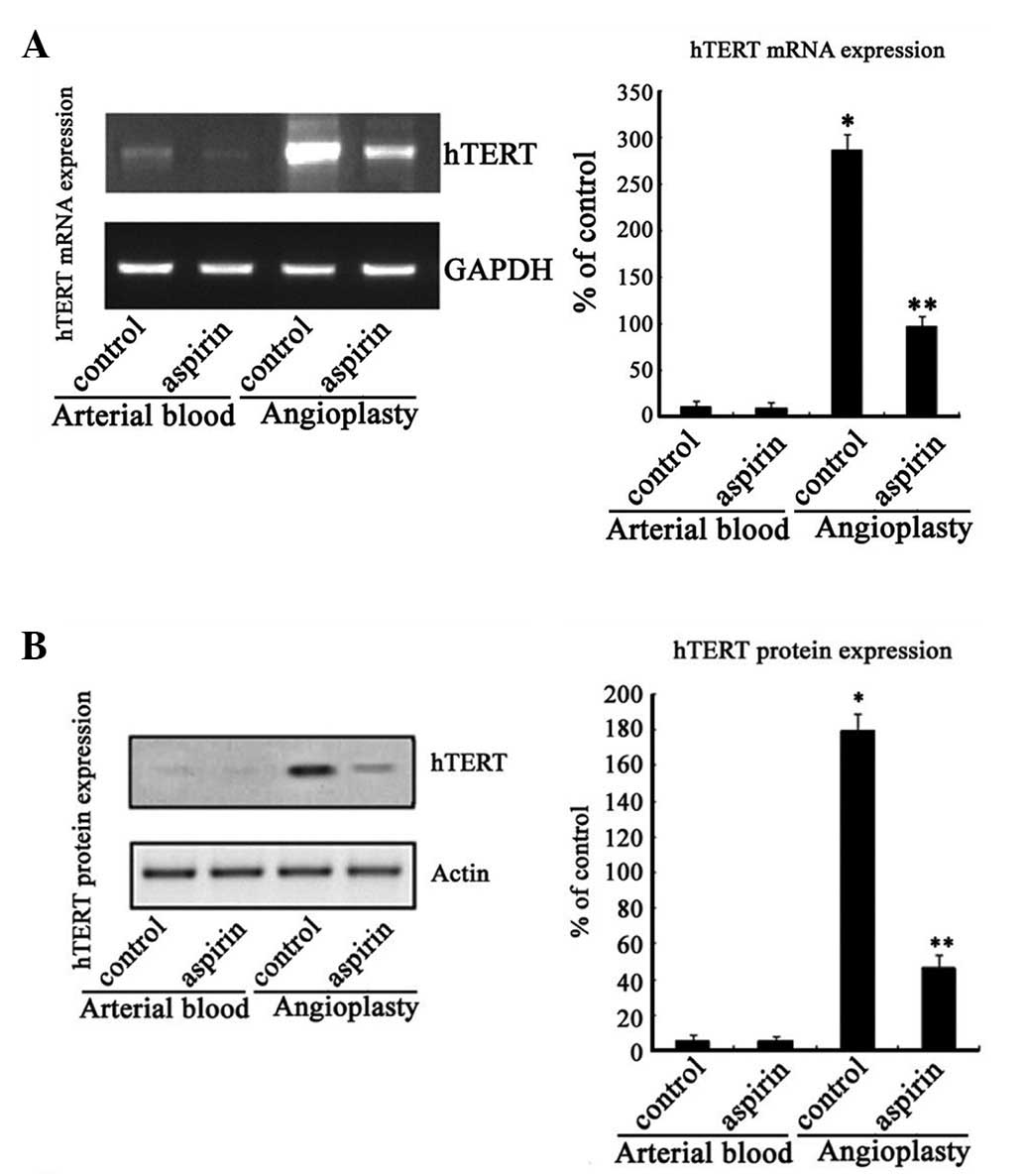
To investigate whether aspirin was able to inhibit
the mRNA and protein expression of hTERT, RT-PCR and western blot
analysis were performed. Extracts of PMNs isolated from the
angioplasty washing medium demonstrated high levels of mRNA and
protein expression of hTERT in the unstable carotid plaques.
However, the levels of mRNA and protein expression of hTERT were
almost undetectable in the PMNs from the arterial blood of patients
who underwent CBAS.
The mRNA expression of hTERT was inhibited by 66.2%,
following aspirin (0.5 mM) treatment, compared with the cells
treated with DMSO (96.7 versus 286.1% expression for
aspirin-treated and control cells, respectively; Fig. 2A). Aspirin treatment (0.5 mM)
inhibited the protein expression of hTERT by 73.8% compared with
the cells treated with DMSO (46.7 versus 178.3% expression for
aspirin-treated and control cells, respectively; Fig. 2B). However, aspirin did not exert
any inhibitory effects on the mRNA and protein expression of hTERT
in the circulating PMNs (Fig.
2).
Discussion
The activation of telomerase, which occurs in the
majority of atherosclerotic plaques, results in the prolonged
lifespan of cells. This is an important feature in the early phases
of instability of atherosclerotic plaques (17–19).
However, there have only been a limited number of studies that have
investigated the effect of aspirin on the telomerase activity in
PMNs. In order to characterize the mechanism of delayed PMN
apoptosis in unstable carotid plaques, and the correlation between
aspirin and telomerase activity, the activity of telomerase was
determined in PMNs isolated from peripheral blood in patients with
lipid-rich plaques and severe (>70%) ICA stenosis. For the same
patients, telomerase activity was also measured in PMNs isolated
from the washing medium used during percutaneous angioplasty and
stenting procedures. In addition, the telomerase activity, and mRNA
and protein expression of hTERT were determined in cells that had
receieved aspirin treatment. TERT possesses the catalytic activity
of telomerase, and is primarily regulated through transcriptional
mechanisms (20).
The present study demonstrated that there was high
telomerase activity in the PMNs from the carotid plaques of
patients with severe ICA stenosis, but not in the PMNs from
peripheral blood. Notably, in patients with severe ICA stenosis,
the sole predictor of telomerase activity in the coronary
atherosclerotic plaques was a reduced time interval from the onset
of the symptoms to PMN collection, which supports the potential
importance of telomerase reactivation in PMN persistence during the
early phases of carotid plaque instability.
Under normal circumstances, PMNs, similar to other
somatic cells, divide a limited number of times, prior to entering
a nondividing state, known as replicative senescence. In general,
telomerase is inhibited in inflammatory cells (21,22).
The present study revealed that telomerase activity was virtually
undetectable in the circulating PMNs, and that the reactivation of
telomerase in these cells was possible. This reactivation
represents a a method of overcoming replicative senescence
(8,23). The high telomerase activity in the
PMNs from the carotid atherosclerotic plaques in the current study
suggested that there was a local process leading to the
reactivation of the intracellular enzyme, which resulted in the
prolonged survival of these inflammatory cells, as observed in
cells retaining a high proliferative potential (24,25).
Furthermore, the telomere dynamics and the changes in telomerase
activity were consistent with a proliferative state. A highly
specific correlation and an early causal interrelation have been
observed between telomerase activation and indefinite cell
proliferation (26,27).
Therefore, it was indicated that in the patients
with severe ICA stenosis and unstable plaques, the survival of
local activated PMNs was prolonged due to the reactivation of
telomerase. It was likely that this exacerbated the tissue damage
and oxidative stress due to PMN activation, and maintained the
active inflammatory process, since neutrophil apoptosis has been
identified as a crtitical mechanism in the cessation of
inflammation. However, as telomerase reactivation appears to be
important in delaying apoptosis and inducing the growth of cancer
cells (28), this intracellular
mechanism may have also prolonged the activation of the PMNs in the
carotid plaques by extending their local lifespans.
Classical NSAIDs, including aspirin, have been
demonstrated to exert chemopreventive effects on atherosclerosis.
The present study revealed that aspirin inhibited the telomerase
activity in PMNs from carotid lipid-rich plaques, but had no
inhibitory effect on the circulating PMNs. Further studies were
performed to determine whether the inhibition of telomerase by
aspirin was attributed to the inhibition of hTERT mRNA and protein
expression. RT-PCR and western blot analysis demonstrated that
aspirin reduced the mRNA and protein expression levels of hTERT in
the PMNs from carotid plaques, but not in circulating PMNs. This
may be attributed to the altered catalytic activity of the
telomerase holoenzyme following aspirin treatment.
Previous studies have observed that the
phosphorylation of hTERT is involved in the regulation of
telomerase (16), and that certain
kinases, including Akt kinase and protein kinase C (PKC), mediate
the phosphorylation of hTERT, leading to telomerase activation
(29,30). Aspirin may downregulate the
expression of telomerase by interfering with these pathways. It is
likely that aspirin inhibits the transcriptional activity of hTERT
by suppressing the activation or binding activity of the previously
mentioned factors. In the present study, the inhibitory effect of
aspirin on hTERT was correlated with the inhibition of telomerase
activity, which suggests that the decreased hTERT expression
accounts for the inhibition of telomerase activity following
aspirin treatment.
In conclusion, aspirin is able to inhibit telomerase
activity, primarily through the suppression of transcriptional
activity, and the mRNA and protein expression of hTERT in the
carotid atherosclerotic plaques. The promoter (−145 to −330 bp) of
hTERT may be the cis-response element to aspirin. Telomerase
activity is an important factor in unstable plaques, and is
predictive of the future occurrence of cerebrovascular diseases.
Thus, an enhanced understanding of the mechanisms involved in these
processes is required to enable the application of telomerase or
telomerase-related compounds as targets in the treatment of
cerebrovascular diseases. Further studies are required to identify
the potential cis-response elements to aspirin.
Acknowledgements
This study was supported by the
Program of the Health Department of Guangdong Province (grant no.
WSTJJ20101025413001197002070553). The authors would like to thank
Dr Qianglin Duan from Tongji Hospital of Tongji University for the
critical reading of the manuscript.
References
|
1.
|
Samani NJ and van der Harst P: Biological
ageing and cardiovascular disease. Heart. 94:537–539. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Greider CW: Telomerase activity, cell
proliferation, and cancer. Proc Natl Acad Sci USA. 95:90–92. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Le S, Sternglanz R and Greider CW:
Identification of two RNA-binding proteins associated with human
telomerase RNA. Mol Biol Cell. 11:999–1010. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bailey SM and Murnane JP: Telomeres,
chromosome instability and cancer. Nucleic Acids Res. 34:2408–2417.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Harley CB and Villeponteau B: Telomeres
and telomerase in aging and cancer. Curr Opin Genet Dev. 5:249–255.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shay JW and Wright WE: Hayflick, his
limit, and cellular ageing. Nat Rev Mol Cell Biol. 1:72–76. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Holt SE, Glinsky VV, Ivanova AB and
Glinsky GV: Resistance to apoptosis in human cells conferred by
telomerase function and telomere stability. Mol Carcinog.
25:241–248. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yang J, Chang E, Cherry AM, et al: Human
endothelial cell life extension by telomerase expression. J Biol
Chem. 274:26141–26148. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Weinmann P, Gaehtgens P and Walzog B:
Bcl-Xl- and Bax-alpha-mediated regulation of apoptosis of human
neutrophils via caspase-3. Blood. 93:3106–3115. 1999.PubMed/NCBI
|
|
10.
|
Sung YH, Choi YS, Cheong C and Lee HW: The
pleiotropy of telomerase against cell death. Mol Cells. 19:303–309.
2005.PubMed/NCBI
|
|
11.
|
Naruko T, Ueda M, Haze K, et al:
Neutrophil infiltration of culprit lesions in acute coronary
syndromes. Circulation. 106:2894–2900. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Husain S, Andrews NP, Mulcahy D, Panza JA
and Quyyumi AA: Aspirin improves endothelial dysfunction in
atherosclerosis. Circulation. 97:716–720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cyrus T, Yao Y, Tung LX and Praticò D:
Stabilization of advanced atherosclerosis in low-density
lipoprotein receptor-deficient mice by aspirin. Atherosclerosis.
184:8–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Biasucci LM, Liuzzo G, Grillo RL, et al:
Elevated levels of C-reactive protein at discharge in patients with
unstable angina predict recurrent instability. Circulation.
99:855–860. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Narducci ML, Grasselli A, Biasucci LM, et
al: High telomerase activity in neutrophils from unstable coronary
plaques. J Am Coll Cardiol. 50:2369–2374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
He H, Xia HH, Wang JD, et al: Inhibition
of human telomerase reverse transcriptase by nonsteroidal
antiinflammatory drugs in colon carcinoma. Cancer. 106:1243–1249.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Poch E, Carbonell P, Franco S, Díez-Juan
A, Blasco MA and Andrés V: Short telomeres protect from
diet-induced atherosclerosis in apolipoprotein E-null mice. FASEB
J. 18:418–420. 2004.PubMed/NCBI
|
|
18.
|
Brouilette SW, Moore JS, McMahon AD, et
al: Telomere length, risk of coronary heart disease, and statin
treatment in the West of Scotland Primary Prevention Study: a
nested case-control study. Lancet. 369:107–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gizard F, Heywood EB, Findeisen HM, et al:
Telomerase activation in atherosclerosis and induction of
telomerase reverse transcriptase expression by inflammatory stimuli
in macrophages. Arterioscler Thromb Vasc Biol. 31:245–252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ducrest AL, Szutorisz H, Lingner J and
Nabholz M: Regulation of the human telomerase reverse transcriptase
gene. Oncogene. 21:541–552. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hiyama K, Hirai Y, Kyoizumi S, et al:
Activation of telomerase in human lymphocytes and hematopoietic
progenitor cells. J Immunol. 155:3711–3715. 1995.PubMed/NCBI
|
|
22.
|
Robertson JD, Gale RE, Wynn RF, Dougal M,
Linch DC, Testa NG and Chopra R: Dynamics of telomere shortening in
neutrophils and T lymphocytes during ageing and the relationship to
skewed X chromosome inactivation patterns. Br J Haematol.
109:272–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Norrback KF, Enblad G, Erlanson M,
Sundström C and Roos G: Telomerase activity in Hodgkin's disease.
Blood. 92:566–573. 1998.
|
|
24.
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wong JM and Collins K: Telomere
maintenance and disease. Lancet. 362:983–988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bodnar AG, Oulette M, Frolkis M, et al:
Extension of life-span by introduction of telomerase into normal
human cells. Science. 279:349–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Satyanarayana A, Manns MP and Rudolph KL:
Telomeres, telomerase and cancer: an endless search to target the
ends. Cell Cycle. 3:1138–1150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Zimmerman S and Martens UM: Telomeres and
telomerase as targets for cancer therapy. Cell Mol Life Sci.
64:906–921. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kang SS, Kwon T, Kwon DY and Do SI: Akt
protein kinase enhances human telomerase activity through
phosphorylation of telomerase reverse transcriptase subunit. J Biol
Chem. 274:13085–13090. 1999. View Article : Google Scholar
|
|
30.
|
Li H, Zhao L, Yang Z, Funder JW and Liu
JP: Telomerase is controlled by protein kinase Cα in human breast
cancer cells. J Biol Chem. 273:33436–33442. 1998.PubMed/NCBI
|