Introduction
Coronary artery bypass grafting (CABG) surgery,
including on-pump CABG and off-pump CABG (OP-CABG) surgery, is one
of the landmark procedures in the history of cardiac surgery and
has saved millions of individuals afflicted by coronary artery
disease. OP-CABG is the more recent of the two techniques, with the
reported benefits of reductions in blood loss and neurological
impairment (1). Moreover, the
OP-CABG procedure avoids the adverse effects of extracorporeal
circulation on the physiological functions of patients, thus
reducing respiratory problems and resulting in fewer complications
and a faster recovery. OP-CABG is also associated with reduced
risk-adjusted mortality and morbidity (2,3).
Since OP-CABG cases require vigilant anticipation of the surgical
steps, the success of this technique relies upon skilled
hemodynamic management, close communication with the cardiothoracic
surgeon and controlled sedation and analgesia. Currently,
remifentanil is widely used in cardiac surgery due to its sedative,
analgesic and anti-tussive properties, which allow a prolonged
infusion without drug accumulation (4). In addition, remifentanil
target-controlled infusion (TCI) may be applied in OP-CABG for
fast-track anesthesia, which combines a real-time pharmacokinetic
model with an infusion pump and allows the administration and
maintenance of a constant blood concentration of remifentanil. TCI
also enables changes in the blood concentration to be made rapidly
and easily. This procedure has a high safety and efficacy and is
suitable for cardiac surgeries (5). In general, as there are no
standardized methods for determining the state of analgesia,
anesthesiologists determine the surgical stimulation intensity
based on their clinical experience and adjust the depth of
anesthesia accordingly (6). The
lack of objective monitoring indices for immediate stress responses
makes it difficult to control the quality of anesthesia. In
clinical practice, individual signs of inadequate analgesia may
include a change in hemodynamic indices, including the heart rate
(HR) and mean arterial pressure (MAP), and a change in the
concentration of plasma epinephrine (E), cortisol (COR), blood
glucose (BG) and lactate (LAC), all of which indicate the intensity
of the stress response to a certain extent (7,8,9).
However, hemodynamic indices may be affected by a number of factors
and thus differ significantly among individuals. The HR and MAP are
classical parameters for registering the adequacy of analgesia,
whereas the non-invasively measured MAP is not suitable for
closed-loop control since it supplies the measured values in
intervals of several minutes only. Similarly, blood biochemical
parameters are more objective but it is not possible to monitor
them conventionally in a real-time manner. The various parameters
of heart rate variability (HRV) offer the possibility of supporting
the autonomic response of the HR without the induction of a further
monitor. HRV measures the variation in the HR and is calculated by
analyzing the beat-to-beat intervals from an electrocardiogram
(ECG) or an arterial pressure tracing. In this way, the
parasympathetic and sympathetic activities of the autonomic nervous
system (ANS) are visualized. Therefore, the non-invasive HRV
analysis may quantitatively and rapidly evaluate the tension and
balance of cardiac sympathetic and vagal nervous activities, as
well as their effects on cardiovascular system activity (10). The overall levels of functioning
and resilience, the degree of adaptability and the amount of
progress that patients are making with therapy may be quantified
using the HRV analysis, which has been widely applied in basic and
clinical research studies (11–13).
Thus, the continuous monitoring of post-operative HRV remains
necessary. The present study aimed to investigate the changes in
the HRV of patients undergoing OP-CABG with remifentanil TCI
anesthesia and to assess the clinical importance of HRV as a
real-time monitoring index of the stress reaction intensity.
Patients and methods
Patients
Patients with coronary heart disease (CHD) who
exhibited stable angina pectoris at room temperature and took
25–100 mg metoprolol daily, with no other β-blockers or only
metoprolol combined with a calcium antagonist, were selected for
the present study. The patients underwent OP-CABG in the Three
Gorges University People’s Hospital (Yichang, China) between June
2008 and May 2010. The exclusion criteria were as follows: i) a
conventional ECG showing irregularities that interfered with the
HRV analysis, including a left or right bundle branch block, left
ventricular hypertrophy, atrial fibrillation or flutter, a second-
or third-degree atrioventricular block or an R-wave to R-wave (RR)
interval of >0.24 sec; ii) a pacing cardiac rhythm; iii) the
presence of diabetes; iv) the presence of central nervous system
(CNS) or ANS diseases; or v) a pre-operative use of CNS drugs. A
total of 65 patients, consisting of 49 males and 16 females aged
68.4±10.2 years, with a mean body weight of 67.3±10.8 kg, were
selected. Of these patients, 33 exhibited hypertension, two
presented with aneurysms, 51 presented with ≥3 lesions, 11 with 2
lesions and three with a single lesion on the left main coronary
artery. Furthermore, 38 patients demonstrated grade II
pre-operative ventricular function and 27 demonstrated grade III
pre-operative ventricular function. The left ventricular ejection
fraction (LVEF) was >50% in 39 patients and 45–50% in 26
patients. The patients were randomized into a target-controlled
group (group I) with 32 patients and a constant-rate infusion group
(group II) with 33 patients. Approval for the present study was
obtained from the medical ethics board of The Three Gorges
University People’s Hospital. Prior to the surgery, the risks were
fully explained and informed consent was obtained from each
patient.
Anesthesia method
All patients were injected with 10 mg morphine and
0.3 mg scopolamine prior to entering the operating room. The HRs
and blood pressures of the patients were monitored continuously
using an ECG and sphygmomanometer, and the pressure in the right
radial artery was measured using a puncture catheter under local
anesthesia. Intravenous anesthesia was induced in the two groups
using the sequential administration of 10 mg dexamethasone, 0.05
mg/kg midazolam, 0.15 mg/kg etomidate, 0.15 mg/kg vecuronium and 10
μg/kg fentanyl. Tracheal intubation was conducted 5 min
later and TCIs of propofol were then performed to maintain the
plasma concentration (Cp) at 1.0–2.5 μg/ml and the
bispectral index (BIS) at 45–60. Remifentanil was infused in a
target-controlled manner at 1.5–5.0 ng/ml for group I and at a
constant–rate of 0.05–1.0 μg/kg/min for group II, with
additional single increments of 1 μg/kg remifentanil when
appropriate. The central venous pressure (CVP), pulmonary artery
pressure (PAP), pulmonary capillary wedge pressure (PCWP) and
partial pressure of end-tidal CO2 were monitored and the
arterial blood gas was intermittently checked. Following the
opening of the central vein, 0.5–2.0 μg/kg/min
nitroglycerin, 1–3 μg/kg/min dopamine and 10–80 ng/kg/min
norepinephrine were continuously infused. Vecuronium was
administered to maintain muscle relaxation. A single injection of
20–100 μg phenylephrine was used to stabilize the blood
pressure when changing the position of the patients and the
parameters of the ventilator were adjusted to maintain the
indicators of the arterial blood gas within the normal range. In
groups I and II, following sternal closure, a single intravenous
injection of 100 mg tramadol and 5 mg tropisetron and a controlled
analgesia pump were used to manage any post-operative pain. The
analgesia pump formula consisted of 1.0 g tramadol, 100 mg
flurbiprofen and 0.9% sodium chloride in 100 ml, which was
administered at a rate of 2 ml/h, controlled to 0.5 ml per infusion
and then locked for 15 min.
Intensive care unit (ICU) monitoring
Following surgery, the patients were sent to the
ICU, where the ECG, arterial blood pressure (ABP), oxygen
saturation (SpO2), CVP, PAP, PCWP, blood gases and
electrolytes were monitored. Vasoactive drugs were infused to
maintain hemodynamic stability and to support respiratory function.
The monitoring was continued following tracheal extubation.
Monitoring indices
The hemodynamic indices, including the HR, MAP, CVP,
PAP and PCWP, were monitored and recorded prior to induction
(T0), following induction (T1), during
endotracheal intubation (T2), 2 min subsequent to the
chest being opened (T3), at exposure and subsequent to
fixing the anterior descending artery (T4), subsequent
to exposing and fixing the right coronary and/or circumflex artery
(T5), at the end of surgery (T6), at tracheal
extubation (T7) and at 24 h post-surgery
(T8). The total amounts of nitroglycerin, dopamine,
norepinephrine, and phenylephrine that were used in the two groups
during the surgery were also measured. Arterial blood was drawn to
check the blood gas levels at T0, T2,
T3, T5, T6, T7 and
T8. Following centrifugation, the plasma concentrations
of E, COR, BG, and LAC were measured. The corrected value was
calculated as follows: Measured value x basal hemoglobin
concentration/actual hemoglobin concentration. A HXD-I
multi-function monitoring system (Huaxiang company, Harbin, China)
was used to measure the HRV indices by frequency domain analysis,
with the frequency range of the total power (TP) as 0–0.5 Hz. The
low frequency (LF) was 0.03–0.15 Hz and the high frequency (HF) was
0.15–0.40 Hz. The LF/HF ratio of power (LF/HF) was calculated.
These indicators were recorded at T0, T2,
T3, T5, T6, T7 and
T8.
Statistical analysis
All the analyses were carried out using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). The TP, HF and LF data
were transformed by a natural logarithm, while the LF/HF was
transformed by the square root. All data are presented as the mean
± standard deviation (SD). The Student’s t-test was used for the
comparison of the measurement data, including the age and weight of
the patients, the drug dosage and the surgery time, between the
groups. The single-factor repeated measures analysis of variance
was used for the comparison of the acquired HRV relevant indices at
each time point for the two groups. Multiple comparisons were
conducted using the least significant difference (LSD) method. The
χ2 test was used to examine the count data. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 65 patients who underwent a CABG
procedure between June 2008 and May 2010 were randomized into a
target-controlled group (group I) consisting of 32 patients and a
constant-rate infusion group (group II) consisting of 33 patients.
During the perioperative period, no patient succumbed, had serious
complications or required changing to extracorporeal circulation.
The basic characteristics of the patients in the two groups,
including gender, age, body weight, LVEF, duration of surgery,
number of bypasses and remifentanil, metoprolol, nitroglycerin and
esmolol dosages, showed no significant differences between the
groups with the exception of the phenylephrine dosage. The patients
in group II were administered greater amounts of phenylephrine than
those in group I (P<0.05); the phenylephrine was mostly used
when moving the heart and anastomosing the right coronary and/or
circumflex artery vascular bridge (Table I).
 | Table I.Comparison of general characteristics
between the groups. |
Table I.
Comparison of general characteristics
between the groups.
| Characteristics | Group I (n=32) | Group II (n=33) |
|---|
| Gender
(male/female) | 25/7 | 24/9 |
| Age (years) | 68.5±10.7 | 67.9±10.5 |
| Body weight
(kg) | 68.2±11.1 | 67.1±10.7 |
| LVEF value (%) | 53.9±7.0 | 54.3±6.9 |
| Remifentanil dosage
(mg) | 1.68±0.29 | 1.65±0.28 |
| Metoprolol dosage
(mg/day) | 55.8±21.2 | 55.4±20.8 |
| Duration of surgery
(min) | 239.0±33.2 | 239.5±33.7 |
| Number of
bypasses | 3.8±1.1 | 3.8±1.2 |
| Nitroglycerin
dosage (mg) | 12.9±2.4 | 13.3±2.2 |
| Dopamine dosage
(mg) | 36.4±8.1 | 35.9±8.8 |
| Norepinephrine
dosage (μg) | 188.3±20.7 | 191.4±19.5 |
| Esmolol dosage
(mg) | 22.4±7.1 | 23.1±7.6 |
| Phenylephrine
dosage (μg) | 56.4±8.2 | 103.8±13.7a |
Changes in hemodynamic indices
Despite the similar characteristics observed in the
two groups following the CABG procedure, changes in the hemodynamic
indices, including the HR, MAP, CVP, PAP and PCWP, were analyzed
during the perioperative period at various time points from
T0 to T8 in the two groups. Among these
parameters, a significant increase was observed in the HR and MAP,
but only between the T0 and T7 time points
for each group (P<0.05; Table
II), whereas no significant changes occurred in CVP, PAP or
PCWP among the various time points during the perioperative
period.
 | Table II.Changes in hemodynamic indices in
group I (n=32) and group II (n=33) during the perioperative period
at various time points. |
Table II.
Changes in hemodynamic indices in
group I (n=32) and group II (n=33) during the perioperative period
at various time points.
| Indices | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 |
|---|
| HR (bpm) | | | | | | | | | |
| I | 56.9±7.4 | 55.6±6.5 | 56.8±6.8 | 58.1±7.0 | 56.4±6.5 | 56.0±6.6 | 56.2±7.0 | 69.8±9.5a | 58.2±6.8 |
| II | 56.8±7.5 | 55.6±6.8 | 56.9±7.0 | 57.9±6.8 | 56.7±6.9 | 56.2±7.0 | 56.4±7.1 | 69.8±9.7a | 58.7±7.3 |
| MAP (mmHg) | | | | | | | | | |
| I | 72.3±11.6 | 69.9±10.2 | 71.1±11.2 | 72.2±10.4 | 71.5±9.9 | 69.3±10.7 | 72.1±10.2 | 82.1±10.8a | 74.1±10.5 |
| II | 72.6±12.0 | 69.7±10.1 | 71.3±10.7 | 72.5±10.3 | 72.0±9.7 | 69.7±10.1 | 71.6±10.5 | 86.2±10.9a | 74.1±10.5 |
| CVP (mmHg) | | | | | | | | | |
| I | 4.7±0.8 | 4.8±0.9 | 4.8±0.6 | 4.8±0.5 | 5.0±0.8 | 5.1±0.9 | 5.1±0.6 | 5.1±0.7 | 5.0±0.9 |
| II | 4.7±1.0 | 4.8±0.8 | 4.9±0.9 | 4.8±0.6 | 5.0±0.6 | 5.2±0.8 | 5.0±0.8 | 5.2±1.1 | 5.1±0.9 |
| PAP (mmHg) | | | | | | | | | |
| I | 18.6±5.1 | 19.1±4.9 | 18.9±4.4 | 18.9±4.8 | 19.3±4.9 | 19.5±5.1 | 18.9±5.1 | 19.1±5.0 | 18.8±5.0 |
| II | 18.7±4.9 | 19.1±5.0 | 18.8±4.8 | 19.0±4.7 | 19.4±4.9 | 19.4±5.1 | 19.2±5.0 | 19.3±5.2 | 18.7±5.1 |
| PCWP (mmHg) | | | | | | | | | |
| I | 10.8±2.2 | 10.7±2.1 | 10.9±2.2 | 11.0±1.8 | 10.7±1.9 | 11.1±2.1 | 11.0±1.8 | 10.7±1.9 | 10.8±2.1 |
| II | 10.7±2.1 | 10.7±2.2 | 10.9±2.3 | 11.0±1.9 | 10.8±2.1 | 10.9±2.1 | 11.0±1.8 | 10.8±1.9 | 10.9±2.2 |
Plasma levels of E, COR, BG and LAC
Similarly, the levels of E and COR in groups I and
II were significantly elevated at the T7 time point
compared with those at T0 (P<0.05). The levels at the
other time points, (T1, T2, T3,
T4, T5, T6 and T8),
were not significantly different from those at T0
(Table III), which was consistent
with the changes in the HR and MAP observed during the
perioperative period (Table II).
The BG and LAC levels increased at T3, T5,
T6, and T7 in the two groups. The levels in
group II were significantly higher than those in group I at
T5, T6 and T7 (P<0.05).
However, the levels were restored to baseline at T8
(Table III).
 | Table III.Changes in the plasma levels of E,
COR, BG and LAC in group I (n=32) and group II (n=33) at various
time points. |
Table III.
Changes in the plasma levels of E,
COR, BG and LAC in group I (n=32) and group II (n=33) at various
time points.
| Indices | T0 | T2 | T3 | T5 | T6 | T7 | T8 |
|---|
| E (pg/ml) | | | | | | | |
| I | 105±25 | 108±29 | 107±22 | 102±22 | 108±29 | 318±86a | 108±25 |
| II | 106±23 | 110±27 | 104±30 | 101±24 | 104±20 | 327±74a | 109±27 |
| COR (ng/ml) | | | | | | | |
| I | 76.6±15.8 | 74.2±14.9 | 79.8±10.6 | 75.8±16.5 | 75.8±15.8 | 98.1±20.9a | 80.1±16.6 |
| II | 78.3±16.1 | 74.8±15.1 | 79.9±10.9 | 76.5±15.9 | 76.0±14.9 | 99.2±21.8a | 80.3±17.8 |
| BG (mmol/l) | | | | | | | |
| I | 5.3±0.8 | 5.1±0.9 | 7.1±1.1a | 7.3±1.4a | 7.4±1.9a | 7.5±1.1a | 5.6±0.9 |
| II | 5.5±0.9 | 5.6±0.7 | 7.4±1.2a |
9.4±2.5ab |
10.4±1.8ab |
10.9±2.1ab | 5.9±1.0 |
| LAC (mmol/l) | | | | | | | |
| I | 0.9±0.3 | 1.0±0.3 | 1.7±0.5a | 1.8±0.4a | 1.7±0.2a | 1.6±0.3a | 1.0±0.4 |
| II | 1.0±0.3 | 1.0±0.4 | 1.8±0.6a |
2.9±0.3ab |
2.4±0.2ab |
2.2±0.3ab | 1.0±0.4 |
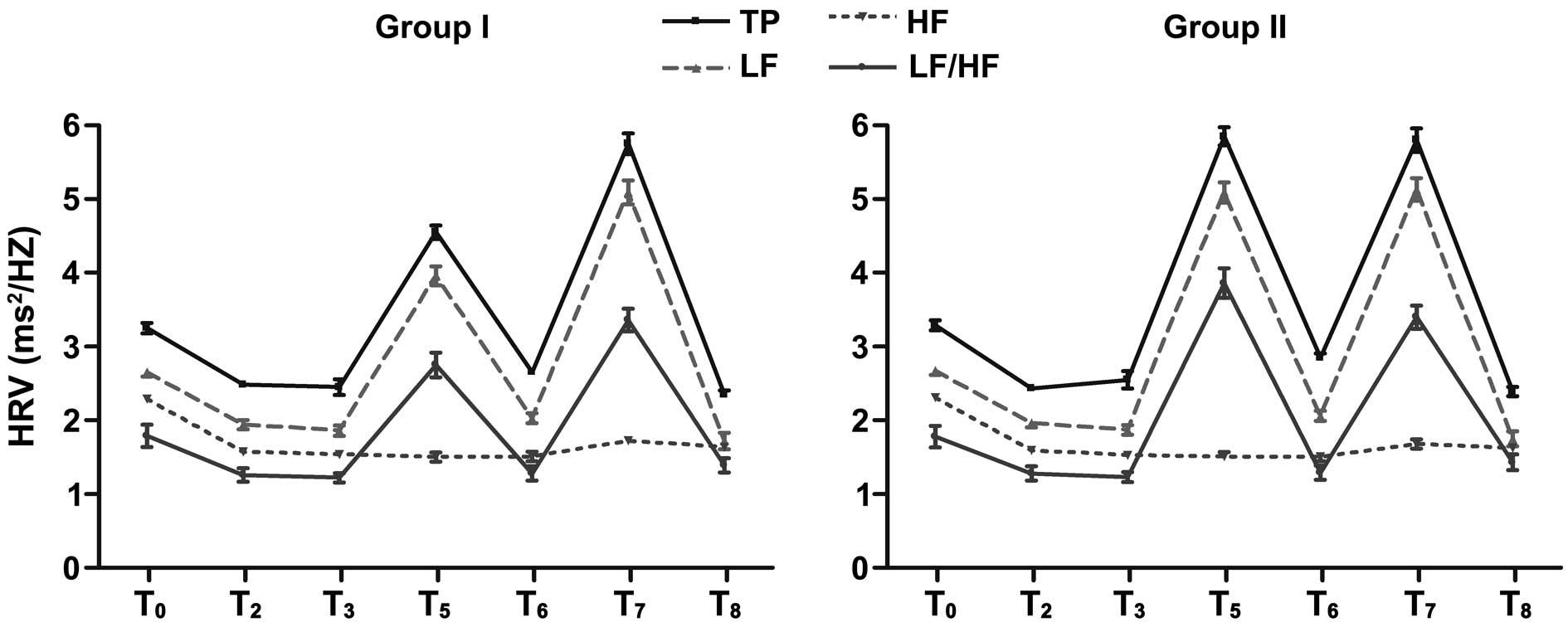
HRV changes in different time points
In a previous study, the BIS, a well-validated
measure of anesthetic depth, did not reflect the level of
intraoperative stress (14).
However, HRV may reflect ANS activity and stress-related
sympathetic activation may induce a similar change in the observed
HRV pattern (15). In the present
study, the HRV indices, including the TP, LF, HF and the LF/HF,
began to decrease following the induction of anesthesia at
T0. These parameters were significantly reduced in each
group at T2, T3, T6 and
T8 when compared with those at T0
(P<0.05). The HRV indices were not restored until 24 h
post-surgery. The values of TP, LF and LF/HF were significantly
increased at T5 and T7 (P<0.05). The
values of TP, LF and LF/HF in group II were significantly higher
compared with those in group I at T5 (P<0.05). At
other time points, there were no significant differences between
the two groups (Table IV). Thus,
the dynamic changes of the TP, LF, HF and LF/HF were observed in
the two groups at each time point. The indices at T5 and
T7 indicated peak values, which also showed significant
differences between the groups (Fig
1).
 | Table IV.Changes in HRV in group I (n=32) and
group II (n=33) at various time points. |
Table IV.
Changes in HRV in group I (n=32) and
group II (n=33) at various time points.
| Indices | T0 | T2 | T3 | T5 | T6 | T7 | T8 |
|---|
| TP
(msec2/Hz) | | | | | | | |
| I | 3.25±0.41 | 2.48±0.29a | 2.45±0.61a | 4.55±0.52a | 2.65±0.31a | 5.75±0.82a | 2.35±0.32a |
| II | 3.29±0.39 | 2.43±0.31a | 2.55±0.67a |
5.85±0.71ab | 2.85±0.34a | 5.80±0.90a | 2.39±0.37a |
| LF
(msec2/Hz) | | | | | | | |
| I | 2.65±0.33 | 1.94±0.35a | 1.86±0.41a | 3.96±0.73a | 2.03±0.38a | 5.09±0.93a | 1.72±0.63a |
| II | 2.67±0.34 | 1.96±0.33a | 1.87±0.39a |
5.09±0.79ab | 2.06±0.39a | 5.13±0.89a | 1.75±0.60a |
| HF
(msec2/Hz) | | | | | | | |
| I | 2.29±0.25 | 1.57±0.29a | 1.54±0.25a | 1.50±0.35a | 1.51±0.37a | 1.72±0.31a | 1.64±0.24a |
| II | 2.31±0.29 | 1.59±0.26a | 1.53±0.23a | 1.51±0.32a | 1.50±0.33a | 1.68±0.35a | 1.62±0.27a |
| LF/HF | | | | | | | |
| I | 1.79±0.86 | 1.26±0.52a | 1.22±0.36a | 2.75±0.96a | 1.28±0.56a | 3.36±0.86a | 1.39±0.56a |
| II | 1.78±0.84 | 1.28±0.56a | 1.23±0.40a |
3.86±1.16ab | 1.29±0.56a | 3.40±0.91a | 1.43±0.61a |
Discussion
Coronary heart disease (CHD) is currently the most
common form of heart disease and an significant cause of premature
mortality throughout the world. Patients with CHD have a lower
vagal tone, increased sympathetic activity and reduced
cardiovascular response to stress and adaptive regulation. These
are significant factors that may trigger ventricular arrhythmia,
myocardial infarction, abnormal changes in heart function and other
cardiac emergencies (15,16). Effective prevention and therapy for
CHD pose a major challenge to the entire medical community. The
perioperative measurement of HRV is a relatively new method of
assessing the balance of the ANS (17), which refers to successive small
differences between the cardiac cycles resulting from the cardiac
autonomic regulation of the sinus node. The TP (0–0.5 Hz) reflects
the general autonomic tone. The LF (0.03–0.15 Hz) is regulated by
the sympathetic and vagal nerves, with the sympathetic nerve having
the dominant role. The HF (0.15–0.40 Hz) is associated with
respiratory rhythm, reflecting the vagal tone, and the LF/HF
represents the balance between the sympathetic and vagal tone
(18). A reduced HRV is an index
of sympathetic nerve predominance, including the parasympathetic
nerve withdrawal state, in the ANS. Moreover, a reduced HRV has
been observed to predict certain cardiovascular events, including
sudden death and myocardial infarction, in patients with coronary
artery disease or in apparently healthy subjects (19,20).
However, the pathophysiological link between a reduced HRV and CHD
is not well understood. Dekker et al observed that a reduced
HRV was the strongest independent predictor of focal coronary
atherosclerosis in patients who had undergone a prior coronary
artery bypass surgery (21).
Similarly, the present study demonstrated that the HRV indices,
including the TP, LF, HF and LF/HF, began to decrease following the
induction of anesthesia at T0 and were not restored
until 24 h post-surgery, suggesting that a reduced HRV may be a
good predictor of pathological changes in patients following
OP-CABG. Therefore, considering the patient’s age, cardiac
function, history of myocardial infarction and other relevant
factors, a reduced HRV is an independent factor predicting sudden
cardiac death and clinical risk (22). Dupliakov et al (23) confirmed that a change in the
frequency domain of HRV was also associated with complications and
the prognosis. Certain β-receptor blockers, including metoprolol,
are often used to improve the LF/HF in such patients. Therefore,
monitoring the changes in HRV in patients with coronary artery
disease is crucial for reducing the incidence of adverse events
during the perioperative period (24–26).
A previous study showed that surgical stress
provoked the hypothalamic activation of the sympathetic ANS and
that HRV reflected sympathetic activation during orthostatic and
mental stress (27). HRV is
affected by anesthesia, and various anesthesia methods and drugs
have differing effects (28,29).
Sato et al (30) described
a reduced LF/HF attributable to a reduction in LF in patients with
sevoflurane or propofol anesthesia. It was concluded that the
choice of the anesthetic did not appear to play a critical role in
HRV. By contrast, Kanaya et al (28) demonstrated more distinct changes in
the HF in patients using propofol versus sevoflurane anesthesia,
concluding that sevoflurane has little effect on the cardiac
parasympathetic tone. However, in the present study, it was
demonstrated that the HRV indices changed with the variations in
the stress response, which indicated that remifentanil anesthesia
was positively correlated with the stress response. Therefore, if
the anesthesia during surgery is too shallow, the body will have
strong stress responses to surgical stimuli, thereby causing an
increase in the body’s sympathetic nerve excitation and anterior
pituitary-adrenal function, and this will therefore alter the
body’s endocrine, metabolic and immune functions. These changes
lead to a significant increase in a variety of stress response
factors, manifesting as high intra-operative levels of COR, glucose
and LAC. A corresponding change in HRV also occurs, in which the
main factor is an increased LF or LF/HF (31). To further understand the
correlation between HRV and the stress response, remifentanil
anesthesia was used in OP-CABG in the present study. The two
delivery methods, including remifentanil TCI and constant-rate
infusion, were used in OP-CABG to compare the changes of indices
when the surgical stimulation was large and the hemodynamic indices
showed severe changes. There were no significant differences in the
intraoperative hemodynamic parameters between the groups, which
indicated that the two delivery methods were able to maintain a
stable cardiac cycle during surgery (Table II). The release of E and COR in the
two groups was effectively inhibited from the time of induction,
and the concentrations of epinephrine and cortisol showed no
significant fluctuation (Table
III). However, the levels of BG and LAC began to increase
significantly in the two groups once the sternum had been opened,
and from T5 the increase was more apparent in group II
than in group I, thus indicating that the internal environment more
stable in the target-controlled group and showing that the
intraoperative catabolism was effectively suppressed in this group
(Table III).
As the hemodynamics may be significantly changed by
the anastomosis of the circumflex artery, diagonal branch and right
coronary artery, the short-term and single-use of α-receptor
agonists, such as phenylephrine, was employed. The results
demonstrated that more phenylephrine was used in group II than in
group I. This may have been due to a clinical requirement against
the increased stress that was induced by the accumulation of
remifentanil following constant infusion, moving the heart and
anastomosing the right coronary and/or circumflex artery. The
levels of nitroglycerin and norepinephrine that were used in the
two groups were approximately the same (Table I). The majority of the
cardiovascular drugs that improve morbidity and mortality,
including β-blockers, ACE-inhibitors and statins, also increase
HRV. Metoprolol, quinapril, captopril, enalapril and atorvastatin
have been shown in a previous study to increase HRV (12). For instance, the clinically
observed increase in HRV with β-blockers was likely to be
associated with the concomitant beneficial effects on the
parasympathetic nervous system and the
renin-angiotensin-aldosterone axis (32). However, the amount of metoprolol
and esmolol used prior to the surgery was similar in the two groups
of the present study. These drugs may have reduced the interference
of the intraoperative HRV analysis. Following general anesthesia,
the tension of the ANS has been shown to decrease along with the
HRV, while the inhibition of the parasympathetic nervous system by
propofol becomes stronger, with the main factors of a decreased HF
and an increased LF/HF (28).
Shinohara et al (29)
showed that through vagal excitation, remifentanil causes
hypotension and bradycardia (decreased LF/HF). In the present
study, remifentanil had little effect on the reliability of HRV as
an indicator. Therefore, following the induction of general
anesthesia, the HRV indices, including TP, LF and HF, began to
decrease and differed significantly from the baseline (P<0.05).
However, differences in the LF/HF were not significant at
T2, T3, T6, T7 and
T8, between groups I and II. When anastomosing the right
coronary and/or circumflex arteries (T5), the levels of
BG and LAC in group II were higher than those in group I. The
corresponding TP, LF and LF/HF values in group II were also
significantly higher than those in group I at T5, thus
indicating that sympathetic activity had increased and vagal
activity remained inhibited. In addition, a weak correlation
between the LF/HF ratio and the plasma level of E, BG and LAC was
observed in the two groups at T5 and T7. This
suggests that changes in HRV are correlated with the stress
response and may also be correlated with stress-related problems,
including heart disease, hypertension, depression and anxiety.
Therefore, HRV training may help the individual to better manage
stress and anxiety. In the present study, the TCI of remifentanil
was used to reduce the imbalance between the sympathetic and vagal
nerves, which is conducive to the regulation of the cardiac
autonomic nervous system.
The hemodynamic indices did not reveal that the
inhibition of the stress response in the target-controlled group
was more effective. Following extubation, the HR and MAP and the
levels of E and COR increased, while the TP, LF, HF and LF/HF
increased significantly. This suggested that despite effective
post-operative analgesia, extubation caused strong stimulation,
which lead to sympathetic excitation.
In OP-CABG, a TCI of remifentanil is more effective
than a constant-rate infusion in the inhibition of the stress
response and the maintenance of the cardiac ANS balance. The change
in HRV corresponds to the intraoperative stress response and is
relevant to the early post-operative recovery of cardiac autonomic
function. HRV may act as a key perioperative monitoring index for
patients who have undergone OP-CABG.
References
|
1.
|
Misfeld M, Brereton RJ, Sweetman EA and
Doiq GS: Neurologic complications after off-pump coronary artery
bypass grafting with and without aortic manipulation: meta-analysis
of 11,398 cases from 8 studies. J Thorac Cardiovasc Surg.
142:e11–e17. 2011. View Article : Google Scholar
|
|
2.
|
Pawlaczyk R, Swietlik D, Lango R and
Rogowski J: Off-pump coronary surgery may reduce stroke,
respiratory failure, and mortality in octogenarians. Ann Thorac
Surg. 94:29–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Godinho AS, Alves AS, Pereira AJ and
Pereira TS: On-pump versus off-pump coronary-artery bypass surgery:
a meta-analysis. Arq Bras Cardiol. 98:87–94. 2012.PubMed/NCBI
|
|
4.
|
Greco M, Landoni G, Biondi-Zoccai G, et
al: Remifentanil in cardiac surgery: a meta-analysis of randomized
controlled trials. J Cardiothorac Vasc Anesth. 26:110–116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Shu A, Zhan L, Fang H, et al: Application
of remifentanil TCI in anesthesia for off-pump coronary artery
bypass grafting. Jiangsu Yi Yao. 35:675–677. 2009.(In Chinese).
|
|
6.
|
Drexler B and Grasshoff C: Is deep
anesthesia dangerous? Anaesthesist. 61:678–681, 684–685. 2012.(In
German).
|
|
7.
|
Desborough JP: The stress response to
trauma and surgery. Br J Anaesth. 85:109–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Monk TG, Ding Y and White PF: Total
intravenous anaesthesia: effects of opioids versus hypnotic
supplementation on autonomic responses and recovery. Anesth Analg.
75:798–804. 1992.PubMed/NCBI
|
|
9.
|
Ledowski T, Bein B, Hanss R, et al:
Neuroendocrine stress response and heart rate variability: a
comparison of total intravenous versus balanced anaesthesia. Anesth
Analg. 101:1700–1705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chon KH, Zhong Y, Wang H, Ju K and Jan KM:
Separation of heart rate variability components of the autonomic
nervous system by utilizing principal dynamic modes. Nonlinear
Dynamics Psychol Life Sci. 10:163–185. 2006.PubMed/NCBI
|
|
11.
|
Thayer JF, Ahs F, Fredrikson M, Sollers JJ
III and Wager TD: A meta-analysis of heart rate variability and
neuroimaging studies: implications for heart rate variability as a
marker of stress and health. Neurosci Biobehav Rev. 36:747–756.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nolan RP, Jong P, Barry-Bianchi SM, Tanaka
TH and Floras JS: Effects of drug, biobehavioral and exercise
therapies on heart rate variability in coronary artery disease: a
systematic review. Eur J Cardiovasc Prev Rehabil. 15:386–396. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bosquet L, Merkari S, Arvisais D and
Aubert AE: Is heart rate a convenient tool to monitor
over-reaching? A systematic review of the literature. Br J Sports
Med. 42:709–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kussman BD, Gruber EM, Zurakowski D,
Hansen DD and Sullivan LJ: Bispectral index monitoring during
infant cardiac surgery: relationship of BIS to the stress response
and plasma fentanyl levels. Paediatr Anaesth. 11:663–669. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kishi T: Heart failure as an autonomic
nervous system dysfunction. J Cardiol. 59:117–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zhou Y, Xie G, Wang J and Yang S:
Cardiovascular risk factors significantly correlate with autonomic
nervous system activity in children. Can J Cardiol. 28:477–482.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wang B, Zhang Z and Wang W: The
development of heart rate variability analysis system. Zhongguo Yi
Liao Qi Xie Za Zhi. 36:333–337. 2012.(In Chinese).
|
|
18.
|
Hanss R, Renner J, Ilies C, et al: Does
heart rate variability predict hypotension and bradycardia after
induction of general anaesthesia in high risk cardiovascular
patients? Anaesthesia. 63:129–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bäcklund M, Toivonen L, Tuominen M, Pere P
and Lindgren L: Changes in heart rate variability in elderly
patients undergoing major noncardiac surgery under spinal or
general anesthesia. Reg Anesth Pain Med. 24:386–392. 1999.
|
|
20.
|
Keyl C, Lemberger P, Palitzsch KD,
Hochmuth K, Liebold A and Hobbhahn J: Cardiovascular autonomic
dysfunction and hemodynamic response to anesthetic induction in
patients with coronary artery disease and diabetes mellitus. Anesth
Analg. 88:985–991. 1999.
|
|
21.
|
Dekker JM, Crow RS, Folsom AR, et al: Low
heart rate variability in a 2-minute rhythm strip predicts risk of
coronary heart disease and mortality from several causes: the ARIC
Study. Atherosclerosis Risk In Communities. Circulation.
102:1239–1244. 2000. View Article : Google Scholar
|
|
22.
|
Buccelletti E, Gilardi E, Scaini E, et al:
Heart rate variability and myocardial infarction: systematic
literature review and metanalysis. Eur Rev Med Pharmacol Sci.
13:299–307. 2009.PubMed/NCBI
|
|
23.
|
Dupliakov DV, Golovina GA, Sysuenkova EV,
Glukhova VL and Gar’kina SV: Heart rate variability in the
prognosis of tilt-testing results. Kardiologiia. 52:61–66. 2012.(In
Russian).
|
|
24.
|
Scheffler P, Muccio S, Egiziano G, et al:
Heart rate variability exhibits complication-dependent changes
postsurgery. Angiology. Oct 21–2012.(Epub ahead of print).
|
|
25.
|
Xhyheri B, Manfrini O, Mazzolini M, Pizzi
C and Bugiardini R: Heart rate variability today. Prog Cardiovasc
Dis. 55:321–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mazzeo AT, La Monaca E, Di Leo R, Vita G
and Santamaria LB: Heart rate variability: a diagnostic and
prognostic tool in anesthesia and intensive care. Acta Anaesthesiol
Scand. 55:797–811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ushiyama T, Nakatsu T, Yamane S, et al:
Heart rate variability for evaluating surgical stress and
development of postoperative complications. Clin Exp Hypertens.
30:45–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kanaya N, Hirata N, Kurosawa S, Nakayama M
and Namiki A: Differential effects of propofol and sevoflurane on
heart rate variability. Anesthesiology. 98:34–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shinohara K, Aono H, Unruh GK, Kindscher
JD and Goto H: Suppressive effects of remifentanil on hemodynamics
in baro-denervated rabbits. Can J Anaesth. 47:361–366. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Sato N, Kawamoto M, Yuge O, et al: Effects
of pneumoperitoneum on cardiac autonomic nervous activity evaluated
by heart rate variability analysis during sevoflurane, isoflurane,
or propofol anesthesia. Surg Endosc. 14:362–366. 2000. View Article : Google Scholar
|
|
31.
|
Haensel A, Mills PJ, Nelesen RA, Ziegler
MG and Dimsdale JE: The relationship between heart rate variability
and inflammatory markers in cardiovascular diseases.
Psychoneuroendocrinology. 33:1305–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Chen WR, Shi XM, Yang TS, Zhao LC and Gao
LG: Protective effect of metoprolol on arrhythmia and heart rate
variability in healthy people with 24 hours of sleep deprivation. J
Interv Card Electrophysiol. 36:267–272. 2013. View Article : Google Scholar : PubMed/NCBI
|