Introduction
Peripheral nerve injury is a frequent and severe
complication following orthopaedic trauma. The treatment of
peripheral nerve injury remains a challenge, despite the focus that
has been placed on it, as none of the traditional therapies have
been demonstrated to produce entirely satisfactory results. One of
the potential methods for improving nerve regeneration and the
restoration of function is electrical stimulation.
Studies have indicated that electrical stimulation
may promote the speed and accuracy of motor and sensory axon
regeneration (1–4). Therefore, there have been
investigations into the use of functional electrical stimulation
(FES) in the induction of peripheral nerve regeneration. The
results of in vitro (5,6) and
in vivo (7,8) studies have revealed that a weak
electric field enhances neurite outgrowth. Numerous investigations
have been performed in this field (1–4,9), and
the histomorphometric and electrophysiological analyses have
demonstrated that electrical stimulation may be used to accelerate
the maturity of regenerated nerves (1–4,7,8).
The expansion of electronic technologies has led to
the rapid development of a number of implantable microsystems that
have been used in the treatment of a variety of diseases, including
deafness (10,11), arrhythmia (12), plegia (13) and Parkinson’s disease (14). However, there has been limited
investigation into the use of implantable electrical stimulators in
the treatment of peripheral nerve injuries.
In the current study, we designed an implantable
electrical stimulator with suitable parameters, and evaluated the
efficacy of the stimulator using an animal model.
Materials and methods
Implantable electrical stimulator
design
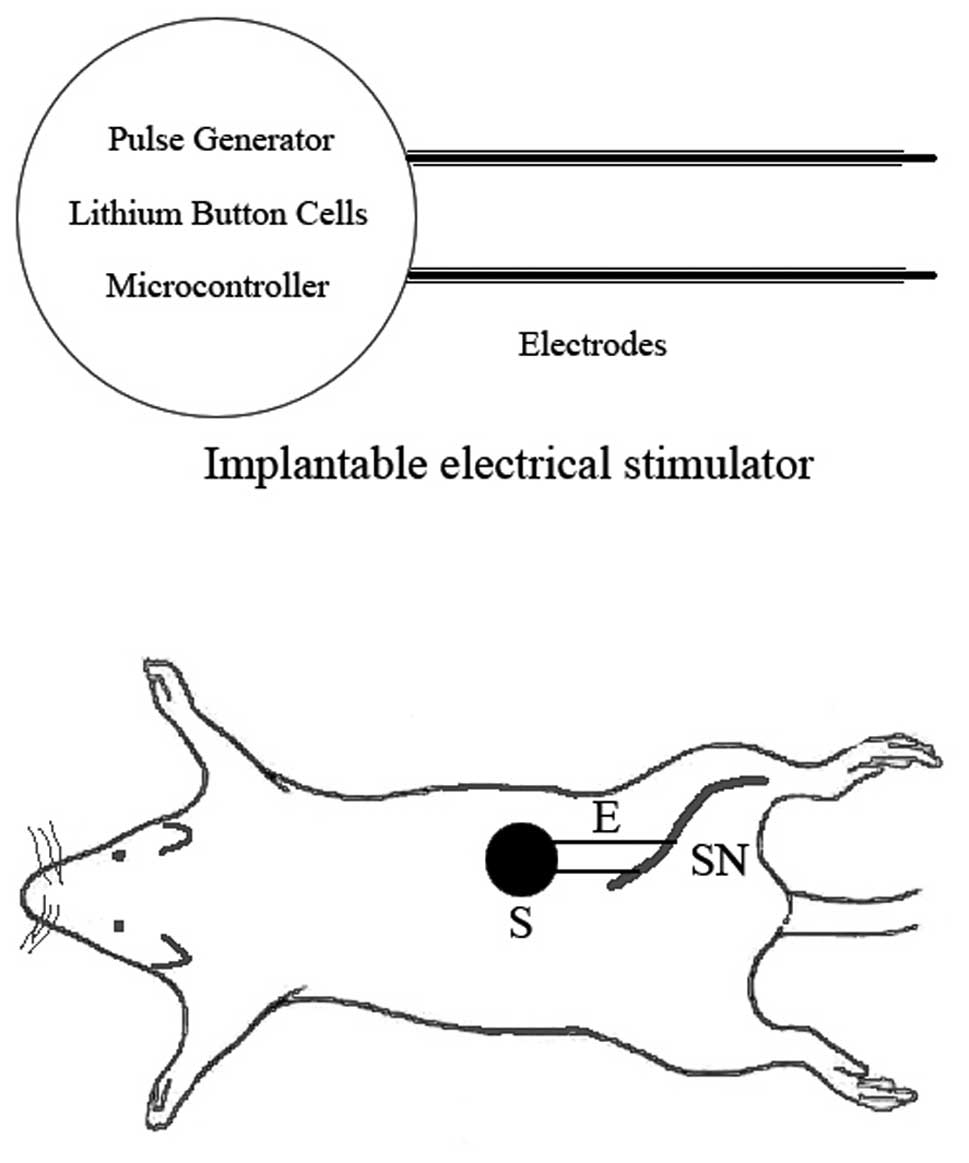
A system block diagram of the implantable electrical
stimulator is displayed in Fig. 1.
The stimulator was designed to be implanted in the backs of the
rats, and was created with batteries, bipolar electrodes and
integrated circuit (IC) chips, including a micro-controller and a
pulse generator chip. The batteries, electrodes and ICs were
integrated into the system. The output pads of the stimulator chip
were connected to the electrodes, and the power pins were connected
to the battery. To supply power to the chip, a 3 V CR2450 lithium
button cell battery was used (Shenzhen Eunicell Battery Co., Ltd.,
Shenzhen, China), which made it possible to operate the device for
>8 weeks. The activity of the stimulator was controlled by an
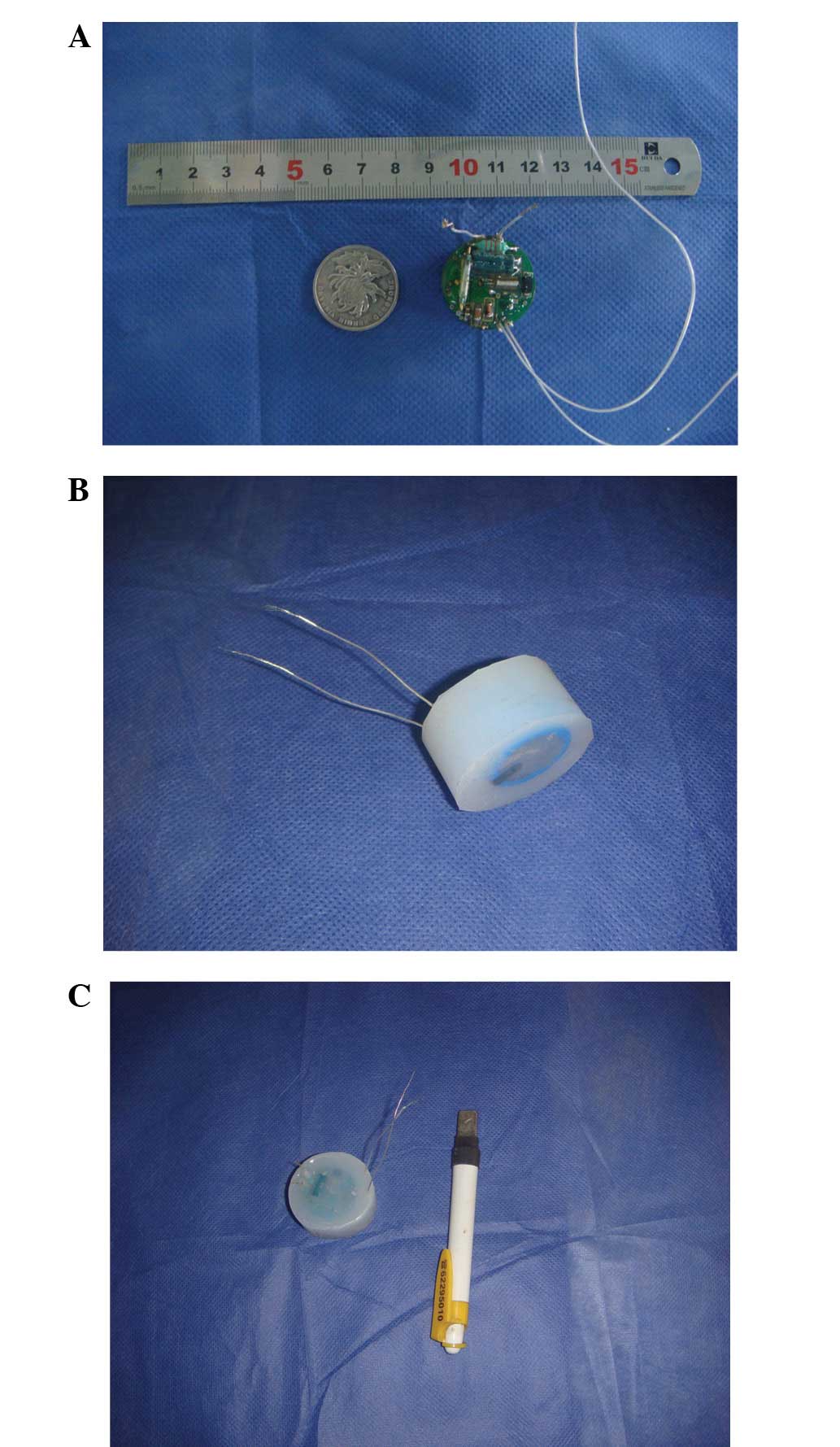
external magnetic switch. To protect the stimulator from bodily
fluids and mechanical forces, the IC chips, batteries and electrode
connector were cast in a medical grade epoxy resin. Following this,
the assembled stimulator was coated with a silicone elastomer, and
the device was sealed in a gas-permeable bag for ethylene oxide
sterilization. Subsequent to the encapsulation, the stimulator
measured 30 mm in diameter and 22 mm in depth and weighed 25 g
(Fig. 2). The stimulation
parameters were selected using the results of our preliminary
experiments, which indicated that a stimulation pattern of bipolar
pulses with a duration of 400 μsec per phase, an amplitude
of 0.8 V and a frequency of 60 Hz was most effective with regard to
peripheral nerve rehabilitation in rats (unpublished data).
Animal models and surgical procedures
for the implantation of the electrical stimulator
All animal experiments were performed in accordance
with the Guide for the Care and Use of Laboratory Animals (15). The procedures in the study were
designed to minimize the pain or discomfort of the animals, in
accordance with the current protocols approved by the Laboratory
Animal Ethics Committee of Shanghai Jiaotong University (Shanghai,
China). A cohort of 40 healthy 8-week-old Sprague-Dawley rats
(weight range, 280–355 g) was recruited for the study. The rats
were randomly divided into two groups: implantation (n=20) and
control (n=20), and each group was then further divided into two
subgroups, according to different time points in the experiment
(three and six weeks).
The animals were anesthetized with an injection of
10% chloral hydrate (0.3 ml/100g; Shanghai Chemical Reagent Co.,
Shanghai, China) into the abdominal cavity. During the surgery,
each animal was placed in a prone position, and the skin was
prepared. A standard longitudinal incision was made in the right
gluteal region, and then the tissue between the subcutaneous and
muscular layers was dissected. Following this, the main stem of
right sciatic nerve was isolated at mid-thigh level, and was
subsequently crushed for 5 min, using micro-vessel clamps with a
strength of 2.0 kg. A 1-0 nylon stitch was sutured to the tissue
adjacent to the crush site as a marker. In the implantation group,
the implantable electrical stimulator was inserted in the back
through the subcutaneous tunnel. The silicone elastomer,
incorporated during the encapsulation process, was sutured to the
subcutaneous tissue to prevent device migration. The two electrodes
were wrapped and sutured to the epineurium proximal and distal to
the crushed sciatic nerve, respectively, prior to the suturing of
the skin incision. In the control group, no measures were taken
subsequent to the crushing of the sciatic nerve. Following the
surgery, the animals were allowed access to food and water ad
libitum, in isolator cages that were maintained under a 12 h
light-dark cycle at 25°C. Stimulation was performed for 120 min per
day in the implantation group. The animals were checked daily, and
the stimulation was observed to be well-tolerated in every
case.
Experimental materials
Ethological evaluation
Ethological methods were used to observe, record and
analyze the behavior of the rats, in terms of the signs and extent
of muscular atrophy in the hind limb, gait and cutaneous
ulceration, at each time point.
Sciatic nerve function index
(SFI)
Three and six weeks following the surgery, the SFI
was calculated using the method described by Reynolds and Weiss
(16). The hind legs of the rats
were dyed with ink, to enable the footprints of the healthy (N) and
wounded (E) feet to be measured when the rats walked across a piece
of white paper. The measurements were taken in three indices, as
follows: length of footprint (IPL, from toe to heel), width of toes
(ITS, from the 1st to the 5th toe) and width of the middle toes
(IIT, from the 2nd to the 4th toe). The results were accurate to
0.1 mm. The SFI was calculated in accordance with the formula
described by Bain et al (17): SFI=−38.3 [(EPL-NPL)/NPL] + 109.5
[(ETS-NTS)/NTS] + 13.3 [(EIT-NIT)/NIT] −8.8, where EPL is the
length of footprint (from toe to heel) of wounded feet, NPL is the
length of footprint (from toe to heel) of healthy feet, ETS is the
width of toes (from the 1st to the 5th toe) of wounded feet, NTS is
the width of toes (from the 1st to the 5th toe) of healthy feet,
EIT is the width of middle toes (from the 2nd to the 4th toe) of
wounded feet and NIT is the width of middle toes (from the 2nd to
the 4th toe) of healthy feet. An SFI value of between 0 and 11%
represented normal nerve function, whereas −100% represented
complete damage of nerve function, and between −11 and −100%
repesented incomplete nerve function recovery.
Electrophysiological assessment
Three and six weeks following the surgery, the
sciatic nerve at the surgical site was exposed under anesthesia.
The proximal site of the crushed section was linked with an
electrode, and the gastrocnemius was connected to a recording
electrode, in order to deter mine the mean conductive velocity
(MCV) of the sciatic nerve.
Macroscopic evaluation
The results were evaluated at three and six weeks
following the surgery, respectively, with 10 rats from each group
assessed at each time point. During the evaluation, the shape,
adhesion between the stimulator and the surrounding tissue, the
corrosion of the electrodes and neuroma formation at the crush site
were observed.
Morphology and morphometry
Sections were cut from the gastrocnemius muscles of
the rats. Two sections, from the experimental and the contralateral
control muscles, respectively, were placed on each slide. The
sections were stained using haematoxylin and eosin (H&E). The
H&E-stained sections were overlaid by a transparent grid of 1×1
mm squares, in order to measure the complete cross-sectional area
(CSA). Ten evenly spaced 1 mm2 fields were selected for
the microscopic analysis, with the convention that the fibers
intersecting the upper and left boundaries were included, whereas
those intersecting the lower and right boundaries were excluded.
The total number of muscle fibers in the muscle was estimated as
the product of the total muscle CSA and the mean number of fibers
per square millimeter. Representative digital photomicrographs of
H&E-stained sections were taken with an adapted camera (Leica
QWin V2.6; Leica Microsystems, Wetzlar, Germany).
Transmission electron microscopy
Specimens were obtained from the sciatic nerve at
the experimental or the equivalent normal site of the rats. These
were fixed in 3.5% glutaraldehyde in 0.1 mol/l sodium cacodylate
buffer (pH 7.2) for 2 h at room temperature, and then stored at 4°C
in the same solution. Small bundles of fibers were postfixed for 1
h in 1% osmium tetroxide in the same buffer, dehydrated through a
graded ethanol series followed by acetone, and then embedded in
epoxy resin. Ultrathin sections (30–40 nm) were cut using a Leica
Ultracut R microtome (Leica Microsystems) and stained with 4%
uranyl acetate and lead citrate. The sections were subsequently
examined with a Hitachi H-600 electron microscope (Hitachi, Tokyo,
Japan), and representative photomicrographs were taken with an
adapted camera.
Statistical analysis
The results are expressed as the mean ± standard
deviation (SD). The electrophysiological and morphological
properties of the control (non-stimulated and contralateral) and
stimulated hind limbs were compared using one-way analysis of
variance (ANOVA), followed by the Tukey-Kramer post-test, using
SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Ethology
All the rats were noted to have normal hind limb
muscles, skin and gait prior to the crushing of the sciatic nerve.
Three weeks following the surgery, the rats in the control group
(n=10) displayed muscular atrophy in the hind limb and marginal
gait changes, and at the six-week time point, the control rats
demonstrated extensive hind limb muscular atrophy, and marked gait
changes. In comparison, at three weeks after surgery, the rats in
the implantation group (n=10) presented with atrophic muscles in
the hind limb, and minor gait changes. At six weeks, the rats with
the implanted electrical stimulator demonstrated marginal atrophy
in the muscles of the hind limb, and normal gait.
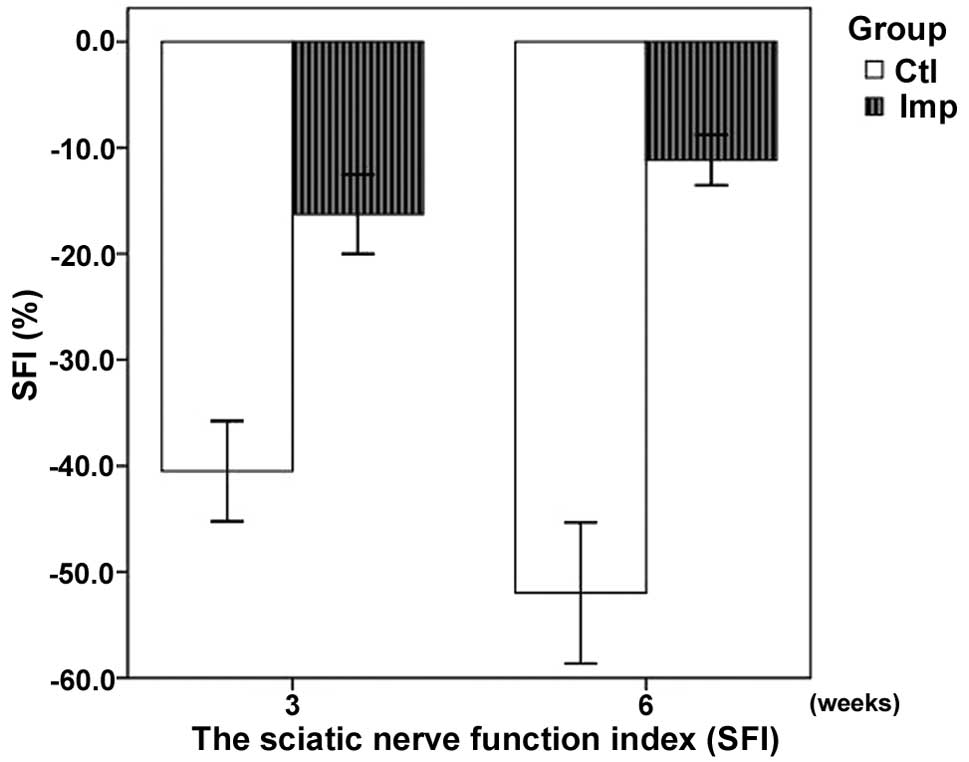
SFI
The results of the SFI assessment are summarized in
Table I. The SFI was significantly
higher in the implantation group compared with the control group,
at the three- and six-week time points (P<0.05). The SFI
demonstrated an improved recovery in the implantation group
(Fig. 3).
 | Table I.Sciatic nerve function index (SFI) in
the implantation and control groups. |
Table I.
Sciatic nerve function index (SFI) in
the implantation and control groups.
| Week | SFI (%)
|
|---|
| Implantation | Control |
|---|
| 3 | −16.27±3.01a | −40.50±3.81 |
| 6 | −11.16±1.90a | −51.98±5.35 |
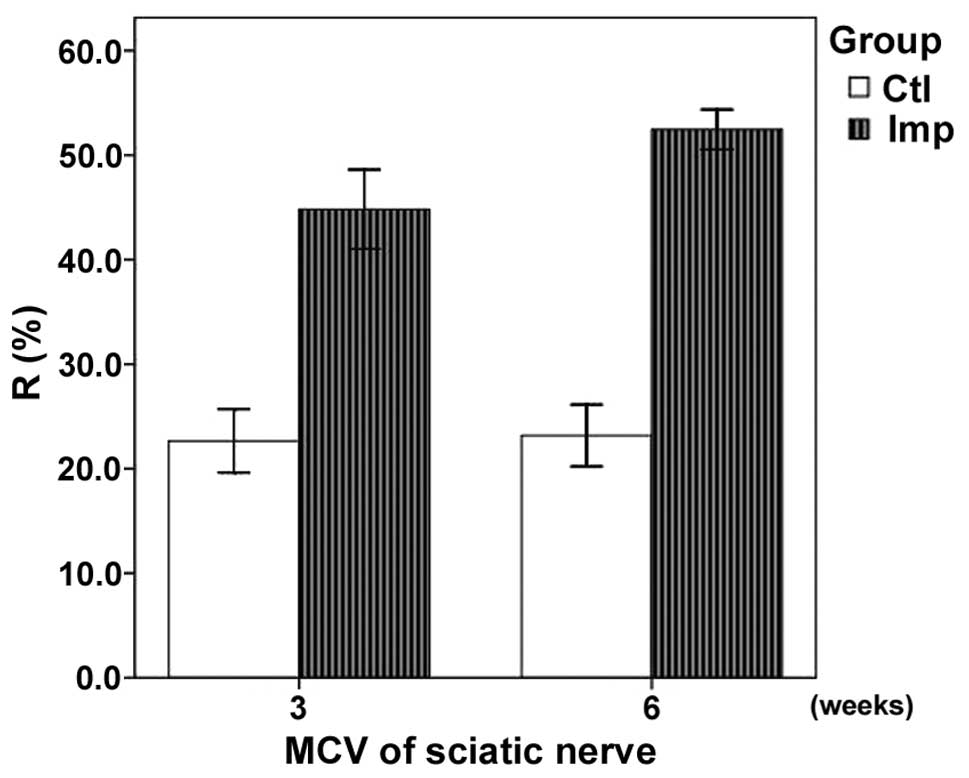
Electrophysiological changes
The results of the electro-physiological assessment
are summarized in Table II. The
recovery rate (R) of the MCV (R=MCV of the experimental side/MCV of
the contralateral side) of the sciatic nerve in the implantation
group was significantly higher compared with that of the control
group at three weeks (P<0.05). In addition, a significant
difference was observed between the Rs of the sciatic nerve MCVs in
the implantation and control groups at six weeks (P<0.05). The
MCV demonstrated an improved recovery in the implantation group
(Fig. 4).
 | Table II.Recovery rate of the motor nerve
conduction velocity in the implantation and control groups. |
Table II.
Recovery rate of the motor nerve
conduction velocity in the implantation and control groups.
| Week | Recovery rate (%)
|
|---|
| Implantation | Control |
|---|
| 3 | 44.8±3.0a | 22.7±2.4 |
| 6 | 52.5±1.5a | 23.2±2.2 |
Macroscopic evaluation
With regard to the implantation group at three
weeks, the electrical stimulator remained in its original size and
shape, although the electrodes had adhered to the surrounding
tissue, and no neuromas were present at the site where the sciatic
nerve had been crushed. Compared with the normal side, the sciatic
nerve expressed a thicker epineurium on the surface of the site of
the crush injury, and the muscles were marginally paler. At six
weeks, no inflamatory reactions were observed in the tissues
surrounding the stimulator, and the adhesion of the electrodes was
reduced. There were no notable differences between the muscles of
the two sides. In the control group, there was extensive
degeneration at the site where the sciatic nerve had been crushed,
and there were no regenerated axons crossing over the site of the
crush injury, or neuroma formation in the proximal nerve. The
muscles appreared paler than those from the normal side.
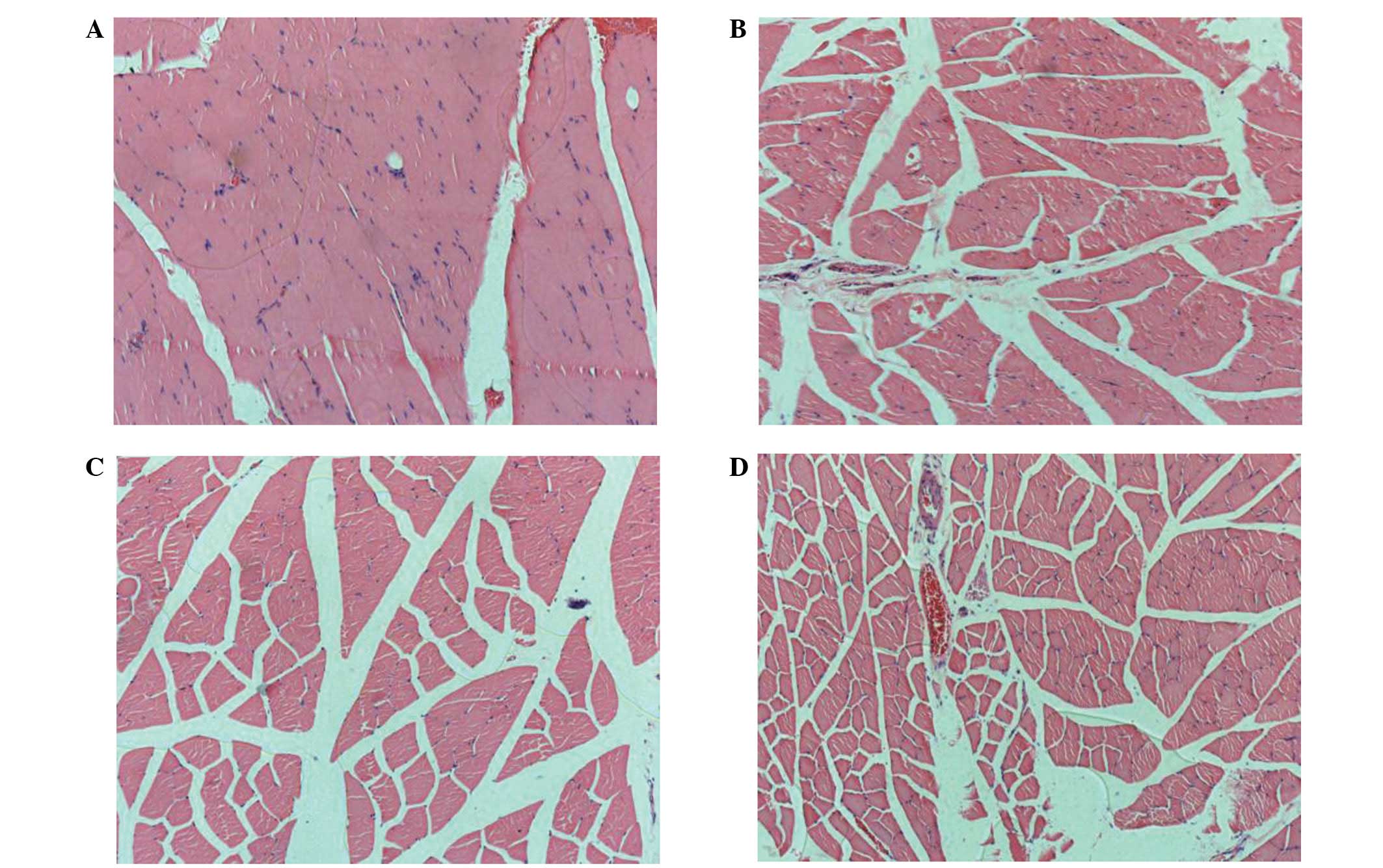
Morphology and morphometry
At three weeks, the muscles of the control group
exhibited the characteristic features of muscle fibre atrophy,
including fibrosis and fatty deposits. The changes were more
evident at six weeks (Figs. 5C and
D). In comparison with the control group, the electrical
stimulation led to a marked improvement in the morphology of the
muscles from three to six weeks. Reductions in the endomysial space
and in the thickness of the perimysium were observed, and the
muscle fibres appeared larger and more tightly packed within the
fascicles (Figs. 5A and B). These
changes were reflected in the results of the morphometric analysis
(Table III). The CSA of the
gastrocnemius muscles and the total number of muscle fibers were
demonstrated to differ significantly between the implantation and
control groups at the three- and six-week time points (P<0.05
for each). These differences were due to the atrophy of the muscles
in control group.
 | Table III.Complete cross-sectional area (CSA) of
muscles and the total number of muscle fibers in the implantation
and control groups. |
Table III.
Complete cross-sectional area (CSA) of
muscles and the total number of muscle fibers in the implantation
and control groups.
| Weeks | CSA (mm2)
| Number of fibers
|
|---|
| Implantation | Control | Implantation | Control |
|---|
| 3 | 52.4±3.5a | 37.8±2.6 | 13685±1024a | 11087±1128 |
| 6 | 66.9±4.7a | 31.3±1.8 | 15373±1198a | 9872±1027 |
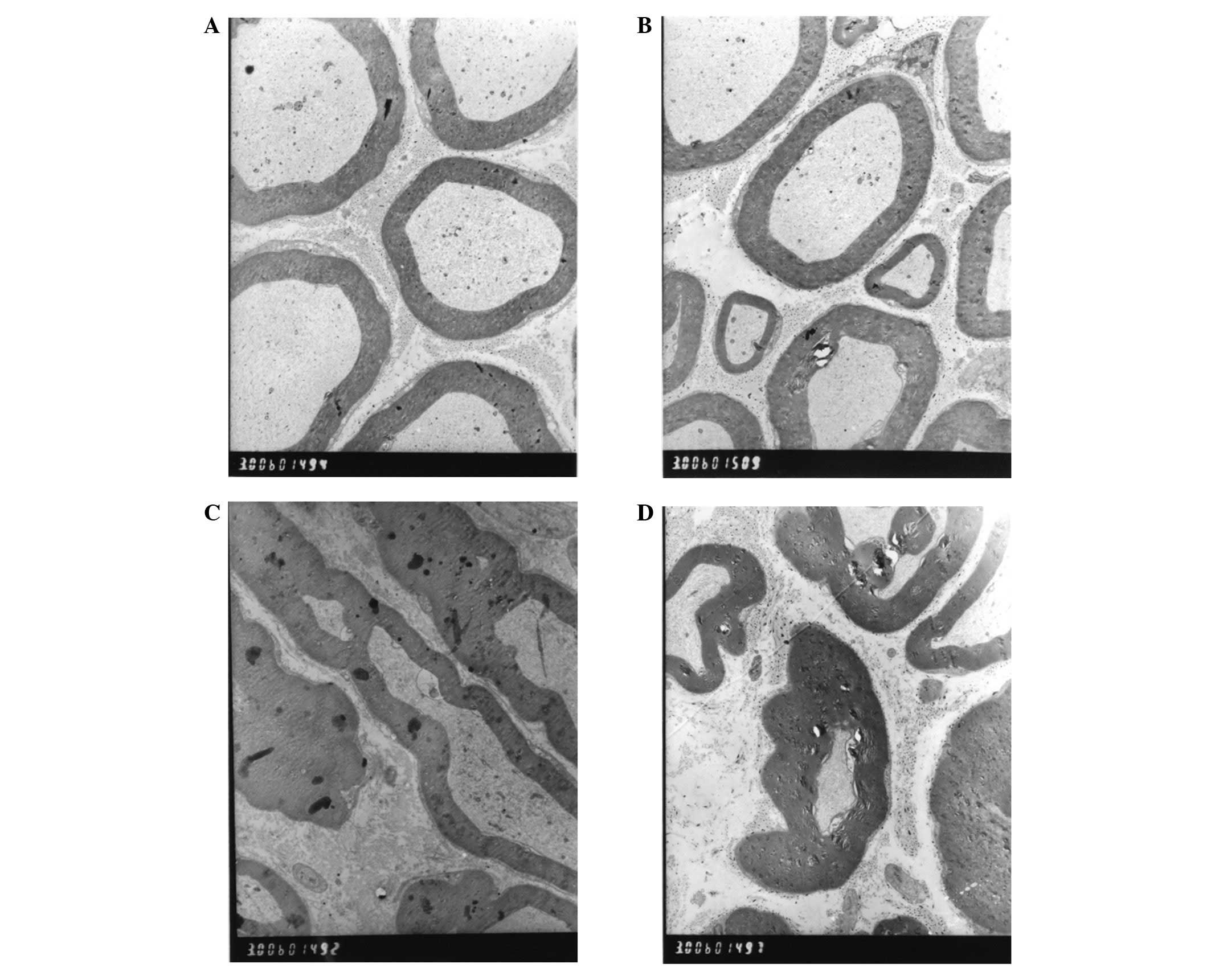
Transmission electron microscopy
Transmission electron microscopy of the control
group, performed three weeks following the surgery, revealed that
the distal axonal portion of the crushed nerve exhibited
microfibrillar breakdown, swollen chondriosomes and myelin
irregularities (Fig. 6C). Six
weeks following the surgery, the breakdown of the microfibrils and
microtubules in the control group still existed, and the
chondriosomes exhibited swelling (Fig.
6D). With regard to the implantation group, from three to six
weeks following the surgery, extensive Schwann cell proliferation
and immature myelin formation were observed in the sciatic nerve
(Figs. 6A and B).
Discussion
Peripheral nerve injuries are common and have a
marked impact on the everyday life of the general population. The
restoration of function following a peripheral nerve injury
continues to present a significant challenge. Although in the last
century there has been an increase in the understanding of
peripheral nerve rehabilitation (18–21),
there remains a lack of totally effective treatment methods, and a
fully functional outcome, particularly of motor function, is rarely
achieved. However, non-surgical approaches, such as FES, have been
developed to enhance nerve recovery, and, at present, FES is widely
used to improve the rehabilitation of patients with neural
impairments (22). A variety of
electrical stimulation techniques have been developed, including
transcutaneous electric nerve stimulation (TENS) (23,24),
spinal cord stimulation (SCS) (25,26)
and deep brain stimulation (DBS) (14,27).
However, there remain disadvantages with the use of these
procedures, for example none of the procedures have exhibited
efficacy in the treatment of peripheral nerve injury, and the use
of certain treatments, such as TENS is limited, particularly in
cases that require the application of electrical stimulation for an
extended period of time. Therefore, there is a requirement for
further fundamental studies.
The development of medical implantable microsystems
has led to a number of potential opportunities for the treatment of
peripheral nerve injuries, particulary as these implants enable the
establishment of a man-machine interface with the peripheral
nervous system. There have recently been investigations into a
variety of techniques for contacting nerves at different anatomical
levels (23–27). In the present study, we developed
an implantable electrical stimulator that incorporated the features
that were considered essential in an ideal device. These
requirements included the stimulator being suitable for a broad
range of applications, including continuous stimulation for
prolonged periods, and being programmable, with easily adaptable
parameters. In addition, there were requirements for
controlled-current sources, a small size and a low power
consumption.
Following the methods described by Beer et al
(28) and Varejão et al
(29), we used Sprague-Dawley rats
to establish an animal model of peripheral nerve injury, and then
used this model to evaluate the previously mentioned requirements
of the implantable electrical stimulator. Following the
implantation of the electrical stimulators into the rats, the
device was demonstrated to be reliable and stable for the duration
of the experiment (≤6 weeks); none of the implanted devices failed.
The ethological and macroscopic evaluations of the model animals
revealed that the stimulator met the requirement of
biocompatibility. Pathological examination of the rats that did not
receive electrical stimulation demonstrated that the crushed nerve
exhibited various degrees of Wallerian degeneration in the 3–6-week
period subsequent to the crush injury. However, in the animals that
received daily in vivo electrical stimulation, transmission
electron microscopy revealed Schwann cell proliferation and
immature myelin formation, in the same 3–6-week period. This result
confirmed the success of the establishment of the peripheral nerve
injury model, in addition to revealing that the selected
stimulation pattern (bipolar pulses with a duration of 400
μsec per phase, an amplitude of 0.8 V and a frequency of 60
Hz) was sufficient and functional. One of the critical factors in
the electrical stimulation technique is the stimulation parameters
that are used. It has been observed that, for short-term electrical
stimulation, a frequency of 20 Hz is effective for the regeneration
of a transected nerve (30).
However, for long-term electrical stimulation, a frequency of 100
Hz was reported to have an enhanced regenerative effect, in
comparison with that of 20 Hz (31). Another important factor in
electrical stimulation is the type of stimulation waveform. A
biphasic current pulse has previously been used in the electrical
stimulation of cells and tissues, and has been demonstrated to
produce safe and effective results (32). In the present study, the
stimulation parameters were selected from the results of our
preliminary experiments, and were revealed to be effective with
regard to peripheral nerve rehabilitation in rats. The results of
the study demonstrated the presence of muscle atrophy in the
control group, a phenomenon that, clinically, is a secondary
occurence to the peripheral nerve injury. Electrical stimulation
may be used in the treatment of this disorder. In the rats in the
implantation group, the gastrocnemius muscle was revealed to have
recovered by the 6-week time point, which indicated that the
electrical stimulation was critical to the recovery. The study,
therefore, demonstrated that the implantable electrical stimulator
was able to prevent denervated muscles from undergoing atrophy, in
addition to accelerating the regeneration of the nerve.
There was, however, a clear disadvantage to the
implantable stimulator, in that there was a lack of direct access
to the electrode waveforms. To resolve this, it may be possible to
add light emitting diodes to the circuit, to provide confirmation
of the delivery of a current pulse to the bipolar electrodes. A
future development of the implantable stimulators may be to include
microsystem stimulation parameters that may be adapted during the
different periods of recovery of the injured peripheral nerve, and
which may be communicated via wireless techniques. In addition,
there is a requirement for investigations into the development of
devices with variable current amplitude outputs and multichannel
stimulation capabilities.
In the current study, we designed an effective
implantable electrical stimulator that was suitable for
implantation in an rat model of peripheral nerve injury, and then
analyzed its use in the electrical stimulation of the peripheral
nerve. With simple modifications to the stimulation parameters,
this stimulator may have clinical applications in numerous other
areas of neuroscience.
References
|
1.
|
Al-Majed AA, Neumann CM, Brushart TM and
Gordon T: Brief electrical stimulation promotes the speed and
accuracy of motor axonal regeneration. J Neurosci. 20:2602–2608.
2000.
|
|
2.
|
Brushart TM, Jari R, Verge V, Rohde C and
Gordon T: Electrical stimulation restores the specificity of
sensory axon regeneration. Exp Neurol. 194:221–229. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
English AW, Schwartz G, Meador W, Sabatier
MJ and Mulligan A: Electrical stimulation promotes peripheral axon
regeneration by enhanced neuronal neurotrophin signaling. Dev
Neurobiol. 67:158–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Geremia NM, Gordon T, Brushart TM,
Al-Majed AA and Verge VM: Electrical stimulation promotes sensory
neuron regeneration and growth-associated gene expression. Exp
Neurol. 205:347–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yamada M, Tanemura K, Okada S, et al:
Electrical stimulation modulates fate determination of
differentiating embryonic stem cells. Stem Cells. 25:562–570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhang Z, Rouabhia M, Wang Z, et al:
Electrically conductive biodegradable polymer composite for nerve
regeneration: electricity-stimulated neurite outgrowth and axon
regeneration. Artif Organs. 31:13–22. 2007. View Article : Google Scholar
|
|
7.
|
Zealear DL, Rodriguez RJ, Kenny T, et al:
Electrical stimulation of a denervated muscle promotes selective
reinnervation by native over foreign motoneurons. J Neurophysiol.
87:2195–2199. 2002.PubMed/NCBI
|
|
8.
|
Al-Majed AA, Tam SL and Gordon T:
Electrical stimulation accelerates and enhances expression of
regeneration-associated genes in regenerating rat femoral
motoneurons. Cell Mol Neurobiol. 24:379–402. 2004. View Article : Google Scholar
|
|
9.
|
Gordon T, Udina E, Verge VM and de Chaves
EI: Brief electrical stimulation accelerates axon regeneration in
the peripheral nervous system and promotes sensory axon
regeneration in the central nervous system. Motor Control.
13:412–441. 2009.PubMed/NCBI
|
|
10.
|
Snapp HA, Fabry DA, Telischi FF, Arheart
KL and Angeli SI: A clinical protocol for predicting outcomes with
an implantable prosthetic device (Baha) in patients with
single-sided deafness. J Am Acad Audiol. 21:654–662. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yanagihara N, Gyo K, Sato H, Yamanaka E
and Saiki T: Implantable hearing aid in fourteen patients with
mixed deafness. Acta Otolaryngol Suppl. 458:90–94. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Leclercq JF, Ménasché P, Piwnica A, Coumel
P and Slama R: The implantable automatic defibrillator. Preventive
treatment of sudden death caused by ventricular arrhythmia. Presse
Med. 12:1707–1710. 1983.(In French).
|
|
13.
|
Chiou YH, Luh JJ, Chen SC, Chen YL, Lai JS
and Kuo TS: Patient-driven loop control for hand function
restoration in a non-invasive functional electrical stimulation
system. Disabil Rehabil. 30:1499–1505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Blomstedt P: Deep-brain stimulation in
Parkinson’s disease. N Engl J Med. 346:452–453. 2002.
|
|
15.
|
National Institute of Health (NIH): Guide
for the Care and Use of Laboratory Animals. Publication no. 85–23.
The National Academies Press; Washington, D.C: 1996
|
|
16.
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bain JR, Mackinnon SE and Hunter DA:
Functional evaluation of complete sciatic, peroneal, and posterior
tibial nerve lesions in the rat. Plast Reconstr Surg. 83:129–138.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lundborg G: A 25-year perspective of
peripheral nerve surgery: evolving neuroscientific concepts and
clinical significance. J Hand Surg Am. 25:391–414. 2000.PubMed/NCBI
|
|
19.
|
Naff NJ and Ecklund JM: History of
peripheral nerve surgery techniques. Neurosurg Clin N Am.
12:197–209. 2001.PubMed/NCBI
|
|
20.
|
Dvali L and Mackinnon S: Nerve repair,
grafting, and nerve transfers. Clin Plast Surg. 30:203–221. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zeng X, Zhang L, Sun L, et al: Recovery
from rat sciatic nerve injury in vivo through the use of
differentiated MDSCs in vitro. Exp Ther Med. 5:193–196.
2013.PubMed/NCBI
|
|
22.
|
Stieglitz T: Neural prostheses and
functional electrical stimulation. Biomed Tech (Berl). 49:70–71.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Tong KC, Lo SK and Cheing GL: Alternating
frequencies of transcutaneous electric nerve stimulation: does it
produce greater analgesic effects on mechanical and thermal pain
thresholds? Arch Phys Med Rehabil. 88:1344–1349. 2007. View Article : Google Scholar
|
|
24.
|
Malm-Buatsi E, Nepple KG, Boyt MA, Austin
JC and Cooper CS: Efficacy of transcutaneous electrical nerve
stimulation in children with overactive bladder refractory to
pharmacotherapy. Urology. 70:980–983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Robaina F and Clavo B: Spinal cord
stimulation in the treatment of post-stroke patients: current state
and future directions. Acta Neurochir Suppl. 97:277–282.
2007.PubMed/NCBI
|
|
26.
|
Hamid S and Hayek R: Role of electrical
stimulation for rehabilitation and regeneration after spinal cord
injury: an overview. Eur Spine J. 17:1256–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hamani C, Schwalb JM, Rezai AR, Dostrovsky
JO, Davis KD and Lozano AM: Deep brain stimulation for chronic
neuropathic pain: long-term outcome and the incidence of
insertional effect. Pain. 125:188–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Beer GM, Steurer J and Meyer VE:
Standardizing nerve crushes with a non-serrated clamp. J Reconstr
Microsurg. 17:531–534. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Varejão AS, Cabrita AM, Meek MF, et al:
Functional and morphological assessment of a standardized rat
sciatic nerve crush injury with a non-serrated clamp. J
Neurotrauma. 21:1652–1670. 2004.
|
|
30.
|
Ahlborn P, Schachner M and Irintchev A:
One hour electrical stimulation accelerates functional recovery
after femoral nerve repair. Exp Neurol. 208:137–144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Baptista AF, Gomes JR, Oliveira JT, Santos
SM, Vannier-Santos MA and Martinez AM: High- and low-frequency
transcutaneous electrical nerve stimulation delay sciatic nerve
regeneration after crush lesion in the mouse. J Peripher Nerv Syst.
13:71–80. 2008. View Article : Google Scholar
|
|
32.
|
Lilly JC, Hughes JR, Alvord EC Jr and
Galkin TW: Brief, noninjurious electric waveform for stimulation of
the brain. Science. 121:468–469. 1955. View Article : Google Scholar : PubMed/NCBI
|