Introduction
Vestibular schwannoma surgery is currently performed
with low morbidity and mortality rates due to improvements in
diagnostic capabilities and advances in perioperative monitoring
and microsurgical techniques (1).
Intraoperative facial nerve interruption may be minimized, but
remains an inevitable complication (2,3),
particularly for patients with certain types of large tumors or
recurrent tumors following stereotactic irradiation (4–7). The
most common techniques for facial reanimation are sural grafting
and hypoglossal-facial (VII–XII) anastomosis. It has been reported
that these techniques produce excellent results, as assessed by the
House-Brackmann grade of facial function, but to date there has
been no long-term comparison of the techniques (6).
The aim of the current study was to compare the
short-and long-term facial recovery results and surgical outcomes
of patients who had undergone sural grafting and VII–XII
anastomosis for the reanimation of interrupted facial nerves during
the removal of translabyrinthine vestibular schwannomas.
Patients and methods
Subject selection
All vestibular schwannoma surgeries performed by
surgeons at Xinhua Hospital (Shanghai, China) between 2003 and 2006
were screened for the following selection criteria: i)
translabyrinthine total tumor removal; ii) normal preoperative
facial nerve function (House-Brackmann grade); iii) interruption of
the facial nerve during surgery and immediate reanimation by sural
graft or VII–XII anastomosis; and iv) data available at all
postoperative points in time (upon discharge from the hospital, at
one year, and at three years following surgery). These criteria
were met in 25 cases. These patients became the subjects of this
study. Written informed consent was obtained from patents and their
families. The study was approved by the Ethics Committee of Xinhua
Hospital.
Patient characteristics
The characteristics of the patients in the two
groups are shown in Table I. There
were no statistically significant differences in the mean age or
tumor size.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | Surgical group
|
|---|
| Sural graft
(n=13) | VII–XII (n=12) | P-value |
|---|
| Age (years) | | | |
| Mean | 46.69 | 47.00 | 0.934 |
| SD | 9.75 | 8.60 | NS |
| Range | 24–57 | 31–57 | |
| Tumor size (cm) | | | |
| Mean | 3.15 | 3.08 | 0.827 |
| SD | 0.88 | 0.70 | NS |
| Range | 2.0–5.0 | 2.0–4.5 | |
The patients were divided into two groups, odd and
even according to the last number of their ID. Odd patients
received sural graft facial reanimation and even patients received
VII–XII anastomosis. The four patients in whom it was not possible
to identify the proximal stumps of the facial nerves were placed in
the even group.
Facial function and surgical data
Facial function and surgical data were obtained from
Xinhua Hospital records. Patients who underwent vestibular
schwannoma removal were routinely followed up for facial function
and surgical outcome by interview approximately one and three years
following surgery. All data were accessible for the purpose of this
study.
Follow-up
During the follow-up period, any complications
developed by the facial reanimation patients, such as synkinesia,
claudication, disarticulation, numbness of the tongue and
dysphagia, were assessed.
Statistical analysis
Statistical comparisons were performed using
parametric and non-parametric tests, as appropriate. Assessments
were performed using two-tailed probabilities. P<0.05 was
considered to indicate a statistically significant difference.
Statements showing no difference between groups indicate that a
statistical test was performed and failed to reject the null
hypothesis.
Results
Observations at discharge
House-Brackmann facial grade VI function was
observed in all 25 patients upon discharge. Significant recovery of
facial nerve function was observed at both postoperative time
intervals: one year (short term) and three years (long term).
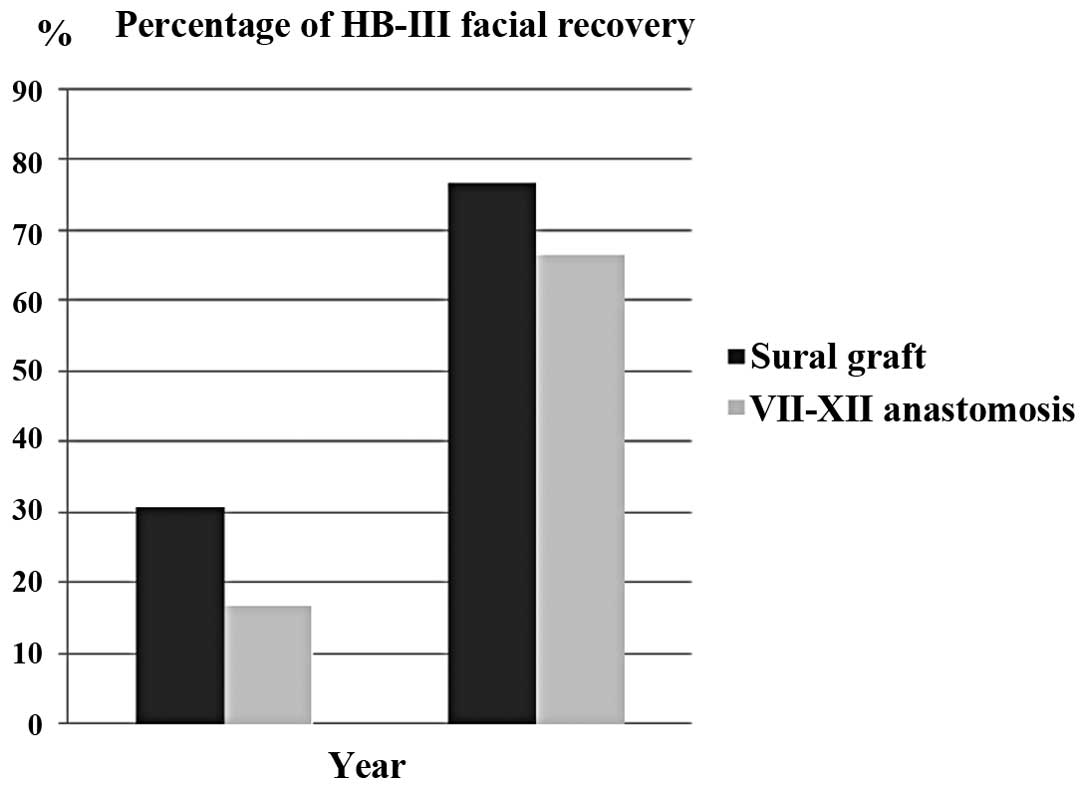
Facial nerve grades for the two groups at the short- and long-term
intervals are shown in Fig. 1 and
Table II. There was a
statistically significant difference in facial nerve recovery
between the short- and long-term follow-up in the two treatment
groups. In the sural graft group, House-Brackmann grade III facial
function was achieved in four (30.8%) and ten (76.9%) patients in
the short and long term, respectively, and House-Brackmann grade IV
facial function was achieved in nine (69.2%) and three (23.1%)
patients in the short and long term, respectively. There was a
statistically significant difference between the facial recovery
results across the short- and long-term follow-up periods in the
sural graft group (P=0.036). In the VII-XII anastomosis group,
House-Brackmann grade III facial function was achieved in two
(16.7%) and eight (66.7%) patients in the short and long term,
respectively and House-Brackmann grade IV facial function was
achieved in ten (83.3%) patients in the short term and four (33.3%)
patients in the long term. There was a statistically significant
difference between the facial recovery results across the short-
and long-term follow-up periods (P=0.047).
 | Table II.Comparison of short-term (1 year) and
long-term (3 year) facial recovery. |
Table II.
Comparison of short-term (1 year) and
long-term (3 year) facial recovery.
| HB | Sural graft
| VII–XII anastomosis
|
|---|
| 1 year | 3 years | 1 year | 3 years |
|---|
| Grade III | 4 | 10 | 2 | 8 |
| Grade IV | 9 | 3 | 10 | 4 |
| P-value | 0.036 | | 0.047 | |
Observations at follow-up
A comparison of the facial recovery results of the
techniques revealed that, upon short-term follow-up,
House-Brackmann grade III facial function was achieved in four
(30.8%) and two (16.7%) cases in the sural graft and VII–XII
anastomosis groups, respectively. Upon long-term follow-up,
House-Brackmann grade III facial function was achieved in ten
(76.9%) and eight (66.7%) cases in the sural graft and VII–XII
anastomosis groups, respectively. It appeared that facial nerve
function recovery was improved in the sural graft group compared
with the VII–XII anastomosis group in both the short- and long-term
follow-up, but no statistically significant difference was observed
between the groups (short term, P=0.645; long term, P=0.673).
Some special outcomes following facial animation
were observed during postoperative follow-up. In the sural graft
group, claudication was common upon discharge (four patients), but
diminished over time. Synkinesia, the most common cause of
complaint, was observed in three patients. In the VII–XII
anastomosis group, disarticulation was the most common
complication, observed in five patients. Numbness of the tongue was
the second most common complication, observed in four patients.
None of the patients developed dysphagia and synkinesia.
Discussion
In this type of large tumor, total tumor removal is
achieved exclusively through the translabyrinthine approach
(8,9). The translabyrinthine approach allows
a positive identification of the distal segment of the facial nerve
(10). Dissection of the plane
between the tumor capsule and the facial nerve may then be extended
medially (11). Following tumor
debulking and the positive identification of the proximal facial
nerve at the surface of the brain stem, the plane may be
established medially. Combined medial and lateral identification
provides the maximal possibility of preserving nerve integrity
(12,13). It has been reported that
particularly poor facial outcomes are observed in patients who
required the surgical excision of previously irradiated vestibular
schwannomas (14–16).
The incidence of facial nerve interruption has been
reduced significantly due to improvements in surgical techniques
and perioperative monitoring. It has been reported that automatic
facial nerve preservation is achieved in >90% of large
vestibular schwannoma patients. However, a certain degree of facial
interruption is inevitable, particularly in patients with large or
recurrent tumors (17).
Various techniques may be used for facial
reanimation depending on the characteristic of the proximal stump
of the facial nerve and the preference of the surgeon (18). Sural graft and VII–XII anastomosis
are the most common facial reanimation techniques. Both surgeries
provide the possibility of House-Brackmann grade III facial
recovery in the long term. These techniques have advantages and
disadvantages. The sural nerve has a history of use as a donor
nerve, but there is evidence that motor nerve grafts are more
appropriate than sensory nerve grafts (19). Acquisition of a sural cable may not
necessarily extend the surgical period. With sural nerve grafting,
the best outcome is House-Brackmann grade III facial function
(20). Claudication is the most
common complication at the time of discharge, but it quickly fades.
Synkinesia is the most prominent complication of sural graft facial
reanimation. VII–XII anastomosis may be achieved by a single
surgical incision and does not require the identification of the
proximal facial stump. The disadvantage of VII–XII anastomosis is
that it sacrifices the function of the hypoglossal nerve (21). Disarticulation and numbness of the
tongue are the most common complaints during follow-up. The best
possible facial recovery is House-Brackmann grade III.
This study compared patients who underwent surgery
during the same period by surgeons with the same level of
experience. The mean patient age was 46.69 years in the sural graft
group and 47.00 years in the VII–XII anastomosis group. No
statistical difference in age was detected between the groups.
Tumor size is the most important factor affecting facial function
following surgery. The mean tumor size was 3.15 cm in the sural
graft group and 3.08 cm in the VII–XII anastomosis group. No
statistically significant difference was detected between the
groups with regard to tumor size.
We compared the short- and long-term outcomes of
facial reanimation. In the sural graft group, House-Brackmann grade
III facial function was achieved in four patients in the short term
and in ten patients in the long term. House-Brackmann grade IV
facial function was achieved in nine patients in the short term and
in three patients in the long term. In the VII–XII anastomosis
group, House-Brackmann grade III facial function was achieved in
two patients in the short term and eight patients in the long term.
House-Brackmann grade IV facial function was achieved in ten
patients in the short term and in four patients in the long term.
There was a statistically significant difference between the facial
recovery results across the short- and long-term follow-up periods.
The outcomes of facial reanimation were acceptable following sural
grafting and VII–XII anastomosis.
We compared the long-term outcomes of facial
recovery following the different surgical facial reanimation
techniques. Sural grafting appeared to produce superior facial
recovery compared with VII–XII anastomosis in the short term
(House-Brackmann grade III 30.8 vs. 16.7%) and in the long term
(House-Brackmann grade III 76.9 vs. 66.7%). However, no
statistically significant difference was observed between the
surgical groups. Selecting the appropriate surgical technique for
facial reanimation requires careful consideration of the
complications and benefits of all available surgeries. A study
sampling a greater number of patients may provide further insight
into the value of various surgical procedures.
Following surgery, synkinesia developed in 23.1%
(3/13) of sural graft cases in the present study. No significant
synkinesia was observed in the VII–XII anastomosis group. This
observation is explained by the fact that the healing of two nerves
led to a greater incidence of incorrect regeneration by the facial
nerve fibers (22).
Loss of function of the hypoglossal nerve led to
numbness of the tongue and disarticulation, which was occasionally
accompanied by severe facial paresis. Following surgery,
rehabilitation training of the hypoglossal and facial nerves may
improve functional recovery.
In conclusion, facial reanimation remains an
effective procedure for the surgical rehabilitation of static and
dynamic facial nerve functions. A significant improvement in facial
nerve function may occur more than three years following surgery.
Despite such morbidities as synkinesia, the sural graft technique
is accompanied by a greater improvement in facial nerve function
compared with VII–XII anastomosis in both the short- and long-term
evaluations, but this requires confirmation by larger studies.
References
|
1.
|
Theodosopoulos PV and Pensak ML:
Contemporary management of acoustic neuromas. Laryngoscope.
121:1133–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Falcioni M, Fois P, Taibah A and Sanna M:
Facial nerve function after vestibular schwannoma surgery. J
Neurosurg. 115:820–826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Han SJ, Oh MC, Sughrue ME, Aranda D,
Rutkowski MJ and Parsa AT: Reporting standard compliance in
publications of vestibular schwannoma patients treated with
microsurgery. Otol Neurotol. 33:648–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Giordano AI, Domènech I, Torres A, et al:
Results in the surgical treatment of giant acoustic neuromas. Acta
Otorrinolaringol Esp. 63:194–199. 2012.(In Spanish).
|
|
5.
|
Bambakidis NC, Lo SS and Selman WR: Large
vestibular schwannomas. J Neurosurg. 115:894–895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Nuseir A, Sequino G, De Donato G, Taibah A
and Sanna M: Surgical management of vestibular schwannoma in
elderly patients. Eur Arch Otorhinolaryngol. 269:17–23. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ahmad RA, Sivalingam S, Topsakal V, Russo
A, Taibah A and Sanna M: Rate of recurrent vestibular schwannoma
after total removal via different surgical approaches. Ann Otol
Rhinol Laryngol. 121:156–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ben Ammar M, Piccirillo E, Topsakal V,
Taibah A and Sanna M: Surgical results and technical refinements in
translabyrinthine excision of vestibular schwannomas: the Gruppo
Otologico experience. Neurosurgery. 70:1481–1491. 2012.PubMed/NCBI
|
|
9.
|
Mamikoglu B, Esquivel CR and Wiet RJ:
Comparison of facial nerve function results after translabyrinthine
and retrosigmoid approach in medium-sized tumors. Arch Otolaryngol
Head Neck Surg. 129:429–431. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kim J, Moon IS, Jeong JH, Lee HR and Lee
WS: What really decides the facial function of vestibular
schwannoma surgery? Clin Exp Otorhinolaryngol. 14:168–173. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gerganov VM, Giordano M, Samii M and Samii
A: Diffusion tensor imaging-based fiber tracking for prediction of
the position of the facial nerve in relation to large vestibular
schwannomas. J Neurosurg. 115:1087–1093. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Charpiot A, Tringali S, Zaouche S,
Ferber-Viart C and Dubreuil C: Perioperative complications after
translabyrinthine removal of large or giant vestibular schwannoma:
Outcomes for 123 patients. Acta Otolaryngol. 130:1249–1255. 2010.
View Article : Google Scholar
|
|
13.
|
Angeli RD, Ben Ammar M and Sanna M:
Perioperative complications after translabyrinthine removal of
large or giant vestibular schwannoma: outcomes for 123 patients.
Acta Otolaryngol. 131:1237–1238. 2011.
|
|
14.
|
Collen C, Ampe B, Gevaert T, et al: Single
fraction versus fractionated linac-based stereotactic radiotherapy
for vestibular schwannoma: a single-institution experience. Int J
Radiat Oncol Biol Phys. 81:e503–e509. 2011. View Article : Google Scholar
|
|
15.
|
Friedman RA, Berliner KI, Bassim M, et al:
A paradigm shift in salvage surgery for radiated vestibular
schwannoma. Otol Neurotol. 32:1322–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gerganov VM, Giordano M, Samii A and Samii
M: Surgical treatment of patients with vestibular schwannomas after
failed previous radiosurgery. J Neurosurg. 116:713–720. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hertzano R and Eisenman DJ: Large,
symptomatic tension pneumocele: 23 years after translabyrinthine
resection of an acoustic neuroma. Otolaryngol Head Neck Surg.
144:477–478. 2011.PubMed/NCBI
|
|
18.
|
Guntinas-Lichius O, Straesser A and
Streppel M: Quality of life after facial nerve repair.
Laryngoscope. 117:421–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Samii M, Koerbel A, Safavi-Abbasi S, Di
Rocco F, Samii A and Gharabaghi A: Using an end-to-side interposed
sural nerve graft for facial nerve reinforcement after vestibular
schwannoma resection. Technical note. J Neurosurg. 105:920–923.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Humphrey CD and Kriet JD: Nerve repair and
cable grafting for facial paralysis. Facial Plast Surg. 24:170–176.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Magliulo G, D’Amico R, Forino M and
Marcotullio D: Facial reanimation: a proposal to reduce
postoperative morbidity. Laryngoscope. 112:183–186. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Filipo R, Spahiu I, Covelli E, Nicastri M
and Bertoli GA: Botulinum toxin in the treatment of facial
synkinesis and hyper-kinesis. Laryngoscope. 122:266–270. 2012.
View Article : Google Scholar : PubMed/NCBI
|