Introduction
Venous malformation (VM) is a common vascular
anomaly in children (1,2), and is mainly composed of abnormally
dilated venous components without cell proliferation
characteristics. Compared with other vascular malformations, the
type, location, scope and tissue invasion of VM are significantly
diverse. There are various treatments for VM, including surgical
excision, laser therapy and interventional sclerotherapy.
Previously, surgical resection had been considered to be the
preferential treatment (3).
However, the drawbacks of surgical resection are clear: it is not
possible to remove the tumors completely, recurrence after surgery
is common, surgical trauma is serious and the peripheral nerve may
be injured. The effects of laser therapy are limited and this
technique is only effective in patients with small superficial
tumors. Recently, interventional sclerotherapy has become the main
method of treatment (4,5). Common sclerosing agents include foam
sclerosing agents, polidocanol, sodium tetradecyl sulfate,
anhydrous ethanol and bleomycin. Anhydrous ethanol is the most
widely used sclerosing agent. There are numerous reports regarding
treating VM with a single dose of anhydrous ethanol or bleomycin
(6–8). However, few reports provide a
comprehensive comparison of treatment effects and adverse reactions
between the two methods. Between February 2009 and February 2012,
138 children with VM were treated with either anhydrous ethanol or
bleomycin. This study was designed in order to compare the
treatment effects and adverse reactions of anhydrous ethanol and
bleomycin and to provide evidence for the selection of sclerosing
agents.
Patients and methods
Patients
All 138 patients with VM in this study were
diagnosed in accordance with the diagnostic criteria of VM in
Clinical Practice Guidelines - Plastic Surgery Volume (9): the lesions existed at birth; the
lesions grew in proportion with the body; when the focus location
was superficial, the lesion area was blue; the texture was soft and
compressible; the skin temperature in the lesion area was not high
(a key point for hemangioma identification); and it tested positive
in postural experiments. Also, characteristic manifestations were
observed by MRI. This study involved 138 patients, 83 males and 55
females, aged between 3 months and 14 years. Signed informed
consent was obtained from guardians of all patients. The study was
approved by the ethics committee of Guangzhou Women and Children’s
Medical Center.
Medical history
The foci of 112 patients existed when they were born
and grew as the patients did; the foci of 26 patients occurred 3
months after birth. In 83 cases (60%), the tumors were located in
the maxillofacial region, in 28 cases (20%) the tumors were located
in the limbs, in 17 cases (12%) the tumors were located in the
trunk and in 10 cases (7%) the tumors were located in the hip.
There were 87 cases of superficial VM; in 55 cases the foci were
located in the subcutaneous tissues and of those, 22 were located
in the mucous membrane. There were 51 cases of deep VM with the
foci located in deep muscle tissue. Foci involving skin or mucosal
surfaces were bluish-violet in color and were raised from the skin
surface or mucous membranes; the foci located in deep muscle tissue
were expressed as masses. The texture of the masses was soft and
tested positive in a postural experiment. The tumor size ranged
from 2.5×2.0×1.0 cm to 15.0×10.0×6.0 cm. There were 66 patients
whose chief complaint of irregular pain at the site of the lesion
could be relieved without further treatment. All patients were
examined using MRI (Philips Achieva 1.5T dual-gradient magnetic
resonance imager; Royal Philips Electronics, Amsterdam, The
Netherlands). The lesion demonstrated a low or iso-signal on the
T1-weighted image (T1WI) and a high signal on the T2WI. The
enhanced scan revealed 39 cases of marked enhancement, 62 cases of
mild to moderate enhancement and 37 cases of no enhancement. Using
ultrasound, the lesion was expressed as an uneven internal echo
with clear boundary and irregular shape and a pipe-like echo was
visible. Blood flow was not probed by a Doppler test. All surgeries
were carried out under the guidance of the large C-arm angiography
machine (GE, Waukesha, WI, USA). Drugs used during surgery included
bleomycin injection (8 mg/tube; Harbin Lebo Pharmaceutical Co.,
Ltd., Harbin, China), lipiodol injection (10 ml/tube; Guerbet,
Aulnay-sous-Bois, France) and iohexol injection (20 ml, 6 mg: GE
Pharmaceutical Co., Ltd., Shanghai, China).
Treatment method
The 138 patients with VM were randomly divided into
group A and group B according to hospitalization number and were
selected on the basis of admission time. Patients whose
hospitalization number ended in an odd number were assigned to
group A with anhydrous ethanol and lipiodol as the sclerosing
agent; patients whose hospitalization number ended in an even
number were assigned to group B with bleomycin and lipiodol as the
sclerosing agent. The ratio of anhydrous ethanol to lipiodol in the
sclerosing agent of group A was 5:1 (v/v). The maximum dosage of
anhydrous ethanol was 1 mg/kg, with a single dosage ≤50 ml. When
the dosage of anhydrous ethanol was assumed to exceed 0.5 ml/kg,
pulmonary artery pressure was monitored. To prepare the sclerosing
agent of group B, 8 mg bleomycin was dissolved in 4 ml contrast
agent; the dosage of bleomycin was calculated at 10
mg/m2 body surface. The solution was mixed with an equal
amount of ultra-liquefied lipiodol in a sterile container and the
mixture was aspirated repeatedly with a syringe to prepare a
bleomycin-lipiodol emulsion. The concentration of bleomycin was 1
mg/ml.
Due to the poor cooperation of children and the
irritating nature of the sclerosing agents, all patients were
treated under general anesthesia. After general anesthesia, the
malformed vascular mass was directly punctured for radiography. The
most protuberant part of the VM was punctured directly using 7th
scalp acupuncture (Venofix (r) A). When the malformed vascular mass
was successfully punctured, venous blood was pumped back. Then, 30%
(iodine content) contrast agent (iohexol injection) was injected
under fluoroscopy and the filling situation of the VM was
continuously observed. Prior to treatment with a sclerosing agent,
the patients were injected intramuscularly with dexamethasone at a
dose of 0.3 mg/kg. In group A, anhydrous ethanol-lipiodol emulsion
was injected into abnormal vessels; in group B, patients were
treated with a bleomycin-lipiodol emulsion. Prior to treatment, the
proximal-end draining veins of the VM patients in the two groups
were pressed directly by assistants to expand the malformed
vascular mass. The sclerosing agent was injected slowly into the
malformed vascular mass under fluoroscopy via scalp acupuncture
which was used for injection of the contrast agent. During the
injection process, embolization agents were carefully observed to
monitor whether they entered the draining veins and assess the
filling situation of the vessel mass. When the vessel mass or the
draining vein was shown to be filled completely, the injection of
sclerosing agent was stopped. If the malformed vascular mass was
unable to be filled completely via one injection site, another
puncture site was used to inject the embolization agent. Patients
who were cured were followed up. If tumors were reduced by <80%,
the treatment was continued. The time interval between treatments
was one month.
Efficacy criteria and follow-up
All patients were reviewed one month after treatment
and the efficacy was evaluated. If patients with superficial VM,
whose efficacy could be judged by the naked eye, were cured, they
were followed up. If the tumors were reduced by <80%, treatment
was continued. For deep VM, efficacy was evaluated by MRI
examination. The final efficacy was the result of an MRI follow-up
visit carried out 6 months after the final treatment. The treatment
efficacy was classified into four levels (7): i) cured, the tumors completely
disappeared after injection, the surface color was normal, no
dysfunction and no recurrence during the follow-up visit. ii)
Markedly effective, the majority of the tumors disappeared after
injection (tumors were reduced by >80%), skin color was close to
normal or there was slight pigmentation, no dysfunction, the
appearance had not yet completely recovered and the treatment
should be continued. iii) Effective (improved), tumors were reduced
by <80% and the treatment should be continued. iv) Ineffective,
tumors were not reduced; they remained unchanged or continued to
increase. The effective rate = (the number of cured cases + the
number of markedly effective cases + the number of effective cases)
/ the number of total cases × 100. Systemic and local adverse
reactions of the patients were recorded. Since all patients
demonstrated swelling and pain, which was relived without treatment
3–7 days after surgery, and swelling was a part of the
sclerotherapy mechanism, swelling and pain were not classified as
adverse reactions.
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. The comparison of effective rates and
incidence of adverse reactions between the two groups were tested
using the χ2 test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Efficacy
The patients in the two groups were followed up for
6–24 months. The observed efficacy is demonstrated in Table I. In group A, the effective rate of
superficial VM was 95% (38/40) and that of deep VM was 94% (33/35).
In group B, the effective rate of superficial VM was 68% (32/47)
and that of deep VM was 56% (9/16). The difference in the effective
rate between the two groups was considered to be statistically
significant. The efficacy rate in group A was greater than that in
group B. In group A, 30 cases were treated once with anhydrous
ethanol embolotherapy, 30 cases were treated twice and 15 cases
were treated three times. In group B, 6 cases were treated once
with bleomycin embolotherapy, 23 cases were treated twice, 31 cases
were treated three times and three cases were treated four times
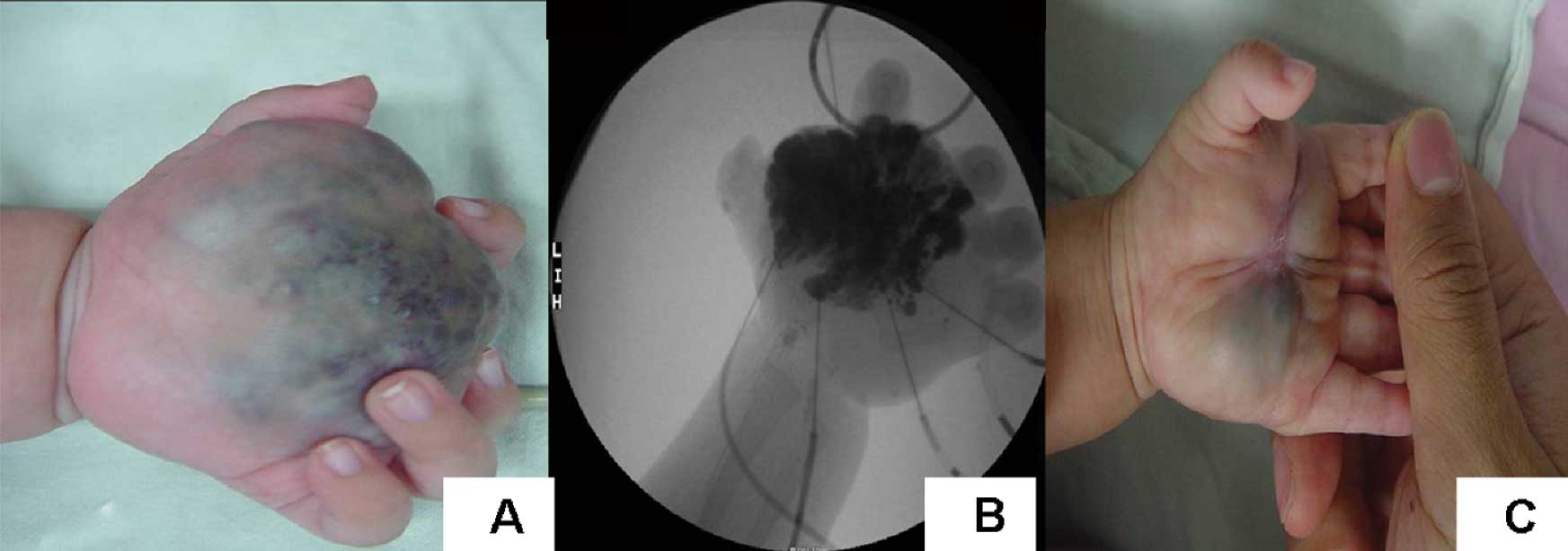
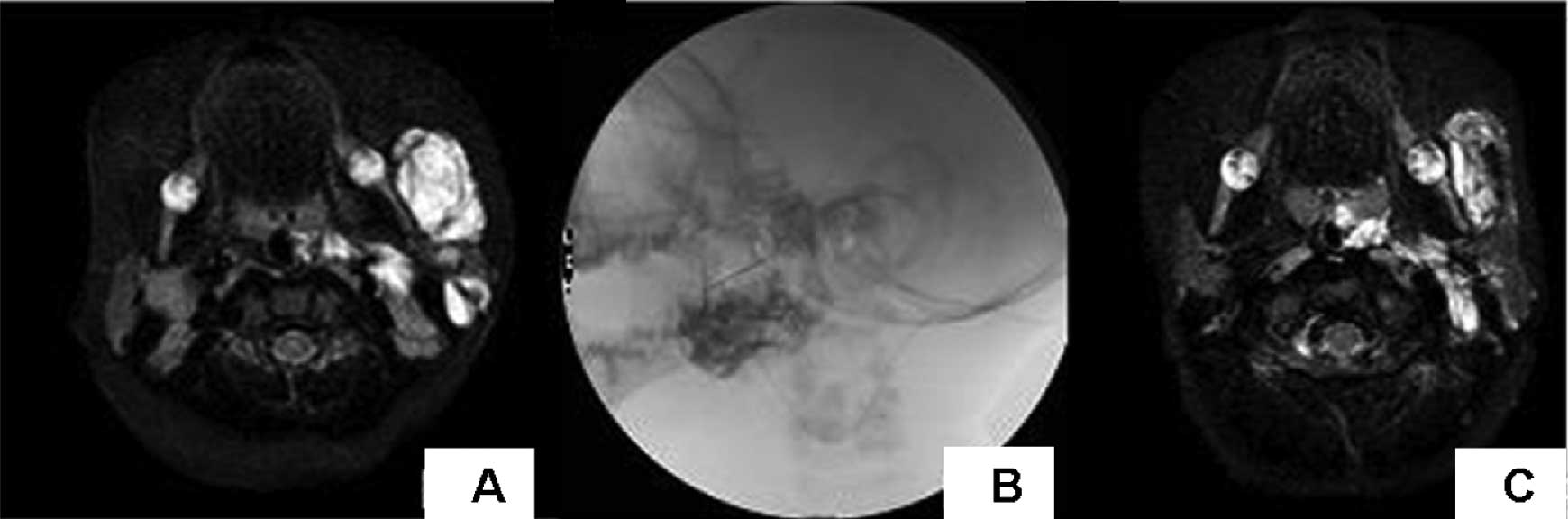
(Table II; Figs. 1–3).
 | Table I.Effect of interventional therapy on
the two groups. |
Table I.
Effect of interventional therapy on
the two groups.
| Group | Cases | Evaluation of
therapeutic effect |
|---|
|
|---|
| Cured | Markedly
effective | Effective | Ineffective |
|---|
| A | 75 | 15 | 33 | 13 | 4 |
| B | 63 | 6 | 19 | 16 | 22 |
| χ2
value | 19.6 | | | | |
| P-value | 0.0001 | | | | |
 | Table II.Therapeutic effect of local injection
treatment for venous malformation. |
Table II.
Therapeutic effect of local injection
treatment for venous malformation.
| Number of
treatments | Group A | Group B | χ2
value | P value |
|---|
|
|
|---|
| Cases | Cured | Markedly
effective | Effective | Ineffective | Cases | Cured | Markedly
effective | Effective | Ineffective |
|---|
| 1 | 75 | 9 | 30 | 28 | 8 | 63 | 3 | 12 | 21 | 27 | 18.7 | 0.0001 |
| 2 | 45 | 4 | 20 | 15 | 6 | 57 | 2 | 15 | 8 | 32 | 19.7 | 0.0001 |
| 3 | 15 | 2 | 9 | 0 | 4 | 34 | 1 | 5 | 6 | 22 | 6.04 | 0.01 |
| 4 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | | ` |
Adverse reactions
The incidence adverse reactions was relatively high
in the patients in group A (45%). In this study, six patients (two
cases of superficial VM and four cases of deep VM) contracted a
fever with nausea and vomiting after surgery, and the symptoms were
relieved with treatment. Skin necrosis occurred in 14 superficial
VM patients, and a scar remained after scabbing. There were 17
patients with serious localized swelling which required additional
treatment, three patients developed muscle fibrosis and one patient
suffered a brain embolism. The symptoms were relieved once the
micro-circulation was improved by the intravenous infusion of
Salvia miltiorrhiza and low molecular weight dextran. Muscle
injury and fibrosis occurred in three cases of deep VM, leading to
clubfoot. Cerebral infarction occurred in one case of deep VM
located in the neck. The manifestations were hyperspasmia and
vomiting. No sequelae remained after symptomatic treatment. The
incidence of patients with an adverse reaction in group B was
relatively low (10%). Fever accompanied by vomiting occurred in six
patients. There were two patients (one case of deep VM and one case
of superficial VM) who demonstrated swelling and tumor pain. The
symptoms were relieved after treatment. A further five patients
suffered from skin ulceration. The difference in the incidence of
adverse reactions between the two groups was considered to be
statistically significant (χ2=18.8, P=0.0001).
Discussion
Sclerotherapy is the first-line therapy for VM.
However, the guidelines for the selection of sclerosing agents were
considered to be insufficient. Anhydrous ethanol and bleomycin are
liquid sclerosing agents, but it is unclear which one should be
used preferentially, as reports offer differing conclusions. It has
been reported that the effective rate of treatment for VM with
anhydrous ethanol was 75–95% (10–13).
In this study, the effective rate of embolotherapy with anhydrous
ethanol for superficial VM was 95% and for deep VM was 94%; the
total effective rate was 95%. It has been reported that the
effective rate of treatment for VM with bleomycin was 82.7%
(14). In the current study the
effective rate of treatment with bleomycin for superficial VM was
68% and for deep VM was 56%, with a total effective rate of 65%.
The efficacy of embolotherapy with anhydrous ethanol was greater
than that with bleomycin. However, the dehydration and denudation
effects of anhydrous ethanol were more serious and the adverse
reaction rate of anhydrous ethanol was high, particularly in the
treatment of superficial VM. It was reported by Berenguer et
al (15) that the rate of
adverse reaction to embolotherapy with anhydrous ethanol was 50%
for VM. The majority of adverse reactions involved vesicle tension
and ulcer and nerve injury. Of the serious adverse reactions, skin
necrosis occurred in 14 patients and cerebral infarction occurred
in one patient. The dosage of anhydrous ethanol in patients who
developed serious adverse reactions exceeded 0.5 ml/kg. Generally,
anhydrous ethanol was injected into the draining vein. If there was
no further lesion increase, the treatment was ended. If the dosage
was excessive, the anhydrous ethanol would flow back to the feeding
artery, leading to skin and tissue necrosis (5,11,16).
Therefore, it is essential that the dosage of anhydrous ethanol is
selected on the basis of tumor size.
Compared with anhydrous ethanol, bleomycin has a
longer shelf life and a lower price. However, the effect of
bleomycin is mild. Severe pain does not occur following the
injection of bleomycin and there are fewer adverse reactions. It
has been reported that the main adverse reaction of bleomycin is
pulmonary fibrosis. However, in previous studies (17), pulmonary fibrosis did not occur
when bleomycin was the sclerosing agent, and severe adverse
reactions were rare. The majority of adverse reactions involved an
increase in body temperature and localized swelling. In this study,
fever and vomiting occurred in six patients in group B, two of whom
demonstrated swelling and tumor pain. A further five patients
developed skin ulceration. The incidence of adverse reaction was
10%.
At present, there is no consensus on the selection
of sclerosing agents for clinical applications. Large scale
randomized clinical trials are insufficient. Most clinicians select
sclerosing agents according to their own familiarity with
sclerosing agents and the focus size. Although there have been many
reports (18–22) regarding treatment of VM with
various sclerosing agents, the efficacy of anhydrous ethanol has
rarely been compared with that of bleomycin. In the current study,
it was demonstrated that the efficacy of anhydrous ethanol was
greater than that of bleomycin. Although treatment with anhydrous
ethanol was effective and fast, the incidence of adverse reactions
was higher. For superficial VM, the efficacy of bleomycin emulsion
is greater, with fewer adverse reactions. For deep VM, anhydrous
ethanol is more effective.
References
|
1.
|
Fishman SJ and Mulliken JB: Hemangiomas
and vascular malformations of infancy and childhood. Pediatr Clin
North Am. 40:1177–1200. 1993.PubMed/NCBI
|
|
2.
|
Greene AK and Alomari AI: Management of
venous malformations. Clin Plast Surg. 38:83–93. 2011. View Article : Google Scholar
|
|
3.
|
Belov S and Loose DA: Surgical treatment
of congenital vascular defects. Int Angiol. 9:175–182.
1990.PubMed/NCBI
|
|
4.
|
Yun WS, Kim YW, Lee KB, et al: Predictors
of response to percutaneous ethanol sclerotherapy (PES) in patients
with venous malformations: analysis of patient self-assessment and
imaging. J Vasc Surg. 50:581–589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Orlando JL, Caldas JG, Campos HG, et al:
Ethanol sclerotherapy of superficial venous malformation: a new
procedure. Dermatology. 220:376–380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ierardi AM, Mangini M, Vaghi M, et al:
Sclerotherapy of peripheral venous malformations: a new technique
to prevent serious complications. Vasc Endovascular Surg.
44:282–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chen WL, Yang ZH, Bai ZB, et al: A pilot
study on combination compartmentalisation and sclerotherapy for the
treatment of massive venous malformations of the face and neck. J
Plast Reconstr Aesthet Surg. 61:1486–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lee CH and Chen SG: Direct percutaneous
ethanol instillation for treatment of venous malformation in the
face and neck. Br J Plast Surg. 58:1073–1078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chinese Medical Association: Clinical
Practice Guidelines - Plastic Surgery volumes. Beijing: People’s
Health Publishing House; pp. 11–12. 2009
|
|
10.
|
Su L, Fan X, Zheng L and Zheng J: Absolute
ethanol sclerotherapy for venous malformations in the face and
neck. J Oral Maxillofac Surg. 68:1622–1627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Spence J, Krings T, TerBrugge KG and Agid
R: Percutaneous treatment of facial venous malformations: a matched
comparison of alcohol and bleomycin sclerotherapy. Head Neck.
33:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Khandpur S and Sharma VK: Utility of
intralesional sclerotherapy with 3% sodium tetradecyl sulphate in
cutaneous vascular malformations. Dermatol Surg. 36:340–346.
2010.PubMed/NCBI
|
|
13.
|
Rautio R, Laranne J, Kähärä V, et al:
Long-term results and quality of life after endovascular treatment
of venous malformations in the face and neck. Acta Radiol.
45:738–745. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sainsbury DC, Kessell G, Fall AJ, et al:
Intralesional bleomycin injection treatment for vascular
birthmarks: a 5-year experience at a single United Kingdom unit.
Plast Reconstr Surg. 127:2031–2044. 2011.PubMed/NCBI
|
|
15.
|
Berenguer B, Burrows PE, Zurakowski D and
Mulliken JB: Sclerotherapy of craniofacial venous malformations:
complications and results. Plast Reconstr Surg. 104:1–11. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Dompmartin A, Vikkula M and Boon LM:
Venous malformation: update on aetiopathogenesis, diagnosis and
management. Phlebology. 25:224–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Barbier C, Martin A, Papagiannaki C, et
al: Superficial venous malformations. Presse Med. 39:471–481.
2010.(In French).
|
|
18.
|
Zochowski CG, Salgado CJ and Jamali AA:
Extensive muscle necrosis and infection following treatment of a
lower extremity vascular malformation with Sotradecol and absolute
ethanol. Blood Coagul Fibrinolysis. 21:480–486. 2010. View Article : Google Scholar
|
|
19.
|
Bisdorff A, Mazighi M, Saint-Maurice JP,
et al: Ethanol threshold doses for systemic complications during
sclerotherapy of superficial venous malformations: a retrospective
study. Neuroradiology. 53:891–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wang YA, Zheng JW, Zhu HG, et al:
Sclerotherapy of voluminous venous malformation in head and neck
with absolute ethanol under digital subtraction angiography
guidance. Phlebology. 25:138–144. 2010.
|
|
21.
|
Berber O, Holt P, Hinchliffe R, et al:
Endovenous therapy for the treatment of congenital venous
malformations. Ann Vasc Surg. 24:e13–e17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Uehara S, Osuga K, Yoneda A, et al:
Intralesional sclerotherapy for subcutaneous venous malformations
in children. Pediatr Surg Int. 25:709–713. 2009. View Article : Google Scholar : PubMed/NCBI
|