Introduction
Recurrent spontaneous abortion (RSA) is defined as
three or more spontaneous abortions of a fetus before 20 weeks of
gestation (1,2). It is one of the most common
complications during pregnancy. Common pathogeny including
infections, endocrine disorders, heritable mutations and immune
deficiencies, have not been identified in RSA (3). Therefore, the most important
pathogeny of RSA remains unclear. Pregnancy-associated plasma
protein A (PAPP-A) is characterized as plasma glycoprotein secreted
by placental syntrophoblastic cells and decidual cells. Previously
PAPP-A was shown to be involved in a number of processes during
embryonic development, including the early development of gametes,
the implantation of zygote and the development of fetus (4). In addition, the expression levels of
PAPP-A in placental tissue increases with the time course of
pregnancy. In this study, we investigated the correlation between
the expression levels of PAPP-A and RSA.
Subjects and methods
Subjects
A total of 39 RSA patients from June 2010 to June
2012, termed the RSA group, were enrolled in this study. The
criteria for enrolment were: i) Normal cytogenetic phenotype
without any heritable disease or spontaneous abortion in the family
history; ii) Negative physical examination of vaginal infection;
iii) Negative for anticardiolipin antibodies, antinuclear
antibodies, antisperm antibodies and antiendometrium antibodies;
iv) No autogenous immune disease or endocrine disease; v) No
vascular disease or infection disease history; vi) No reproductive
disorder or sperm impairment of the fetus’ father; vii) No
addiction to cigarettes or alcohol. The age range of the RSA group
was 27–41 years, with an average of 33.1±5.4 years. The pregnancy
time period was 5–7 weeks, with an average of 6.1±0.8 weeks. In
addition to the RSA group, 30 patients who were experiencing normal
pregnancy, but who were subjected to induced abortion, were
enrolled as a control group. The age range of the control group was
23–40 years, with an average of 32.1±5.2 years. The pregnancy
period time was 5–8 weeks, with an average of 6.5±0.9 weeks. No
significant difference concerning age and pregnancy time period
between the two groups was observed (P>0.05). This study was
conducted in accordance with the declaration of Helsinki and with
the approval from the Ethics Committee of the Third Affiliated
Hospital of Zhengzhou University. Written informed consent was
obtained from all the participants.
qPCR results of PAPP-A mRNA
Basal decidual tissue was obtained using vacuum
suction and stored in liquid nitrogen. This tissue was then removed
from liquid nitrogen and resolved in 1 ml TRIzol (Invitrogen,
Carlsbad, USA). After resolving, 200 μl chloroform was added
into the tissue suspension and mixed well. The mixture was then
incubated on ice for 5 min and centrifuged at 7342 × g for 15 min.
The supernatant liquid was moved to another new tube to which an
equal volume of isopropanol was added and mixed well. The tube was
incubated on ice for 10 min and centrifuged at 7342 × g for 15 min.
Following aspiration of the supernatant, the tissue was washed in 1
ml chilled 75% alcohol. The tube was gently tapped five times,
centrifuged at 4828 × g for 5 min and the supernatant was then
carefully aspirated. DEPC-treated water was added to resolve the
RNA pellet and the total concentration of RNA was measured. RNA was
reversed using a reverse transcriptional kit (Takara, Dalian,
China). The resulting cDNA was employed in qPCR analysis performed
using the primers: PAPP-A, forward: 5′-CTACTTGGATGTTAATGAGC-3′, and
reverse: 5′-TCCTGCCAACTCCTCCTCTG-3′; actin, forward: 5′-AGC
GGGAAATCGTGCGTGACA-3′, and reverse: 5′-GTGGA CTTGGGAGAGGACTGG-′3.
The primers were synthesized by the Shanghai Yingjun Biotechnology
Co. Ltd., Shanghai, China. The primers were resolved at a
concentration of 10 μM. The reaction system included 10
μl SYBR® Premix Ex TaqTM (Applied Biosystems,
Milan, Italy), 0.6 μl forward primer, 0.6 μl reverse
primer, 8.8 μl cDNA diluted at a 1:100 ratio. The total
volume of 20 μl for each qPCR reaction was added into a
96-well plate and gently centrifuged at 150 × g for 2 min.
Following the initial step of 95°C for 5 min, 40 PCR cycles of 95°C
for 30 sec and then 60°C for 1 min, were carried out. The
expression levels of PAPP-A mRNA were measured according to the
2−ΔΔCt relative quantification analysis method.
Immunostaining for PAPP-A protein
Basal decidual tissue from the RSA and control
groups were paraffin-embedded. Deparaffinization treatment of a 5
μm section slide was performed prior to staining.
Subsequently, the slide was preincubated in PBST three times
followed by 3% H2O2-methanol treatment at
95°C for 15 min. The slide was then washed again in PBST three
times and blocked with a medium containing 5% normal goat serum for
1 h at room temperature. The slides were then incubated overnight
at 4°C with the primary antibodies (Abcam, Cambridge, UK) diluted
in blocking medium. On the second day, the slides were rinsed in
PBST and the sections incubated for 30 min at room temperature with
the secondary antibodies (Santa Cruz Biotechnology, Santa Cruz CA,
USA) diluted in blocking medium. Following rinsing of the slides in
PBST three times, the slides were incubated in the DAB exposure
medium. DAB exposure medium was then washed away and the slides
incubated in hematoxylin for 30 sec. The slides were gradient
dehydrated and mounted.
Immunostaining analysis for PAPP-A
The stained sections were analyzed using Motic Med
6.0 digital medicine image software. For each section, 10 views
were randomly chosen under a microscope (magnification, ×400). The
PAPP-A-positive cells were counted in all 10 views and the
percentage of positive cells in the entire population was
calculated. Four grades were defined to analyze the results:
Negative(−): % of positive cells, <5%; weak positive(+): % of
positive cells, 5–25%; medium positive (++): % of positive cells
with a range of, 5–25%; positive(+++): % of positive cells was
25–50%; strong positive (++++): % of positive cells, >50%.
Quantitative evaluation was achieved using Motic Med 6.0 digital
medicine image software.
Statistical analysis
Data were shown as mean ± SD. For statistical
analyses, the Student’s t-test and the Chi-Square test were used as
appropriate by using SPSS 13.0 software (SPSS Inc., Chicago, IL,
USA). Multivariate non-conditional logistic regression analysis was
used to evaluate the correlation between PAPP-A and RSA. A
difference at P<0.05 was considered statistically
significant.
Results
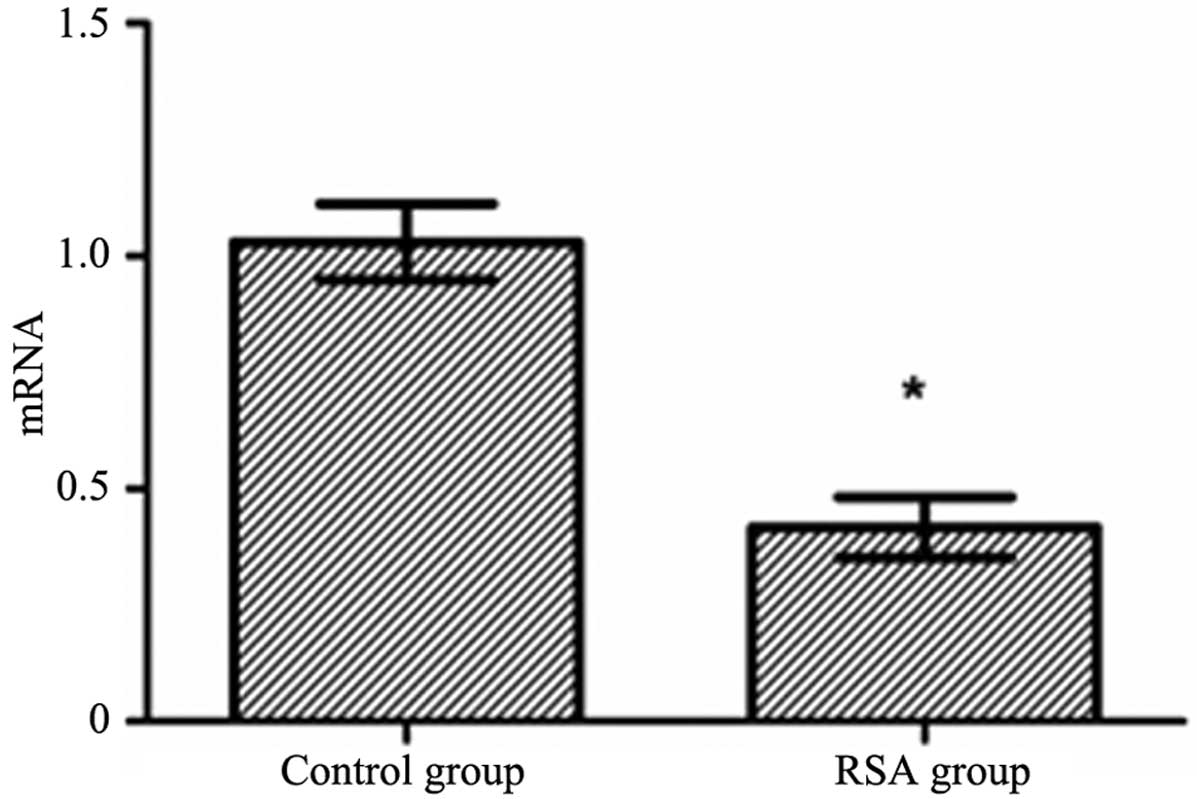
Comparison of the PAPP-A mRNA levels
between the RSA and control groups
The total RNA in decidual tissue was measured using
ultraviolet spectrophotometry. The optical density (OD)260/280
values were in the range of 1.9–2.0. qPCR results demonstrated that
the mRNA levels of PAPP-A in the RSA group were significantly
decreased compared with the control group (t=5.204, P<0.05)
(Fig. 1).
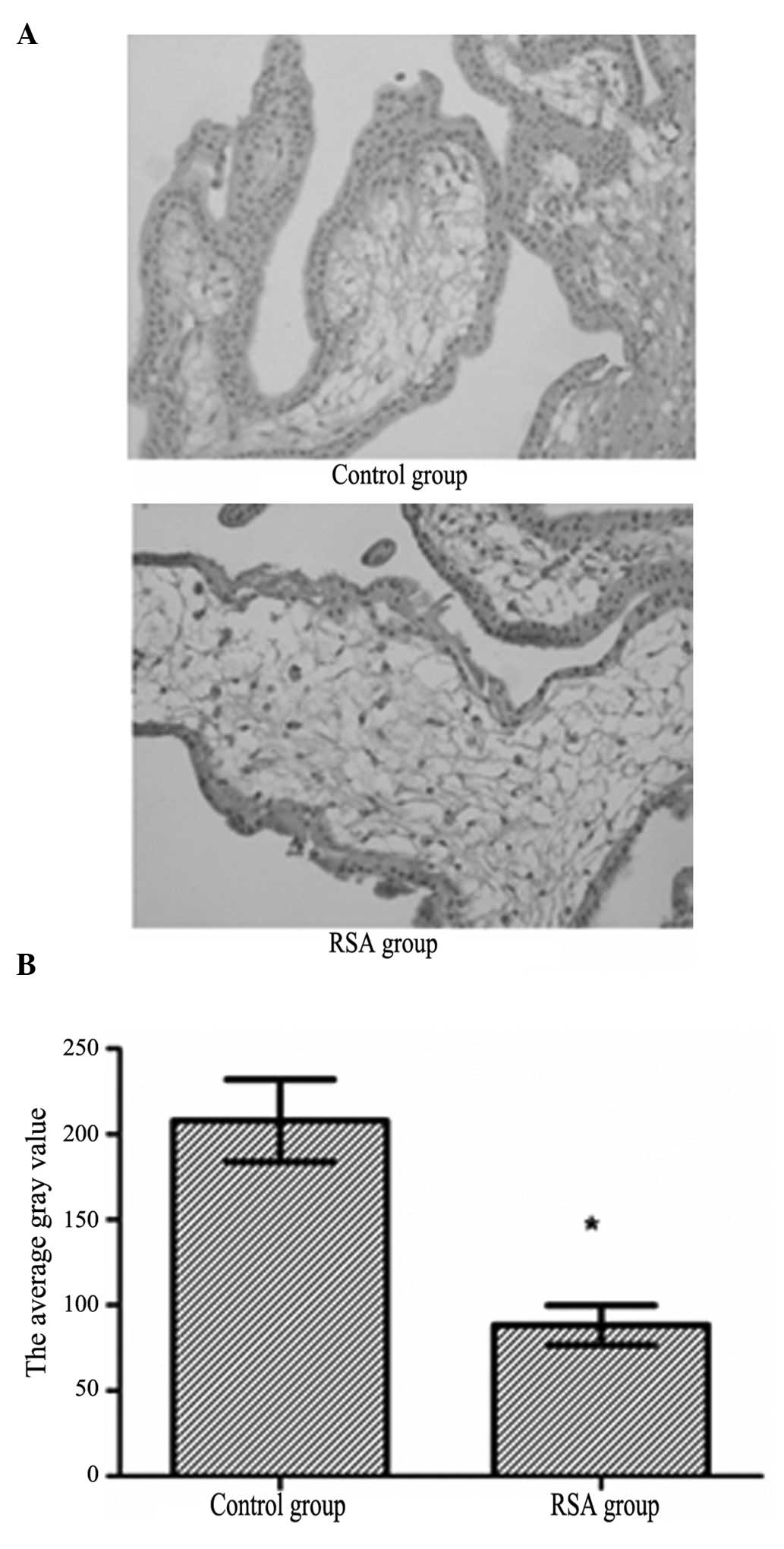
Comparison of the PAPP-A protein levels
between the RSA and control groups
Immunostaining showed that PAPP-A protein was
expressed in the cytoplasm of basal decidual cells (Fig. 2A). The general PAPP-A protein level
of the RSA group was weak positive, whereas the general PAPP-A
protein level of the control group was strong positive (Table I). The quantitative measurement of
PAPP-A protein showed that the protein expression levels were
significantly decreased in the RSA group compared with the control
group (t=4.464, P<0.05) (Fig.
2B).
 | Table I.PAPP-A protein expression level in the
RSA and control groups. |
Table I.
PAPP-A protein expression level in the
RSA and control groups.
| Group | − | + | ++ | +++ |
|---|
| RSA | 2 | 19 | 8 | 1 |
| Control | 0 | 7 | 11 | 21 |
Correlation analysis between the PAPP-A
protein level and RSA
The correlation analysis between the PAPP-A protein
levels and RSA was evaluated using multivariate logistic analysis.
The results showed that the protein expression level of PAPP-A was
highly related to RSA, indicating that PAPP-A is one of the major
risk factors of RSA. The Hosmer-Lemeshow analysis showed that the
protein expression level of PAPP-A is likely a good prognosis
reference for RSA (χ2=3.158, P<0.05).
Discussion
The incidence of RSA is approximately 1% in all
females experiencing pregnancy. Therefore, it has been a hot topic
in clinical and scientific research in recent years (5). Since its complicated pathologic
mechanisms include cytogenetic abnormality (6), endocrine disorder (7), immunodeficiency (8), reproductive disease, infection
(9), trauma, unhealthy behaviors
and environmental factors, the major pathogeny of RSA remains to be
determined (10). Previous studies
have shown that PAPP-A is abnormally expressed in RSA patients
(11,12). In this study, more detailed data
that support the correlation between RSA and PAPP-A levels were
obtained. The results may provide a further useful prognosis method
for this disease.
PAPP-A, which is secreted by placental
syntrophoblastic cells and basal decidual cells, is one of the
plasma glycoproteins involved in pregnancy. PAPP-A has previously
been shown to regulate embryonic development (13,14).
It can first be detected in serum during the fifth week of
pregnancy, and then increases with time subsequently. Peak PAPP-A
expression levels are observed at the end of pregnancy and levels
are then downregulated subsequent to delivery (15,16).
At present, PAPP-A levels are used as one of the major references
for monitoring early pregnancy and evaluating the health of the
fetus. During clinical investigations, a low level of PAPP-A
expression in RSA patients compared to patients experiencing normal
pregnancy has been observed. Similar phenotypes have also been
reported by other groups (17). In
the present study, the specific transcription and expression levels
of PAPP-A were evaluated in an RSA group.
The results have demonstrated that the level of
PAPP-A mRNA in basal decidual tissue was significantly decreased in
the RSA group patients compared with the control group. To obtain
more detailed information on the difference in PAPP-A protein
expression level, immunostaining with PAPP-A antibodies was
performed. In agreement with the qPCR result, the PAPP-A protein
expression levels were also decreased in the RSA group compared
with the control group. Furthermore, results of the correlation and
Hosmer-Lemeshow analyses suggested that the protein levels of
PAPP-A could be used as an important reference in clinical RSA
prognosis. The decrease in PAPP-A levels may also be a critical
risk factor in RSA.
In conclusion, PAPP-A levels were significantly
decreased in RSA patients compared with patients experiencing
normal pregnancy. This abnormal expression may be one of the risk
factors leading to RSA.
References
|
1.
|
Li TC, Makris M, Tomsu M, Tuckerman E and
Laird S: Recurrent spontaneous abortion: aetiology, management and
prognosis. Hum Reprod Update. 8:463–481. 2002. View Article : Google Scholar
|
|
2.
|
Ribas-Maynou J, García-Peiró A,
Fernandez-Encinas A, et al: Double stranded sperm DNA breaks,
measured by Comet assay, are associated with unexplained recurrent
miscarriage in couples without a female factor. PLoS One.
7:e446792012. View Article : Google Scholar
|
|
3.
|
Wu Z, You Z, Zhang C, Li Z, Su X, Zhang X
and Li Y: Association between functional polymorphisms of Foxp3
gene and the occurrence of unexplained recurrent spontaneous
abortion in a Chinese Han population. Clin Dev Immunol.
2012:8964582012.PubMed/NCBI
|
|
4.
|
Shen XN, Tang SH, Yang LW, Li YY and Mao
YJ: Diagnostic value of pregnancy - associated plasma protein A and
free β-hCG in the forecast of missed abortion and ectopic
pregnancy. Chin J Fam Plann. 17:107–108. 2009.(In Chinese).
|
|
5.
|
Sugiura-Ogasawara M, Ozaki Y, Katano K,
Suzumori N, Kitaori T and Mizutani E: Abnormal embryonic karyotype
is the most frequent cause of recurrent spontaneous abortion. Hum
Reprod. 27:2297–2303. 2012.PubMed/NCBI
|
|
6.
|
El-Dahtory FA: Chromosomal abnormalities
as a cause of recurrent abortions in Egypt. Indian J Hum Genet.
17:82–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Todorova-Ananieva K: Autoimmune thyroid
disorders and reproductive failures. Akush Ginekol (Sofiia).
48(Suppl 2): S26–S30. 2009.(In Bulgarian).s.
|
|
8.
|
Nakashima A, Shima T, Inada K, Ito M and
Saito S: The balance of the immune system between T cells and NK
cells in miscarriage. Am J Reprod Immunol. 67:304–310. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nigro G, Mazzocco M, Mattia E, Di Renzo
GC, Carta G and Anceschi MM: Role of the infections in recurrent
spontaneous abortion. J Matern Fetal Neonatal Med. 24:983–939.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fatima N, Ahmed SH, Salhan S, Rehman SM,
Kaur J, Owais M and Chauhan SS: Study of methyl transferase (G9aMT)
and methylated histone (H3-K9) expressions in unexplained recurrent
spontaneous abortion (URSA) and normal early pregnancy. Mol Hum
Reprod. 17:693–701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Conover CA: Key questions and answers
about pregnancy-associated plasma protein-A. Trends Endocrinol
Metab. 23:242–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhong Y, Bradshaw R, Stanley AP and Odibo
AO: The impact of assisted reproductive technology on the
association between first-trimester pregnancy-associated plasma
protein a and human chorionic gonadotropin and adverse pregnancy
outcomes. Am J Perinatol. 28:347–354. 2011. View Article : Google Scholar
|
|
13.
|
Wen J, Ren HY and Zhai JJ: The diagnostic
of combination measurement of pregnancy-associated plasma protein A
and progesterone in diagnosis of early pregnancy. J Cap Univ Med
Sci. 28:649–651. 2007.
|
|
14.
|
Tarim E, Cok T, Hacivelioglu S and Bagis
T: Low molecular weight heparin and first trimester maternal PAPP-A
and hCG levels, fetal nuchal translucency in the first trimester of
pregnancy. Clin Exp Obstet Gynecol. 38:81–83. 2011.PubMed/NCBI
|
|
15.
|
Wang J, Qiu Q, Haider M, Bell M, Gruslin A
and Christians JK: Expression of pregnancy-associated plasma
protein A2 during pregnancy in human and mouse. J Endocrinol.
202:337–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tong S, Ngian GL, Onwude JL, et al:
Diagnostic accuracy of maternal serum macrophage inhibitory
cytokine-1 and pregnancy-associated plasma protein-A at 6-10 weeks
of gestation to predict miscarriage. Obstet Gynecol. 119:1000–1008.
2012. View Article : Google Scholar
|
|
17.
|
Suzuki K, Sata F, Yamada H, Saijo Y,
Tsuruga N, Minakami H and Kishi R: Pregnancy-associated plasma
protein-A polymorphism and the risk of recurrent pregnancy loss. J
Reprod Immunol. 70:99–108. 2006. View Article : Google Scholar : PubMed/NCBI
|