Introduction
Animal models mimicking human diseases are important
tools, and provide an understanding of the underlying mechanisms of
many diseases. A number of surgical models that mimic human
myocardial infarction (MI) have been described in recent years.
However, there are very few detailed descriptions regarding the
surgical conditions used when performing these surgical techniques
in mice (1). At present, animals
that are frequently being used to establish MI models include rats,
rabbits, dogs, sheep and swine (2–7).
Expense and a lack of inbred varieties limit the use of these
larger animals for application in the MI models. However, the use
of mice for MI models has been widely explored in recent years, due
to their many advantages, such as small size, low cost and the
availability of many inbred varieties. Furthermore, mice exhibit
numerous similarities to human beings, including genetic,
physiological, biochemical and developmental characteristics.
Moreover, there are similarities in the structure of the food
consumed by mice and humans (1,8,9).
However, establishing a successful MI model in mice presents many
challenges, since the size of the mice results in a small surgical
field. This creates difficulties in exposing the left anterior
descending artery, as well as in the ligation of the artery. It has
been suggested that these disadvantages, in addition to the lack of
standard operating procedures (SOP), may be the main reasons why
mouse MI models have not been widely used in human MI research
(1,10–14).
In this study, we have described suitable,
cost-effective and detailed surgical conditions for coronary artery
ligation (CAL), which may be used to establish an MI model in mice.
These conditions have been based on previous studies, in
combination with the experience of the authors in preparing an MI
model in mice.
Materials and methods
Materials
C57BL/6 male mice were purchased from the Shanghai
Silaike Experimental Animal Company, Ltd. (license no. SCXK,
Shanghai 2007-0005; Shanghai, China). An XTL continuous zoom
stereomicroscope (Shenzhen Ruiwode Life Technology Company, Ltd.,
Shenzhen, China) and a Microvent 1 small animal ventilator
(Hallowell Engineering and Manufacturing Corp., Pittsfield, MA,
USA) were used in the microsurgical procedure, while a Hitachi
7600-110 autoanalyzer (Hitachi, Tokyo, Japan) was used for the
biochemical analyses. Chloral hydrate and isoflurane were obtained
from Sun Chemical Technology (Shanghai, China). The microsurgical
instruments, endotracheal intubations and disposable intravenous
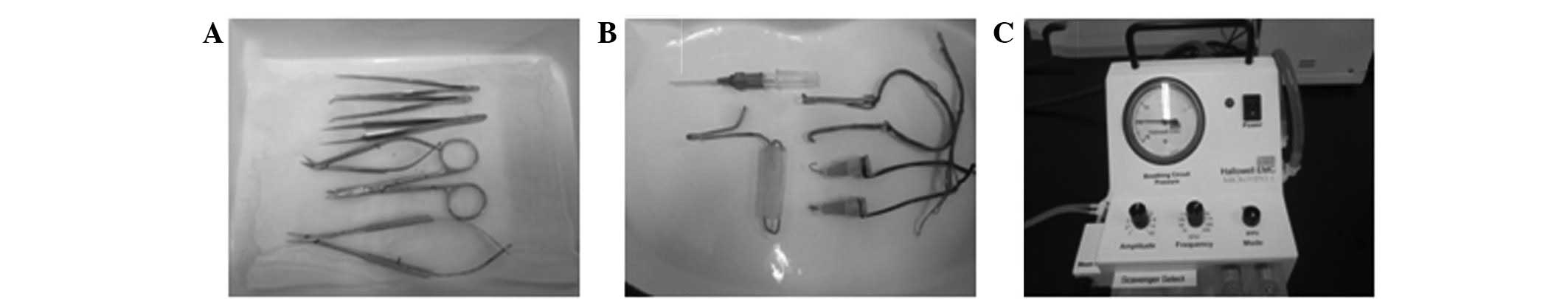
catheter (22 G) used in this study are shown in Fig. 1A and B).
Methods
Establishment of the MI model
A total of 30 healthy adult C57BL/6 male mice were
randomly divided into two groups: control (n=10, sham group) and
experimental (n=20, ligation group). Mice were housed in conditions
with a controlled temperature and humidity, a 12-h light-dark cycle
and free access to chow and water. The mouse experiments were
approved by the Ningbo University Institutional Animal Care and Use
Committee (Ningbo, China).
Anesthesia. Prior to the surgery, the mice
were accurately weighed, and then anesthetized by intraperitoneal
injection with 7% chloral hydrate (250 mg/kg body weight).
Endotracheal intubation. Each anesthetized
mouse was placed on a flat foam board on the surgical platform and
fixed with disposable tape. The mouth of the mouse was opened with
a self-prepared mouth opener (Fig.
1B) and then the glottis was located with the aid of a cold
light source under the stereomicroscope. An intravenous indwelling
needle was inserted into the trachea along the glottis and was
connected to the ventilator (Fig.
1C) with a respiratory rate of 130–140 beats/min (tidal volume
of 4–5 ml). The respiratory parameters were marginally adjusted
during the surgery, according to the conditions of the anesthetized
animals.
Surgical position and procedure. The mouse
was placed on the surgical platform in a right lateral position. A
warm soapy water solution was used to wet the left breast fur,
prior to the fur in the surgical area being scraped with a scalpel.
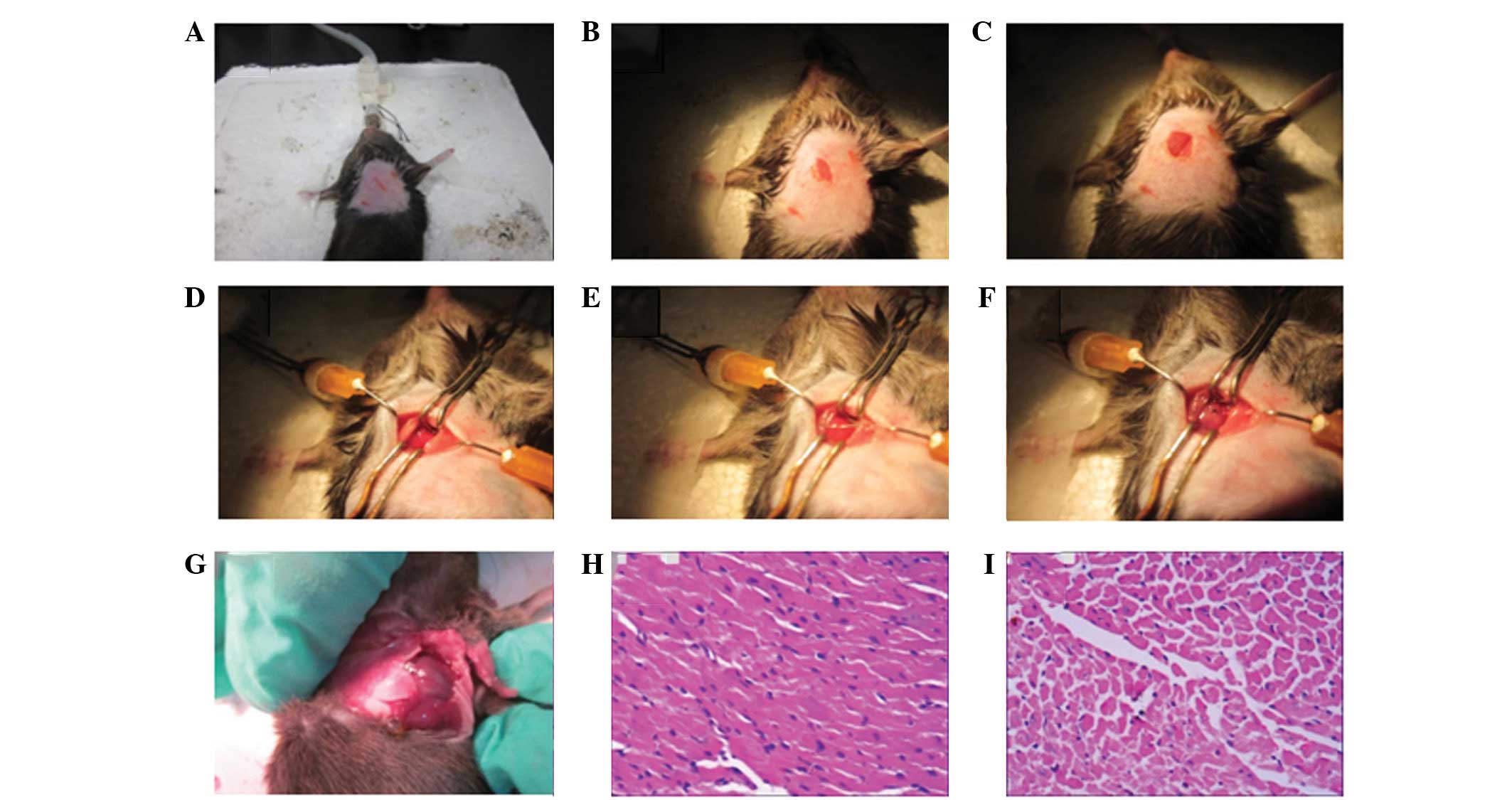
The skin was then disinfected with 75% alcohol (Fig. 2A). Following cutting an incision
(∼1 cm long) in the skin in an obliquely upward direction at the
midpoint on the connection line between the lower edge of the
sternum and the left armpit, a subcutaneous vein in the
longitudinal direction was exposed (Fig. 2B). Blunt separation of the muscles,
layer by layer, was performed with small curved forceps from the
medial side of the vein along the venous direction (Fig. 2C). A self-prepared small retractor
was used to fix the muscle along the left-right direction. Curved
surgical scissors were used to cut an incision in the muscle (<1
cm) at 4–5 intercostals. Following this, self-prepared retractors
were used to pull and open the incision up or down in a vertical
direction, respectively. The retractors were fixed on the foam on
the surgical platform, which left the heart fully exposed. The
pericardium was then carefully lifted, separated and fixed with a
retractor, prior to the retractor also being fixed (Fig. 2D).
Coronary artery ligation: Locating the coronary
artery. The heart was initially exposed by pushing the right
side of the chest gently with small curved forceps. A pink coronary
artery (the left anterior descending coronary artery) was located
along the edge of the left atrial appendage (Fig. 2D). In cases when the myocardial
surgery took longer than expected, the coronary artery became
difficult to locate. Lifting the myocardium by the suture helped
expose the branch of the left coronary artery, located between the
pulmonary artery cone and the left atrial appendage.
Coronary artery ligation. Holding a needle
(7/0 G) in the right hand and using the left hand, the beating
heart was stabilized with tweezers by pushing against the right
ventricle. The coronary artery and a small area of the surface
layer of the myocardium were then ligated with a 7/0 no-damage silk
suture (Fig. 2E). To prevent the
knots from coming untied, the coronary artery was wrapped twice
with the silk suture before the first knot was tied. If the color
of the local area surrounding the ligation and the myocardium
downstream of the ligation simultaneously became gray, in addition
to the movement of the corresponding myocardial region becoming
minimized (Fig. 2F), the ligation
was a success. These were the early signs for validating the
success of the CAL procedure.
In the experimental mice, the left anterior
descending coronary artery was ligated with a fast knot. In the
control mice, all surgical procedures for the ligation were the
same as for the experimental mice, except a slipknot was used
instead of the fast knot.
Thoracic cavity closure. Following ligation,
the self-prepared retractors were released. The heart was covered
with the pericardium, and the ribs were sutured with number 5 nylon
sutures. The intercostals were fully closed, in order to discharge
the air from the chest. During suturing, care was taken not to
damage the myocardium or the left lung. Following this, two layers
of muscle were pulled together with the skin and sutured with
number 5 nylon sutures. Subsequent to the removal of the breathing
machine, tweezers were used to stimulate the foot of the mouse to
promote spontaneous breathing. Five to ten minutes later, the
endotracheal intubation was extracted, following the return of
spontaneous breathing.
Postoperative care
Following the completion of the surgery, the mice
were placed on a warm platform (32–35°C). When consciousness was
completely regained, the mice were transferred into a cage to
resume normal activity.
Biochemical and histopathological
analyses
On the seventh day subsequent to surgery, the mice
were weighed and then anesthetized with inhaled 2% isoflurane.
Following anesthesia, blood samples were collected via the femoral
artery. Serum was obtained by centrifugation at 1,150 × g for 10
min and stored at −80°C until use. The heart tissues were obtained
as rapidly as possible and were rinsed in saline. Following
weighing, the heart tissues were fixed in a 10% formalin solution
for 24 h, embedded in paraffin and sliced. The tissue sections were
stained with hematoxylin and eosin (HE) staining and observed under
the microscope. The activities of aspartate aminotransferase (AST),
creatine kinase (CK) and lactate dehydrogenase (LDH) in the serum
were detected using the Hitachi 7600-110 autoanalyzer.
Statistical methods
Data are expressed as the mean ± standard deviation
(SD). SPSS statistical software version 13.0 (SPSS, Inc., Chicago,
IL, USA) and the Student’s t-test were used for the data analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Animal survival rate following
surgery
The CAL procedure was performed on 30 mice,
including 10 in the control group and 20 in the experimental group.
Of these, five mice in the control and experimental groups,
respectively, were administered 350 mg/kg body weight of 7% chloral
hydrate for anesthesia. Three deaths occurred in each group due to
anesthetic overdose. To all other animals, 250 mg/kg body weight of
7% chloral hydrate was administered. The five mice in the control
group that were anesthetized with 250 mg/kg body weight of 7%
chloral hydrate survived the procedure, while among the 15 mice
anesthetized with 250 mg/kg body weight of 7% chloral hydrate in
the experimental group, three deaths occurred 2–3 days subsequent
to the procedure. Autopsies revealed one case with pleural effusion
and two cases with cardiac rupture. The total 7-day survival rate
following the surgery was 70% (seven and 14 cases in the control
and experimental groups, respectively).
Change in animal body weight following
surgery
The average preoperative weight of the mice was
29.00±3.23 g and seven days subsequent to the surgery it was
24.78±2.39 g (t=4.45, P<0.0001). This showed that the surgical
trauma decreased the weight of mice significantly. Further analysis
revealed no significant difference in the body weight change
between the experimental and control groups.
MI occurrence
Evidence of MI occurrence was observed under the
naked-eye (Fig. 2G) and under the
microscope (Fig. 2H) in all the
experimental mice. No MI was observed in the control group
(Fig. 2I).
The heart weight/body weight coefficients of the
control and experimental groups were (5.15±0.80)×10−3
and (6.52±1.13)×10−3, respectively, which indicated that
the CAL procedure resulted in a significant enlargement of the
heart (t=−2.25, P=0.0385).
Activities of AST, CK and LDH in the
serum
The activities of AST, CK and LDH in the serum are
shown in Table I. The activity
levels in the serum of the ligated mice were higher than those in
the control mice, which may have been a result of the release of
AST, CK and LDH enzymes into the blood stream following myocardial
necrosis.
 | Table I.Activities of AST, CK and LDH in the
serum. |
Table I.
Activities of AST, CK and LDH in the
serum.
| Detection
indicators | Group | No. of animals | Value (U/l,
10−3) | P-value |
|---|
| AST | Control | 7 | 56.70±14.19 | 0.048a |
| Experimental | 14 | 89.32±40.04 |
| LDH | Control | 7 | 293.33±64.66 | 0.024a |
| Experimental | 14 | 470.73±185.92 |
| CK | Control | 7 | 45.33±23.09 | 0.027a |
| Experimental | 14 | 98.23±41.44 |
Discussion
Previous studies have demonstrated that simultaneous
increases in AST, CK and LDH are frequently observed following MI
(15,16). The present results showed that the
serum AST, CK and LDH concentrations in the experimental mice were
significantly increased compared with those in the control mice.
These results indicated the onset of myocardial necrosis in the
ligated mice. Using histopathological analysis, it was observed
that different degrees of MI occurred in the heart tissues of the
experimental mice, and the heart weight/body weight coefficient of
the experimental mice was significantly higher than that of the
control mice. This demonstrated that the MI model in mice was
successfully established by CAL under the selected surgical
conditions.
The methods for preparing an animal MI model include
cryoinfarction (17–19), low amperage electrical injury
(20) and CAL (9,21).
Although the electrical injury and cryo-methods are simple to
perform and result in relatively light damage and a constant
infarct area, their clinical relevance is poor. In comparison with
the electrical injury and the cryo-methods, the CAL method is more
relevant to the pathological process of MI in the clinic. However,
certain characteristics of mice, including their small size and low
ability to resist trauma, as well as the difficulties encountered
in locating the anterior descending artery and the frequent
surgical complications, such as pneumothorax (22), limit the use of mice for the MI
model. In this study, based on previous studies (11) and the experience of the authors
(9), the aim was to optimize the
surgical conditions for CAL, in order to establish an MI model in
mice. The following describes the procedures used during the CAL
surgery and discusses the rationale behind the selection of these
procedures in the establishment of a simple, fast and successful MI
model in mice.
There were several factors to consider when
selecting the anesthetic agents and doses. A variety of anesthetic
agents have been used in the production of an MI model in mice,
including sodium pentobarbital (23), ketamine (24), chloral hydrate (25) and urethane (26). In China, sodium pentobarbital and
ketamine are very expensive and complicated procedures are required
in order to obtain approval prior to purchase. Chloral hydrate and
urethane may be easily purchased and are cheaper than sodium
pentobarbital and ketamine; as a result, they are widely used for
experiments in China. In preliminary experiments, chloral hydrate
and urethane were used to assess their suitability as anesthetics
for the procedure. At 1,000–1,250 mg/kg body weight, urethane led
to a very long duration of anesthesia (more than two hours).
Following this, chloral hydrate was used to anesthetize the mice
instead, which led to a fast and smooth anesthesia; however, the
use of chloral hydrate also caused oral secretions in the animals.
Following further studies, it was revealed that it was possible to
gradually alleviate the oral secretions by reducing the anesthetic
dosage. At 250 mg/kg body weight, chloral hydrate showed the
optimum anesthetic results, with an anesthesia duration of 1.5–2.0
h. This fully met the surgical requirements.
In our previous experiments, sodium pentobarbital
was used for the anesthesia of the mice (9). Sodium pentobarbital was more suitable
for anesthesia when considering the respiratory secretion problems
and spontaneous breathing recovery time. However, it was
demonstrated that with the proper dosage of chloral hydrate, it was
possible to eliminate the respiratory secretions. Furthermore, it
was possible to minimize the recovery time for spontaneous
breathing with the correct chest closure tightness. For these
reasons, as well as the cost, chloral hydrate was selected over
sodium pentobarbital for use as the anesthetic.
Of note is the fact that it was necessary to
accurately weigh the mice prior to anesthesia and to anesthetize
the mice with accurate doses. Moreover, it was desirable to
administer the anesthetic drug as a single injection, since it was
demonstrated that a second injection often resulted in
unsatisfactory anesthetic effects. In addition, the site of
intraperitoneal injection was very important. Our experience
demonstrated that it was desirable to perform the anesthetic
injection of chloral hydrate with a 1 ml syringe needle (26 G),
piercing ∼0.5 cm into the abdominal cavity (no visible blood on the
withdrawal of the needle) on the side at the midpoint of the line
between the external genitalia and xiphoid. This procedure
anesthetized the mice with an anesthesia induction time of 1–5 min
and provided the best results for the surgery.
With regard to the securing of the mice and the
intubation methods, the mice were placed in a right lateral
position on the surgical platform and the lower limbs of the mice
were tightly fixed. The upper limbs were loosely fixed, which
alleviated muscle tension as much as possible, in order to reduce
any influence on breathing.
Ventilator-assisted breathing by intubation through
the mouth is a common method used during open-heart surgery when
preparing an MI model in animals, since this may aid in avoiding
trauma and complications, such as postoperative airway constriction
caused by tracheotomy surgery, and therefore improves the quality
of life of the animals. It is worth noting that intubation results
in unavoidable stimulation of the larynx and airway and may damage
the trachea. Occasionally, intubations may stray into the
esophagus. Therefore, full exposure of the mouse glottis and a
prompt, precise and delicate technique by the surgeon are all
important factors for reducing respiratory complications caused by
intubations, and for enhancing the survival rate of the MI model.
This study demonstrated that a 22-G venous indwelling needle was
suitable for tracheal intubations in mice.
With regard to the anatomical site of the surgery,
the mouse was placed on the surgical platform in a right lateral
position and an incision was performed along the ribs. Following
the principle of blunt dissection, the dissection of the muscle was
performed layer by layer. When opening the intercostal muscle to
enter the chest, special attention was taken to prevent damage to
the thoracic blood vessels and the heart and lungs, which may have
resulted in the death of the mice. The method used in the present
study did not shear the pectoralis major muscle or the ribs, which
ensured that the normal anatomical structures of the chest were
retained. This was beneficial for the survival of the mouse
subsequent to the surgery.
Mice are very small in size and have a limited
pleural area, which inevitably results in a narrow surgical field
and an insufficient exposure of the surgical site. At present,
there is no commercial equipment available in the Chinese domestic
market for the rib distraction of mice. To resolve this, a
self-prepared rib-retractor, made with a paperclip, was used, which
was demonstrated to aid in exposing the surgical field during the
experiments.
The surface of the mouse heart is covered by the
pericardium and the thymus. The thymus, as a lymphoid organ, is
particularly important for the body’s immune function. To preserve
the integrity of the immune system of the mouse, the pericardium
was separated and the thymus was subsequently pulled to the left
side and carefully attached to a surgical retractor. Following the
CAL, the thymus was placed back in its original position. In this
manner, the thymus was preserved effectively. In addition, this
method fully protected the left lung during the surgery.
There were numerous factors to be considered with
regard to the recognition of the coronary artery and the ligation
method. The blood circulation of the left ventricular myocardium is
predominantly supplied by the collateral of the left anterior
descending coronary. Therefore, left anterior descending coronary
ligation may result in a large area of infarction in the left
ventricular myocardium. The anterior descending coronary artery in
the shallow myocardium is small and may be difficult to distinguish
from the heart surface veins. These factors may all result in
ligation failure. Therefore, the identification of the anterior
descending coronary artery is a key point for a successful MI
operation. The left atrial appendage is an important anatomical
marker for locating the anterior descending coronary artery. With
regard to the difference in the distribution direction, there are
two typical distributions of the anterior descending coronary
artery: The first distribution starts from the center of the left
atrial appendage, while the second starts from between the center
of the left atrial appendage and the pulmonary cone. It was
observed that in the C57BL/6 male mice, the first distribution was
more common than the second, and, therefore, the anterior
descending coronary artery was most often accurately found by
starting from the center and following the edge of the left atrial
appendage, in the direction of the pulmonary artery cone. However,
exceptions may occur in certain mice due to variations in the
distribution directions of the coronary artery.
The light source of the stereomicroscope was
important in the location of the anterior descending coronary
artery. Under ordinary light, particularly under white light, it is
difficult to distinguish between myocardium, arteries and veins.
However, under a high-brightness yellow cold light source, the
myocardium, arteries and veins appear bright red, pink and dark
red, respectively. These three different colors were easily
distinguished by the surgeons. The anterior descending coronary
artery travels under the shallow myocardium. Prior to ligation,
only one-third of it may be visible, with the remaining two-thirds
lying beneath the myocardium. This remaining section was only
observed when the anterior descending coronary artery was slightly
lifted with a suture needle.
Attention is required to limit the puncture depth of
the needle. The present study demonstrated that one-third to
one-half of the ventricular wall penetration was a suitable depth.
Ligating an appropriately sized area of myocardium and tying it
securely was important, in order to prevent the tearing of the
myocardium. Following the ligation of the anterior descending
coronary artery, the color around the ligation point of the
myocardium rapidly turned white, indicating successful ligation.
However, only pathological detection was able to serve as the final
and gold standard for a successful MI ligation.
An additional factor to be considered was the
prevention, identification and treatment of pneumothorax.
Pneumothorax is a common complication in the modeling process and
may frequently result in the death of the mice following the
surgical wound closure. The tolerance of mice to pneumothorax is
poor, due to their small pleura. It was demonstrated that
incomplete air exhaustion prior to chest closure was the primary
reason for the pneumothorax. By increasing the tidal volume to
expand the lung lobe and performing an appropriate repeated
chest-squeezing to drain the residual air in the thoracic cavity,
the incidence of the pneumothorax may be significantly reduced. A
failure to tightly close the chest cavity following surgery may
lead to air exhaustion failure. Therefore, during muscle separation
in the thoracotomy, it was necessary to focus on reducing muscle
injury, in order to enhance postoperative chest tightness.
Following chest closure, the majority of the mice were able to
restore spontaneous breathing without prolonged mechanical
ventilation. However, there were a few mice that developed the
symptoms of pneumothorax. Therefore, it was considered desirable
for the endotracheal intubations to remain for 5–10 min following
chest closure, in order to prevent the occurrence of
pneumothorax.
With regard to the administration of antibiotics
following surgery, certain studies of MI models have (11) suggested that an intramuscular
injection of penicillin be used to prevent postoperative infection.
It was demonstrated in the present study that surgical instrument
sterilization and disinfection with 75% alcohol during the
procedure was a suitable method of preventing infection.
Maintaining the warmth of the animals during and
following the surgery was crucial, as a complete procedure to
establish an MI model normally lasted 1–2 h. This was due to the
fact that while the surgery itself may only take 20–30 min, the
mice required 1–2 h to wake following the surgery, due to the
anesthesia.
Following the surgery, the recovering anesthetized
mice were placed in a cage. The cage was then placed on a warm
thermostatic plate (30–35°C) until the animals regained
consciousness.
In conclusion, the results of the study showed that
the mouse MI model prepared by ligation of the anterior descending
coronary artery under the selected surgical conditions was
reliable, cost effective and successful. The procedure has been
described in detail to enable easy and effective replication, even
in less well-equipped labs. Further studies are required to
simplify, optimize and standardize the surgical conditions for
preparing a mouse MI model to facilitate the wider use of the mouse
MI model in MI research.
Acknowledgements
The authors would like to thank Mrs.
Linda Bowman for her assistance in the preparation of this
manuscript. This study was partly supported by the National Nature
Science Foundation of China (grant no. 81273111), the Foundations
of Innovative Research Team of Educational Commission of Zhejiang
Province (grant no. T200907), the Nature Science Foundation of
Ningbo city (grant no. 2012A610185), the Ningbo Scientific Project
(grant no. SZX11073), the Scientific Innovation Team Project of
Ningbo (grant no. 2011B82014), the Innovative Research Team of
Ningbo (grant no. 2009B21002) and the K.C. Wong Magna Fund of
Ningbo University.
References
|
1.
|
Tarnavski O, McMullen JR, Schinke M, Nie
Q, Kong S and Izumo S: Mouse cardiac surgery: comprehensive
techniques for the generation of mouse models of human diseases and
their application for genomic studies. Physiol Genomics.
16:349–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cameron VA, Rademaker MT, Ellmers LJ,
Espiner EA, Nicholls MG and Richards AM: Atrial (ANP) and brain
natriuretic peptide (BNP) expression after myocardial infarction in
sheep: ANP is synthesized by fibroblasts infiltrating the infarct.
Endocrinology. 141:4690–4697. 2000.
|
|
3.
|
Clark C, Foreman MI, Kane KA, McDonald FM
and Parratt JR: Coronary artery ligation in anesthetized rats as a
method for the production of experimental dysrhythmias and for the
determination of infarct size. J Pharmacol Methods. 3:357–368.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Deten A, Hölzl A, Leicht M, Barth W and
Zimmer HG: Changes in extracellular matrix and in transforming
growth factor beta isoforms after coronary artery ligation in rats.
J Mol Cell Cardiol. 33:1191–1207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mahaffey KW, Raya TE, Pennock GD, Morkin E
and Goldman S: Left ventricular performance and remodeling in
rabbits after myocardial infarction: effects of a thyroid hormone
analogue. Circulation. 91:794–801. 1995. View Article : Google Scholar
|
|
6.
|
Takano H, Qin Y, Hasegawa H, et al:
Effects of G-CSF on left ventricular remodeling and heart failure
after acute myocardial infarction. J Mol Med (Berl). 84:185–193.
2006.PubMed/NCBI
|
|
7.
|
Zhou S, Chen LS, Miyauchi Y, et al:
Mechanisms of cardiac nerve sprouting after myocardial infarction
in dogs. Circ Res. 95:76–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kumar D, Hacker TA, Buck J, et al:
Distinct mouse coronary anatomy and myocardial infarction
consequent to ligation. Coron Artery Dis. 16:41–44. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
McGaffin KR, Zou B, McTiernan CF and
O’Donnell CP: Leptin attenuates cardiac apoptosis after chronic
ischaemic injury. Cardiovasc Res. 83:313–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Huang Y, Ma Y, Yang Y, et al: Effect of
nuclear factor-κB inhibitor pyrrolidine dithiocarbamate on left
ventricular remodeling after acute myocardial infarction in old
mice. Chinese Circulation Journal. 26:378–381. 2011.(In
Chinese).
|
|
11.
|
Liu B, Du X and Zhou Y: Establishment of
myocardial infarction mode in young mice of low body weight.
Zhonghua Shi Yan Wai Ke Za Zhi. 29:132–134. 2012.(In Chinese).
|
|
12.
|
Nie L, Li QG, Fan FD and Wang DJ:
Establishment of mouse cardiac patch model. Zhonghua Shi Yan Wai Ke
Za Zhi. 27:1703–1704. 2010.(In Chinese).
|
|
13.
|
Yun W, Yu Y, Lu X, et al: Discussion of
establishing mice model with myocardial infarction successfully.
Journal Of China Medical University. 36:631–633. 2007.(In
Chinese).
|
|
14.
|
Zhao X, Zhou J, Ma R, et al: Study of
establishing myocardial infarction model of mice. Sichuan Dong Wu.
25:627–629. 2006.(In Chinese).
|
|
15.
|
Seta Y, Kanda T, Tanaka T, et al:
Interleukin 18 in acute myocardial infarction. Heart. 84:668–669.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Suchalatha S and Shyamala Devi C: Effect
of arogh - A polyherbal formulation on the marker enzymes in
isoproterenol induced myocardial injury. Indian J Clin Biochem.
19:184–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
van Laake LW, Passier R, Monshouwer-Kloots
J, et al: Monitoring of cell therapy and assessment of cardiac
function using magnetic resonance imaging in a mouse model of
myocardial infarction. Nat Protoc. 2:2551–2567. 2007.PubMed/NCBI
|
|
18.
|
Wang H, Zhou J, Liu Z and Wang C:
Injectable cardiac tissue engineering for the treatment of
myocardial infarction. J Cell Mol Med. 14:1044–1055.
2010.PubMed/NCBI
|
|
19.
|
Kolk MVV, Meyberg D, Deuse T, et al:
LAD-ligation: a murine model of myocardial infarction. J Vis Exp.
14382009.PubMed/NCBI
|
|
20.
|
Romson JL, Haack DW and Lucchesi BR:
Electrical induction of coronary artery thrombosis in the
ambulatory canine: a model for in vivo evaluation of
anti-thrombotic agents. Thromb Res. 17:841–853. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
McGaffin KR, Sun CK, Rager JJ, et al:
Leptin signalling reduces the severity of cardiac dysfunction and
remodelling after chronic ischaemic injury. Cardiovasc Res.
77:54–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wang J, Bo H, Meng X, Wu Y, Bao Y and Li
Y: A simple and fast experimental model of myocardial infarction in
the mouse. Tex Heart Inst J. 33:290–293. 2006.PubMed/NCBI
|
|
23.
|
Feng Q, Lu X, Jones DL, Shen J and Arnold
JM: Increased inducible nitric oxide synthase expression
contributes to myocardial dysfunction and higher mortality after
myocardial infarction in mice. Circulation. 104:700–704. 2001.
|
|
24.
|
Creemers EE, Davis JN, Parkhurst AM, et
al: Deficiency of TIMP-1 exacerbates LV remodeling after myocardial
infarction in mice. Am J Physiol Heart Circ Physiol. 284:H364–H371.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Orlic D, Kajstura J, Chimenti S, Bodine
DM, Leri A and Anversa P: Bone marrow stem cells regenerate
infarcted myocardium. Pediatr Transplant. 7(Suppl 3): S86–S88.
2003. View Article : Google Scholar
|
|
26.
|
Janssens S, Pokreisz P, Schoonjans L, et
al: Cardiomyocyte-specific overexpression of nitric oxide synthase
3 improves left ventricular performance and reduces compensatory
hypertrophy after myocardial infarction. Circ Res. 94:1256–1262.
2004. View Article : Google Scholar
|