Introduction
There is increasing evidence that the endothelium
plays a central and pathogenic role in sepsis. When exposed to
certain agonists, including lipopolysaccharide (LPS), endothelial
cells become activated. The activation state is manifested by
enhanced permeability, increased leukocyte adhesion, a shift in the
hemostatic balance towards a procoagulant phenotype and altered
regulation of vasomotor tone (1).
Hyperglycemia, be it secondary to diabetes, impaired glucose
tolerance or impaired fasting glucose, or stress-induced, is common
in individuals with critical illnesses, including sepsis. Thus, the
concurrent existence of hyperglycemia and endotoxemia is common.
Chronic hyperglycemia (diabetes mellitus) is itself known to
activate the endothelium and individuals with this condition have a
higher rate of mortality from sepsis compared with their
non-diabetic septic counterparts. Chronic diabetes causes
endothelial systems to have higher sensitivity to septicemia
(2–3). For instance, diabetes is associated
with exacerbated LPS-induced immune and hemostatic responses. The
increased glucose concentration in diabetes mellitus is associated
with enhanced LPS-stimulated transcriptional activator protein
(AP)-1 and nuclear factor-κB (NF-κB) activity, which are important
in the transcriptional activation of genes involved in inflammation
(4). In addition, the exposure of
mononuclear cells to high glucose (HG) augments the LPS-stimulated
expression of matrix metalloproteinase (MMP) (5) and pro-inflammatory cytokines,
including tumor necrosis factor (TNF)-α and interleukin (IL)-8
(6–8). HG and LPS levels also increase the
generation of reactive oxygen species by peritoneal macrophages
(9). Stegenga et al
demonstrated that hyperglycemia enhances coagulation whereas
hyperinsulinemia inhibits fibrinolysis during human endotoxemia
(10). It remains unclear,
however, whether brief hyperglycemic episodes alter the function of
vascular endothelial cells in response to endotoxins. We
hypothesize that brief hyperglycemic episodes enhance the
permeability of microvascular endothelial cells induced by LPS
in vitro.
The endothelium constitutes the inner lining of
blood vessels and regulates the exchange of fluids, macromolecules
and leukocytes between blood and interstitial tissues. Precise
control of the endothelial barrier function strongly depends on
endothelial nitric oxide (NO) production, since inhibition of NO
production and excessive amounts of NO induce vascular leakage
(11). Basal NO levels are
necessary for vasodilation, platelet aggregation and the modulation
of inflammatory cell adhesion to the endothelium (12–14).
The effects of NO on the cardiovascular system depend on the amount
of NO produced, the local environment and the redox state of NO.
While low NO levels are necessary for endothelial integrity,
excessive NO is pathogenic, compromising barrier function (15). NO is produced by three different NO
synthase (NOS) isoforms: neuronal (nNOS), endothelial (eNOS) and
inducible NOS (iNOS). NOS activity is regulated by endogenous
inhibitors, including asymmetric dimethylarginine (ADMA), which is
metabolized by dimethylarginine dimethylaminohydrolase (DDAH). Two
distinct DDAH isoforms have been described; DDAH-1 is typically
located in tissues expressing nNOS, whereas DDAH-2 predominates in
tissues containing eNOS (16).
Since previous data demonstrated that endothelial dysfunction may
be related to reduced activity of DDAH (17), we hypothesize that there is a
dynamic balance between the protective and pathogenic roles of NO.
This balance may be regulated by the location, time and magnitude
of NO release. We also hypothesize that the DDAH/eNOS/iNOS stress
reaction to insults of brief hyperglycemic episodes and LPS is
aggravated. The current study was conducted with the aim of
investigating these hypotheses.
Materials and methods
Cell culture
Human pulmonary microvascular endothelial cells
(PMVECs) were purchased from American Type Culture Collection
(ATCC; Manassas, VA, USA) and grown in Dulbecco’s modified Eagle’s
medium (DMEM) with 10% fetal bovine serum (Gibco-BRL, Invitrogen,
Carlsbad, CA, USA). Cells were incubated at 37°C in a 5%
CO2 humidified atmosphere and maintained at
subconfluency by passaging with trypsin-ethylenediaminetetraacetic
acid (EDTA; Gibco-BRL). Cells were incubated with normal (5.5 mM)
or high (33 mM) D-glucose concentrations (Sigma, St. Louis, MO,
USA) for 5 days in medium with 2% serum (to maintain the cells in
the quiescent state) and then incubated with LPS (0.00, 0.01, 0.10,
1.00, 10 or 100 μg/ml) for 0, 8, 12, 24 or 36 h. To evaluate
the potential cytotoxicity of these agents, MTT assays were
performed to assess cell viability. Briefly, cells were plated in
96-well plates (0.4×105 cells/well) and treated with the
agent. The supernatant was then replaced by fresh medium containing
10% MTT. The formazan product was dissolved in dimethyl sulfoxide
(DMSO) and the absorbance at 592 nm was measured.
F-actin staining
Cells were washed with phosphate-buffered saline
(PBS) to remove cell debris and fixed in 4% paraformaldehyde (v/v)
for 10 min. Following fixation, the cells were permeabilized with
0.5% Triton X-100 (v/v) in PBS and stained with
phalloidin-fluorescein isothiocyanate (FITC; Sigma) to label actin
and 4′,6-diamidino-2-phenylindole (DAPI) to label cell nuclei in
PBS for 45 min at room temperature (22–24°C). Following the final
rinse, the cells were mounted on a glass slide with fluorescence
mounting medium (Invitrogen Life Technologies, Carlsbad, CA, USA).
Stained cells were photographed with an Olympus Provis fluorescence
microscope (Olympus, Hamburg, Germany). The images shown are
representative of at least three separate experiments.
Scanning electron microscopy (SEM)
Human PMVEC layers grown on fibronectin-coated
dishes (Corning Life Sciences, Corning, CA, USA) were washed with
PBS at room temperature. The cells were prefixed with 2.5%
glutaraldehyde and post-fixed with 1% osmium tetroxide in 0.05 M
PBS for 1 h at 48°C. The cells were then dehydrated in a graded
ethanol series and dried using the 100% t-butyl alcohol and finally
dried in a JFC-310 freeze drying device (JEOL, Tokyo, Japan). After
being coated with gold in a JFC-1100 ion sputter coater (JEOL),
samples were examined under a JSM-T300 SEM (JEOL).
Permeability assay
Human PMVECs were cultivated on polycarbonate
membrane Transwell inserts (6.5 mm diameter, 0.4 μm pore
size; Corning Life Sciences) coated with ProNectin F until
confluent. The amount of culture medium in the upper and lower
compartments of the Transwell were 100 and 600 μl,
respectively. Prior to the experiment, the culture medium in the
two compartments was replaced with fresh medium and 0.126 mM
horseradish peroxidase (HRP; type VI-A, molecular weight 44,000;
Sigma-Aldrich) was added to the upper compartment. The cells were
then incubated under normal culture conditons. After 1 h, the
medium in the lower compartment was collected and stored on ice
until the HRP enzymatic activity was assayed. Briefly, 60 μl
medium was incubated with 860 μl reaction buffer (50 nM
NaH2PO4 with 5 nM guaiacol) for 25 min at
room temperature. The reaction was initiated by adding 100
μl hydrogen peroxide. HRP activity was calculated from the
increase in absorbance at 470 nm.
Nitrite/nitrate
(NO2−/NO3−)
quantification
NO production was measured by determining the
concentration of nitrite (a stable metabolite of NO) using the
Griess reagent system from Jiancheng Institute of Biotechnology
(Nanjing, China) according to manufacturer’s instructions. Cell
culture medium aliquots (100 μl) were incubated for 30 min
at room temperature with 50 mM nicotinamide adenine dinucleotide
phosphate (NADPH) and 24 mU nitrate reductase; then, the samples
were treated with 0.2 U lactate dehydrogenase and 0.5 mmol sodium
pyruvate for 10 min. The color was developed by adding the Griess
reagent (1:1, v/v). Finally, after 10 min at room temperature, the
absorbance was recorded on a 96-well plate Multiskan Microtiter
Plate Reader (Thermo Labsystems, Philadelphia, PA, USA) at 540 nm.
Nitrite levels were determined using a standard curve.
Western blotting
Cells were washed with ice-cold PBS and lysed in
RIPA buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate and 0.1% sodium dodecyl sulfate (SDS)] on ice
for 30 min. The total protein concentration was determined using a
Protein Assay kit (Bio-Rad, Hercules, CA, USA). Protein samples
(30–80 μg) were loaded on a 12% SDS-polyacrylamide gel and
separated by electrophoresis prior to transfer to polyvinylidene
difluoride membranes. After blocking with Tris-buffered saline with
Tween-20 [TBST; 20 mM Tris (pH 7.5), 150 mM NaCl and 0.01%
Tween-20] containing 5% non-fat dry milk for 1 h at room
temperature, the membranes were then incubated with polyclonal
anti-DDAH-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA),
monoclonal anti-eNOS (Sigma) and anti-iNOS (Santa Cruz
Biotechnology) antibodies overnight at room temperature with
constant agitation. The filters were then washed and probed with
secondary HRP-linked goat anti-mouse or anti-rabbit antibodies
(1:500; Santa Cruz Biotechnology) at room temperature for 1 h. The
proteins were detected using an enhanced chemiluminescence (ECL)
detection system. Western blots were quantified by densitometric
analysis followed by normalization with actin. Results are
expressed as arbitrary units (AU).
Statistical analysis
Data are presented as mean ± standard deviation
(SD). One-way analysis of variance and Student’s t-tests were
performed to determine the statistically significant differences
among different experimental groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cell viability assay
The MTT assay indicated that the viability of PMVECs
was significantly increased by treatment with LPS (0.1 and 1
μg/ml) for 12 h compared with the untreated control (LPS, 0
μg/ml; all P<0.05); however, the viability of the PMVECs
was significantly reduced by treatment with higher concentrations
of LPS (10 and 100 μg/ml) for 12 h, compared with that of
the PMVECs treated with 1 μg/ml LPS in the normal glucose
(NG) and HG groups (Fig. 1A; all
P<0.05). Then, 10 μg/ml LPS was selected to treat PMVECs
for different incubation times. The results indicated that the
viability of PMVECs was significantly increased by treatment with
LPS (10 μg/ml) for 8 and 12 h compared with the viability
prior to treatment with LPS (all P<0.05). However, the viability
of the PMVECs was significantly reduced by treatment with LPS (10
μg/ml) for 24 and 36 h compared with the viability following
a 12-h treatment with LPS (Fig.
1B; all P<0.05). We therefore used LPS (10 μg/ml) to
stimulate cells for 24 h in subsequent experiments.
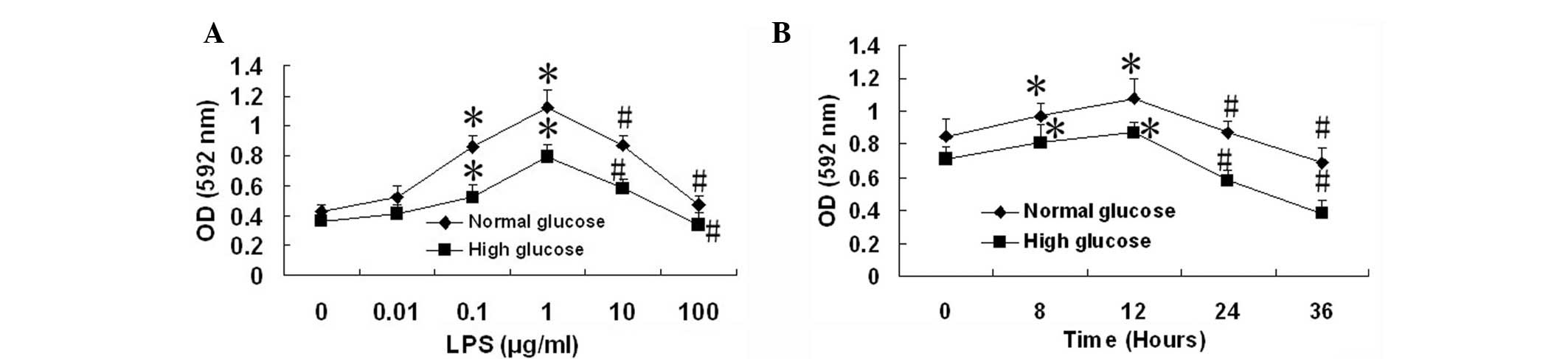 | Figure 1.Effect of lipopolysaccharide (LPS) on
the cell viability of human pulmonary microvascular endothelial
cells (PMVECs). Following the incubation of human PMVECs with
normal (5.5 mM) and high (33 mM) D-glucose concentrations for 5
days, the PMVECs were incubated with (A) LPS (0, 0.01, 0.1, 1, 10
and 100 μg/ml) for 12 h or (B) with LPS (10 μg/ml)
for 0, 12, 24 and 36 h, respectively. Data are expressed as mean ±
standard deviation (SD; n=5). *P<0.05, compared with
the LPS (0 μg/ml) group or 0 h group. #P<0.05,
compared with the LPS (1 μg/ml) group or 12 h group. OD,
optical density. |
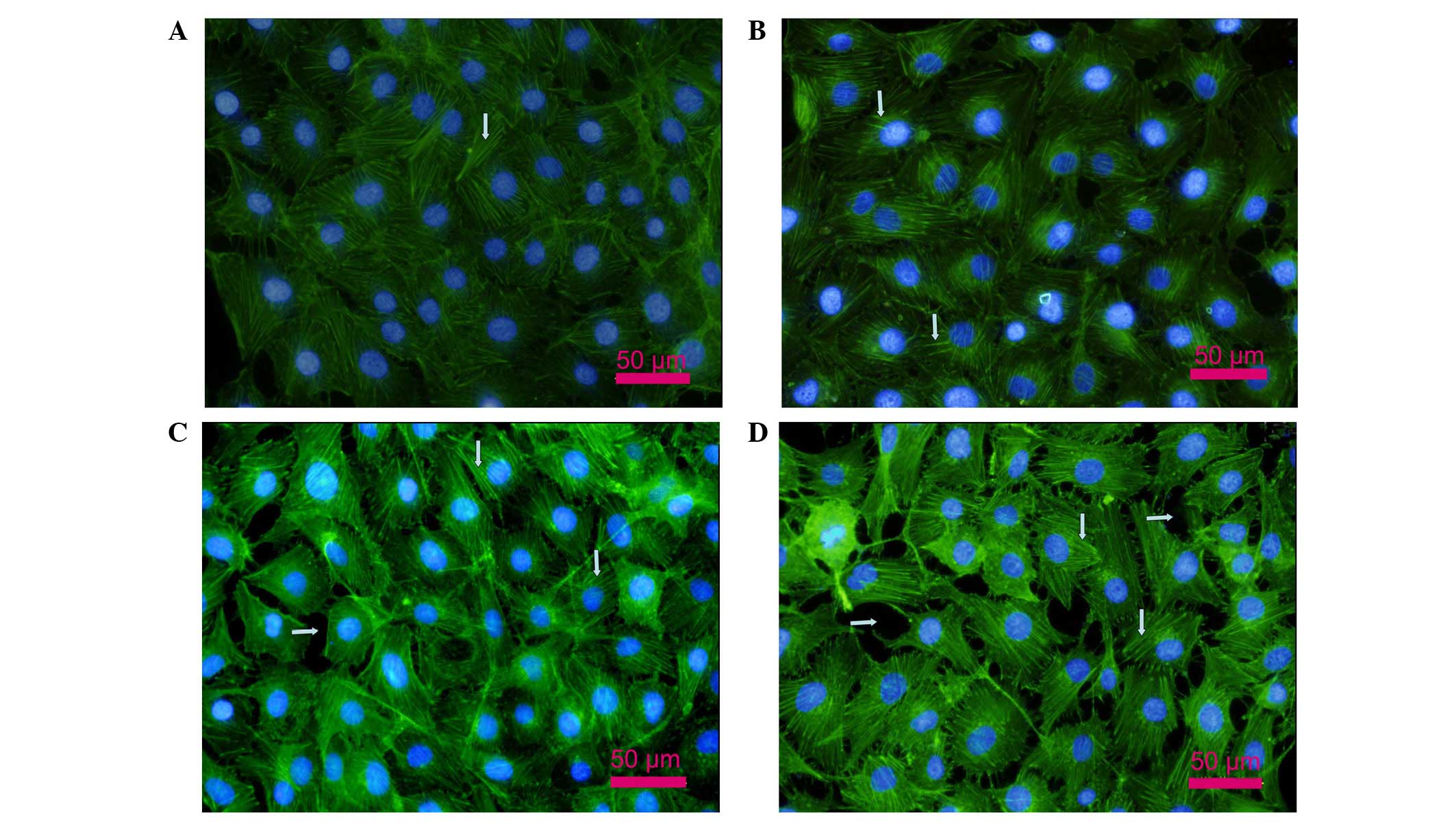
Changes of the actin cytoskeleton in
PMVECs
As shown in Fig.
2A, the actin cytoskeleton of PMVECs consisted of regular fiber
distribution in the cytoplasm and almost continuous peripheral
actin fibers at the cell-cell junctions in the NG group. Stress
fiber formation was observed in the HG group (vertical arrows in
Fig. 2B). In the presence of LPS,
cells incubated in NG concentrations had an activated cell
phenotype, with thin or no cortical actin and abundant stress
fibers (vertical arrows in Fig.
2C). By contrast, when cells incubated in HG concentrations
were exposed to LPS, a marked effect on F-actin filament
organization was observed, with evident and robust stress fiber
formation and intracellular gap formation (parallel arrows in
Fig. 2D).
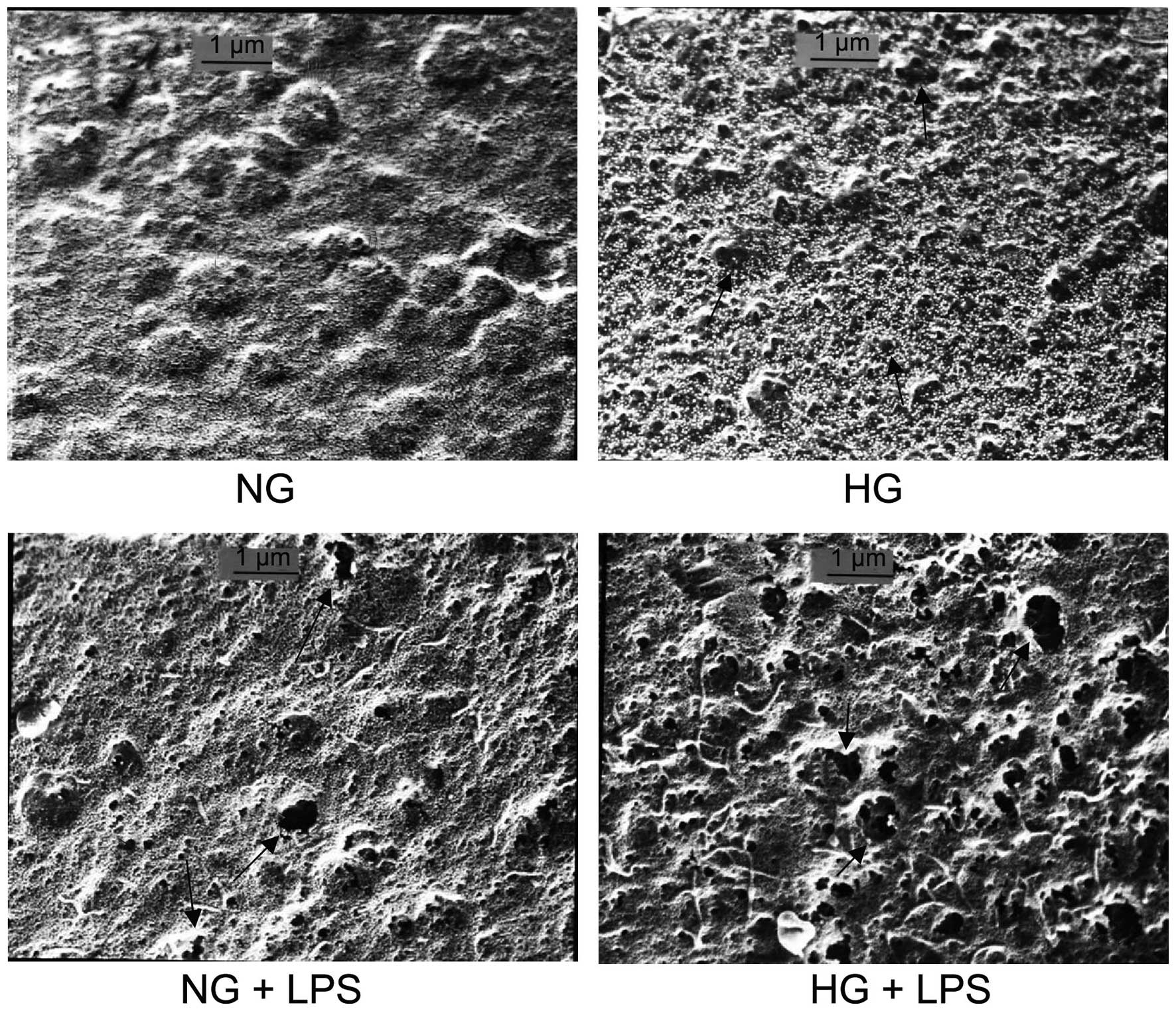
Formation of fenestrae in PMVECs
To examine the effect of HG concentrations and LPS
on the formation of fenestrae in human PMVECs, cells were incubated
with normal (5.5 mM) or high (33 mM) D-glucose concentrations for 5
days, then cells were incubated with 10 μg/ml LPS for 12 h.
The samples were prepared for SEM. Results demonstrated that the
number and average diameter of fenestrae were significantly
increased in the HG and NG + LPS groups compared with those in the
NG group (both P<0.05). Compared with the HG or NG + LPS groups,
the number and average diameter of fenestrae were significantly
increased in the HG + LPS group (both P<0.05; Fig. 3 and Table I).
 | Table I.Number and size of fenestrae in human
PMVECs. |
Table I.
Number and size of fenestrae in human
PMVECs.
| Group | No. of fenestrae per
μm2 | Average diameter of
fenestrae (nm) |
|---|
| NG | 4.87±0.74 | 8.29±1.76 |
| HG | 12.89±2.32a | 17.60±5.59a |
| NG + LPS | 24.0 0±2.19a | 31.25±9.57a |
| HG + LPS | 38.43±5.08b | 52.07±16.40b |
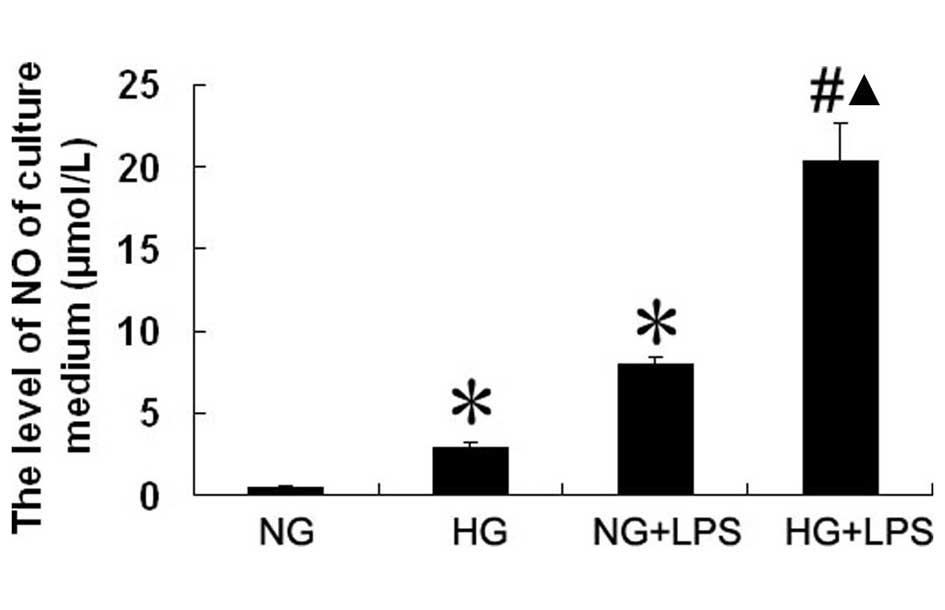
Effect of LPS and HG on NO production in
human PMVECs
As shown in Fig. 4,
LPS (10 μg/ml) increased the level of NO produced by PMVECs
in NG conditions by ∼15-fold compared with the level in the NG
group (7.99±0.33 vs. 0.54±0.12 μmol/l, respectively;
P<0.05). Compared with the NG group, NO production by PMVECs was
significantly increased in the HG group (0.54±0.12 vs. 2.91±0.30
mol/l, respectively; P<0.05). The NO production level of PMVECs
was significantly higher in the HG + LPS group than in the NG + LPS
group (20.36±2.25 vs. 7.99±0.33 μmol/l, respectively;
P<0.05).
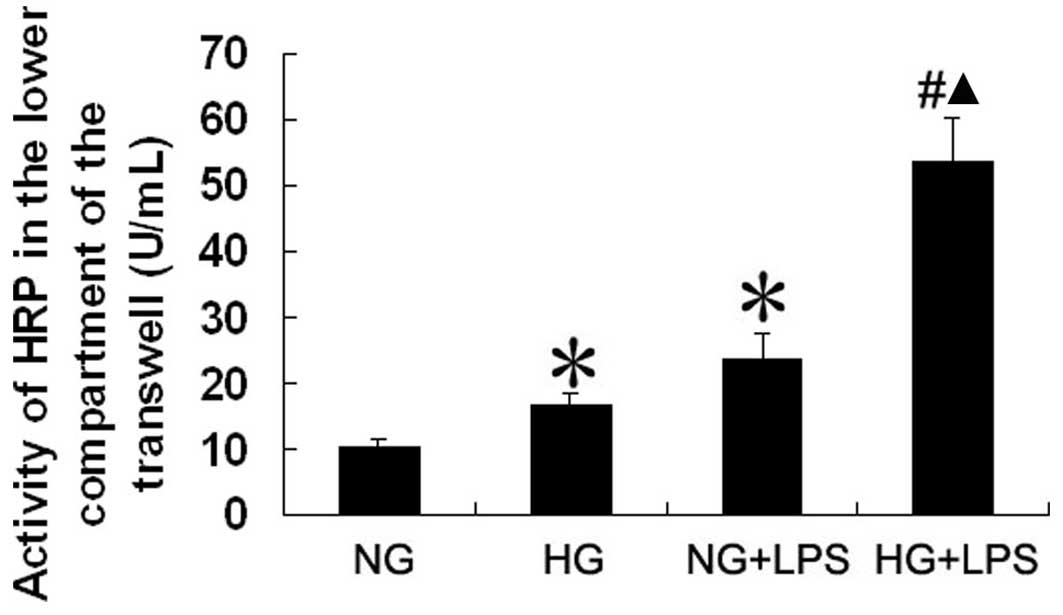
Effect of LPS and HG on the permeability
of human PMVECs
We used HRP as a marker for the endothelial cell
barrier properties against macromolecules. Human PMVECs were
cultured on polycarbonate Transwell membrane inserts. The PMVECs
were incubated with normal (5.5 mM) or high (33 mM) D-glucose
concentrations for 5 days in medium with 2% serum (to maintain the
cells in a quiescent state) and then incubated with 10 μg/ml
LPS for 12 h. The amount of HRP passing from the upper to the lower
compartment was measured by enzymatic assay. As shown in Fig. 5, compared with the HG control
group, the amount of HRP that passed through the membrane was
significantly increased in the HG + LPS group (both P<0.05). The
amount of HRP that passed through the membrane was also
significantly higher in the HG + LPS group than in the NG + LPS
group, (P<0.05).
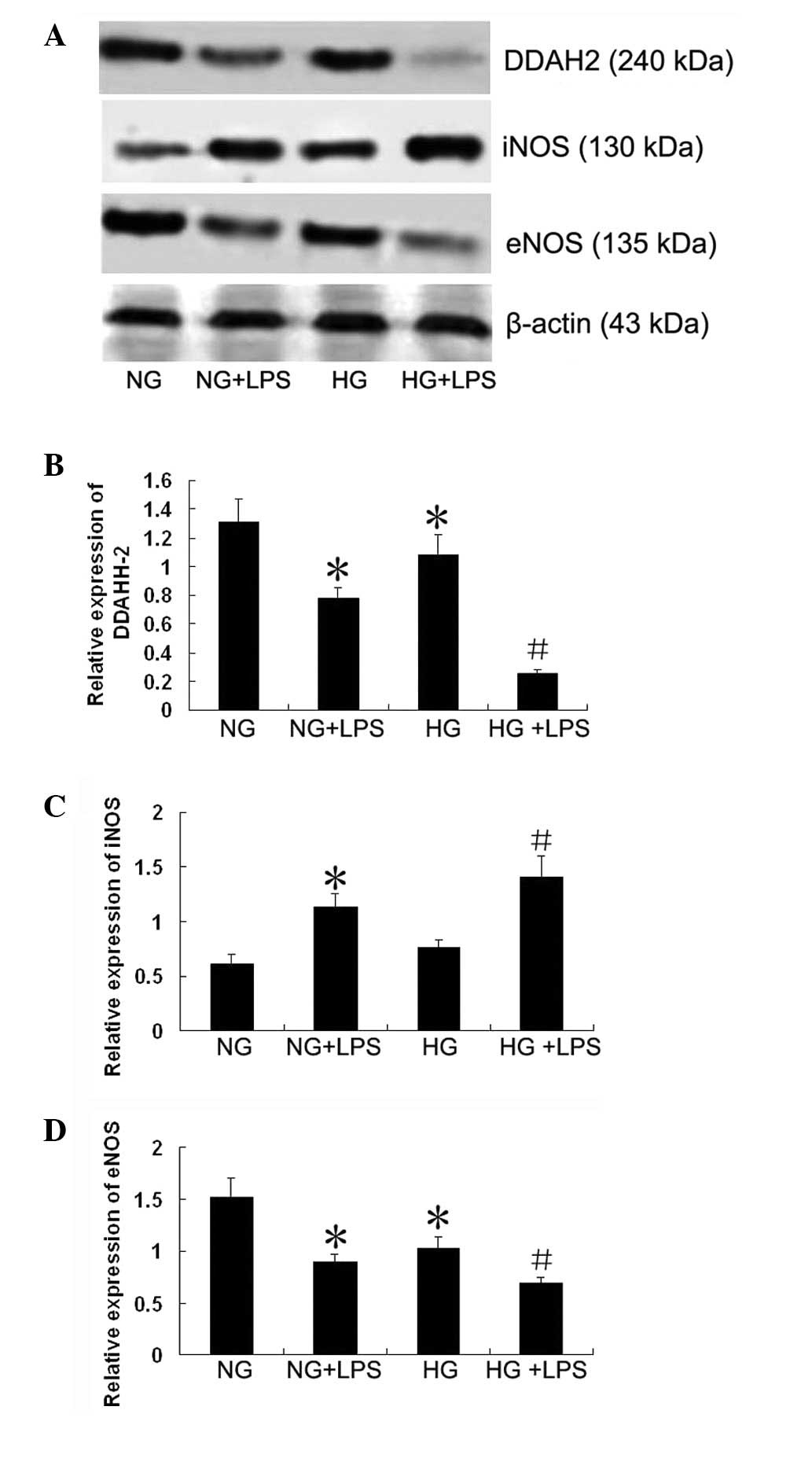
Expression of DDAH-2, eNOS and iNOS in
human PMVECs
As shown in Fig. 6,
compared with the NG group, the protein expression level of DDAH-2
was significantly downregulated in the HG + LPS group (P<0.05).
The protein expression level of DDAH-2 was also significantly lower
in the HG + LPS group than in the NG + LPS group (P<0.05). The
expression level of iNOS in the NG + LPS group was significantly
upregulated compared with that in the NG group (P<0.05).
Compared with the NG + LPS group, the expression level of iNOS was
significantly increased in the HG + LPS group (P<0.05). The
expression level of eNOS in the HG + LPS group was significantly
downregulated compared with that in the HG group (P<0.05).
Compared with the NG + LPS group, the expression level of eNOS was
significantly reduced in the HG + LPS group (P<0.05).
Discussion
The pathway for water and small hydrophilic solutes
through the walls of fenestrated vessels is through the fenestrae.
Under specific conditions, the permeability of the microvasculature
is increased such that macromolecules cross the endothelial barrier
in three ways: through the intercellular junctions, through the
endothelial cell fenestrae and transcellularly by shuttling
vesicles and specific receptors (18). Observations made in large vessel
endothelial cells have demonstrated that disrupting the actin
cytoskeleton enhances vascular permeability (19). Fluorescent staining of human PMVECs
revealed a marked effect on the organization of the F-actin
filaments, the formation of stress fibers and intracellular gap
formation in the presence of NG and LPS. However, HG concentrations
and LPS strongly effaced actin at the cell periphery with thin or
short cortical actin, induced the formation of abundant quanties of
stress fibers and resulted in the appearance of small paracellular
gaps. The damage to these actin and junctional protein bonds
results in the dissociation and redistribution of proteins, which
affects the cell-to-cell barrier functions. In the present study we
observed a significant augmentation of the number and diameter of
fenestrae of endothelial cells during a 24-h period of treatment
with LPS. Moreover, a similar but significantly stronger increase
in the number and diameter of fenestrae was observed following a
24-h HG and LPS treatment; this facilitated the passing of water
through the cell monolayer and ultimately increased macromolecule
permeability.
The results of the current study also demonstrated
that the HG concentration and LPS induced an increase in the
permeability of the cultured endothelial monolayer to
macromolecules, including HRP, which is a marker for determining
the endothelial barrier permeability. HRP flux across the
endothelial cell monolayer was 2.3-fold higher in the HG + LPS
group compared with the NG + LPS group. Evidently, there is a
strong synergy between the effects of HG concentration and LPS on
endothelial barrier permeability. These findings indicate that
hyperglycemia associated with infection may create an early window
of vulnerability allowing secondary insults to activate deleterious
endothelial functions.
Injury to endothelial cells is a key mechanism for
acute lung injury (ALI) and its most severe form, acute respiratory
distress syndrome or sepsis, by which microvasculature permeability
increases (20–22). Under such hyperglycemic
circumstances, such damage may be more evident.
NO is synthesized from L-arginine by NOS. nNOS and
eNOS are referred to as constitutive NOS (cNOS). NO production by
cNOS modulates several aspects of intestinal physiology and is
considered to be required for maintaining epithelial cell barrier
integrity (23). In the present
study, compared with a NG concentration, a HG concentration
resulted in a reduction of LPS-stimulated eNOS expression. Reduced
levels of NO formation by eNOS may be due to reductions in eNOS
expression levels, deficiency of its cofactor, tetrahydrobiopterin
(BH4) or eNOS translocation to a membrane compartment distant from
the plasma or Golgi membranes (24). Moreover, eNOS activity is regulated
by endogenous inhibitors, particularly ADMA, which is metabolized
by DDAH. Therefore, DDAH is a determinant for ADMA concentrations
and its dysregulation may have an important role in ADMA elevation
and NOS pathway modulation in pathological conditions, including
hyperglycemia. In the mouse lung, eNOS protein expression is
reduced 12 h after LPS treatment (25).
By contrast, NO is produced by iNOS under
pathological conditions, including inflammation, and it reduces the
integrity of the intestinal epithelium. Several studies have shown
that iNOS is induced by bacterial products, microbes and certain
cytokines, resulting in the production of high NO levels. NO
overproduction and the attendant oxidative injury to key proteins
are necessary for dysregulation of the monolayer barrier and
barrier hyperpermeability (26–28).
Additional studies have identified that increased lung NO
production is associated with increased iNOS expression and/or iNOS
activity in various ALI models (29,30).
In the current study, we demonstrated that LPS increased NO
production and iNOS expression but reduced eNOS expression,
suggesting that the increased NO levels may be iNOS derived.
Previous studies have demonstrated that the ADMA/
DDAH pathway regulates pulmonary endothelial barrier function by
modulating Rac1 signaling (31). We demonstrated that hyperglycemia
boosts LPS-initiated NO production by enhancing iNOS expression, as
well as reducing DDAH-2 expression. However, enhanced ADMA levels
are not sufficient to limit NO production by iNOS; however, it may
contribute to reducing NO production by eNOS (17). iNOS may compete with eNOS by
limiting BH4 availability. Furthermore, nitrosative stress induced
by iNOS overexpression reduces DDAH activity.
In summary, in the current study we demonstrated
that the exposure of human PMVECs to a HG concentration increased
LPS-stimulated permeability. This may be linked to dysregulation of
the NO pathway. Therefore, monitoring glucose control may be
important for those in acute stress to protect endothelial cells
from secondary damage processes.
References
|
1.
|
Shapiro NI, Schuetz P, Yano K, et al: The
association of endothelial cell signaling, severity of illness, and
organ dysfunction in sepsis. Crit Care. 14:R1822010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Muller LM, Gorter KJ, Hak E, et al:
Increased risk of common infections in patients with type 1 and
type 2 diabetes mellitus. Clin Infect Dis. 41:281–288. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shah BR and Hux JE: Quantifying the risk
of infectious diseases for people with diabetes. Diabetes Care.
26:510–513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nareika A, Im YB, Game BA, et al: High
glucose enhances lipopolysaccharide stimulated CD14 expression in
U937 mono-nuclear cells by increasing nuclear factor kappaB and
AP-1 activities. J Endocrinol. 196:45–55. 2008. View Article : Google Scholar
|
|
5.
|
Maldonado A, He L, Game BA, et al:
Pre-exposure to high glucose augments lipopolysaccharide-stimulated
matrix metalloproteinase-1 expression by human U937 histiocytes. J
Periodontal Res. 39:415–423. 2004. View Article : Google Scholar
|
|
6.
|
Song MK, Um JY, Jang HJ and Lee BS:
Beneficial effect of dietary Ephedra sinica on obesity and
glucose intolerance in high-fat diet-fed mice. Exp Ther Med.
3:707–712. 2012.
|
|
7.
|
Sherry CL, O’Connor JC, Kramer JM and
Freund GG: Augmented lipopolysaccharide-induced TNF-alpha
production by peritoneal macrophages in type 2 diabetic mice is
dependent on elevated glucose and requires p38 MAPK. J Immunol.
178:663–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Noda A, Kinoshita K, Sakurai A, et al:
Hyperglycemia and lipopolysaccharide decrease depression effect of
interleukin 8 production by hypothermia: an experimental study with
endothelial cells. Intensive Care Med. 34:109–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
de Souza LF, Barreto F, da Silva EG, et
al: Regulation of LPS stimulated ROS production in peritoneal
macrophages from alloxan-induced diabetic rats: involvement of high
glucose and PPARgamma. Life Sci. 81:153–192. 2007.PubMed/NCBI
|
|
10.
|
Stegenga ME, van der Crabben SN, Blümer
RM, et al: Hyperglycemia enhances coagulation and reduces
neutrophil degranulation, whereas hyperinsulinemia inhibits
fibrinolysis during human endotoxemia. Blood. 112:82–89. 2008.
View Article : Google Scholar
|
|
11.
|
Durán WN, Breslin JW and Sánchez FA: The
NO cascade, eNOS location, and microvascular permeability.
Cardiovasc Res. 87:254–261. 2010.PubMed/NCBI
|
|
12.
|
Akaike T and Maeda H: Nitric oxide and
virus infection. Immunology. 101:300–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jiang WG, Sanders AJ, Ruge F and Harding
KG: Influence of interleukin-8 (IL-8) and IL-8 receptors on the
migration of human keratinocytes, the role of PLC-γ and potential
clinical implications. Exp Ther Med. 3:231–236. 2012.PubMed/NCBI
|
|
14.
|
van der Vliet A, Eiserich JP, Shigenaga MK
and Cross CE: Reactive nitrogen species and tyrosine nitration in
the respiratory tract: epiphenomena or a pathobiologic mechanism of
disease? Am J Respir Crit Care Med. 160:1–9. 1999.PubMed/NCBI
|
|
15.
|
Pope AJ, Karuppiah K and Cardounel AJ:
Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial
nitric oxide production. Pharmacol Res. 60:461–465. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sharma S, Smith A, Kumar S, et al:
Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced
acute lung injury: Role of asymmetric dimethylarginine. Vascul
Pharmacol. 52:182–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Vu DM, Yokoyama TA, Sawada K, et al:
Enhancement of permeability in endothelial cells for the
development of an anti-thrombogenic bioartificial hemofilter.
Biotechnol Bioeng. 101:634–641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Birukova AA, Arce FT, Moldobaeva N, et al:
Endothelial permeability is controlled by spatially defined
cytoskeletal mechanics: atomic force microscopy force mapping of
pulmonary endothelial monolayer. Nanomedicine. 5:30–41. 2009.
View Article : Google Scholar
|
|
19.
|
Schwarz MA: Acute lung injury: cellular
mechanisms and derangements. Paediatr Respir Rev. 2:3–9. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yang B, Huang W, Han J and Liang Z: Study
of the role of epidermal growth factor on lung fluid transport in
rabbits with acute lung injury caused by endotoxin. Exp Ther Med.
4:611–614. 2012.PubMed/NCBI
|
|
21.
|
Groeneveld AB: Vascular pharmacology of
acute lung injury and acute respiratory distress syndrome. Vascul
Pharmacol. 39:247–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Vallance BA, Dijkstra G, Qiu B, et al:
Relative contributions of NOS isoforms during experimental colitis:
endothelial-derived NOS maintains mucosal integrity. Am J Physiol
Gastrointest Liver Physiol. 287:G865–G874. 2004. View Article : Google Scholar
|
|
23.
|
Nuszkowski A, Gräbner R, Marsche G, et al:
Hypochlorite-modified low density lipoprotein inhibits nitric oxide
synthesis in endothelial cells via an intracellular dislocalization
of endothelial nitric-oxide synthase. J Biol Chem. 276:14212–14221.
2001.
|
|
24.
|
Chatterjee A, Snead C, Yetik-Anacak G, et
al: Heat shock protein 90 inhibitors attenuate LPS-induced
endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol.
294:L755–L763. 2008.PubMed/NCBI
|
|
25.
|
Han X, Fink MP, Yang R and Delude RL:
Increased iNOS activity is essential for intestinal epithelial
tight junction dysfunction in endotoxemic mice. Shock. 21:261–270.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Forsyth CB, Banan A, Farhadi A, et al:
Regulation of oxidant-induced intestinal permeability by
metalloprotease-dependent epidermal growth factor receptor
signaling. J Pharmacol Exp Ther. 321:84–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Farley KS, Wang LF, Law C and Mehta S:
Alveolar macrophage inducible nitric oxide synthase-dependent
pulmonary microvascular endothelial cell septic barrier
dysfunction. Microvasc Res. 76:208–216. 2008. View Article : Google Scholar
|
|
28.
|
Huffman LJ, Prugh DJ, Millecchia L, et al:
Nitric oxide production by rat bronchoalveolar macrophages or
polymorphonuclear leukocytes following intratracheal instillation
of lipopolysaccharide or silica. J Biosci. 28:29–37. 2003.
View Article : Google Scholar
|
|
29.
|
Farley KS, Wang LF, Razavi HM, et al:
Effects of macrophage inducible nitric oxide synthase in murine
septic lung injury. Am J Physiol Lung Cell Mol Physiol.
290:L1164–L1172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Wojciak-Stothard B, Torondel B, Zhao L, et
al: Modulation of Rac1 activity by ADMA/DDAH regulates pulmonary
endothelial barrier function. Mol Biol Cell. 20:33–42. 2009.
View Article : Google Scholar : PubMed/NCBI
|