Introduction
Phytoestrogens, including isoflavones, lignans and
coumestans, are components of plants and seeds (1). The molecular structures of
phytoestrogens and mammalian estrogens are similar. Phytoestrogens
aid the cure of hormone-associated diseases, including breast
cancer, prostate cancer, menopausal syndromes, cardiovascular
diseases and osteoporosis (2–6).
Due to the reduction in ovarian function for women
during their perimenopausal stages, estrogen levels are often
decreased, leading to secondary hyperparathyroidism and reduced
calcitonin secretion. Therefore, bone resorption rates are often
greater than bone formation rates, resulting in osteoporosis. For
women with osteoporosis, bone material density (BMD) is decreased
and the bone microstructures begin to degenerate. Thus, the bones
become fragile and may easily fracture (7). Estrogen supplements may attenuate the
long-term lack of estrogen caused by atrophic diseases of the
genitourinary system, skin and brain. Supplements may also prevent
osteoporosis, fractures and cardiovascular and cerebrovascular
diseases. Bone estrogen promotes increased bone density and the
length of the bone (8,9). Estrogen is involved in the formation
of bone; when estrogen levels decrease, a gradual loss of calcium
in the bones occurs. Osteocalcin loss results in osteoporosis.
Hormone replacement therapy is often used
clinically. However, the application of hormone replacement therapy
is limited due to numerous contraindications and adverse responses.
The gardenia oil is extracted from seeds of the plant Gardenia
jasminoides. Gardenia oil has been observed to affect the
learning ability and memory of mice (10–12).
COX-2 is a protein associated with the functions of estrogen in
numerous diseases (13,14). In the current study, gardenia oil
was used to treat ovariectomized rats. The effects of gardenia oil
on 17β-estradiol (also termed E2) levels, BMD and bone
biomechanics in ovariectomized female rats were investigated.
Quantitative PCR (qPCR) and immunoblot assays were performed to
study the associated molecular mechanisms. We observed that
gardenia oil significantly increases the expression levels of COX-2
protein by increasing the mRNA levels.
Materials and methods
Experimental animals
SD rats with the average age of 3 months and weight
of 280±20 g were provided by the experimental animal center,
Medical College, Xi’an Jiaotong University (Xi’an, China). The SD
rats (n=53) were divided into 6 groups (sham group, n=10; untreated
group, n=12; diethylstilbestrol group, n=9; high dose group, n=7;
middle dose group, n=7; low dose group, n=8). All animal
experiments were conducted according to the ethical guidelines of
Yan’an University (Xi’an, China).
The ovariectomized animal models were established as
follows. Sodium pentobarbital anesthesia (3%) was administered to
the healthy female SD rats (1 ml/kg) by intraperitoneal injection
and ovariectomy surgery was performed. Following surgery, 400,000
units of penicillin was intramuscularly injected daily for 3 days
to prevent infection. The rats receiving ovariectomies were
randomly divided into the following groups: the high (4.50 g/kg),
middle (1.80 g/kg) and low (0.72 g/kg) dose gardenia oil groups,
the diethylstilbestrol group and the untreated control group. The
rats treated with diethylstilbestrol (the diethylstilbestrol group)
or saline (the untreated control group) served as the positive and
the untreated controls, respectively. In addition to the five
groups described above, the sham group served as the false-surgery
control, since the rats in this group received the same surgery as
others, with the exception of the ovariectomy. Fat of the same
volume as the ovaries was removed from the sham group. The rats in
the sham group were also administered saline.
The control and sham-operated groups were medicated
with an equal volume of saline. After 12 weeks of administration
and 12 h of fasting, the anesthetized animals were sampled.
Experimental drug
The gardenia oil was provided by Xi’an Dongsheng
Pharmaceutical Research Institute (Xi’an, China).
Diethylstilbestrol, estrogen, follicle-stimulating hormone and
luteinizing hormone were determined by electrochemical immunoassay.
The diagnostic reagents, standards and quality controls were
provided by Roche (Basel, Switzerland). Detection kits for serum
alkaline phosphatase (ALP), calcium and phosphorus were purchased
from Nanjing Jiancheng Bioengineering Company (Nanjing, China).
Experimental equipment
The DEXA Dual Energy X-ray Absorptiometry equipment
(Hologic QDR, 2000 type) was purchased from Hologic (Bedford, MA,
USA). The fully automated chemiluminescence immunoassay analyzer
COBASE411 and the SWD-10 microcomputer control electronic bone
biomechanical analyzer were purchased from Roche.
Blood collection and testing
After weighing, 3% sodium pentobarbital anesthesia
(1 ml/kg) was administered to rats by intraperitoneal injection.
Blood (3 ml) was collected via a carotid artery catheter. The
levels of serum ALP, calcium and phosphorus were determined.
Determination of 17β-estrogen, follicle-stimulating hormone and
luteinizing hormone levels was also performed.
Determination of BMD
Prior to the treatment, rat bone samples were
collected. A dual-energy X-ray absorptiometry scan was performed.
In order to ensure the quality of the scan, the Hologic QDR 2000
automatic internal quality control system was applied to maintain
the precision to 0.4%.
Determination of bone mechanics
Following measurement of the bone density of left
femur specimens, a femoral three-point bending test was performed
to determine the maximum stress and maximum strain. The space
between two force points (L)=20 mm. The bending force (F) was
measured. Two femoral central diameters, which were perpendicular,
were selected as d1 and d2. The average diameter (d) was
calculated. The maximum stress relative to the bending strength
(ultimate strength) was calculated using the formula: σ=My/L=(8
LF)/(πd3). Bending moment on the section (M) and the
distance to the neutral axis (y) were used. The load measurement
accuracy was 0.1 N. The maximum strain measurement accuracy was
0.001 mm.
Quantitative (qPCR)
The ovary-adjacent tissues of the rats were
collected for RNA isolation using the RNeasy FFPE kit (Cat. no.,
73504, Qiagen, Valencia, CA, USA). qPCR analysis of COX-2 mRNA and
GAPDH mRNA levels was performed. The RT-PCR experiments were
repeated at least 3 times. RNA was reverse transcribed into cDNA
using random primers in a Reverse Transcription II system (Promega,
Madison, WI, USA) according to the manufacturer’s instructions.
Expression of COX-2 mRNAs was quantified by qPCR using an ABI Prism
Sequence Detection system (Applied Biosystems, Foster City, CA,
USA). An assay reagent containing premixed primers and a
VIC-labeled probe (Applied Biosystems; cat. no. 4310884E) was used
to quantify expression of endogenous GAPDH mRNA. The levels (mean
value) of COX-2 mRNA transcripts were calculated. The COX-2
upstream and downstream primers were 5′-CGGGATCCTGCCAGCTCCACCG and
5′-GCTCTAGAACAAACTGAGTGAGTCC, respectively. The GAPDH upstream and
downstream primer sequences were 5′-TGAAGGTCGGAGTCAACGGATTTGGT and
5′-CATGTGGGCCATGAGGTCCACCAC, respectively.
Immunoblot assays
The total proteins were harvested from the
ovary-adjacent tissues of the rats. The proteins were separated on
10% SDS/PAGE gels and then subjected to immunoblot analyses. The
primary antibodies against COX-2 (∼70 kDa) and β-actin were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA;
anti-COX-2, cat. no. sc-70879, 1:200; anti-β-actin, cat. no.
sc-130301, 1:10,000). The secondary antibodies used in this study
were goat anti-mouse IgG-HRP (cat. no. sc-2005, 1:10,000, Santa
Cruz). The bound antibodies were detected using the ECL system
(Pierce Biotechnology, Rockford, IL, USA). The immunoblot
experiments were repeated at least 3 times.
Statistical analysis
Each set of data was expressed as mean ± SEM.
Significance was analyzed using SPSS 16.0 statistical analysis
software (SPSS, Inc., Chicago, IL, USA) for one-way ANOVA and
Newman-Keuls comparison test. P<0.01 and P<0.05 were
considered to indicate a statistically significant difference.
Results
Gardenia oil increases 17β-estradiol
levels in ovariectomized female rats
To determine if the gardenia oil affects
17β-estradiol levels in the ovariectomized female rats, the
ovariectomized rats were administered various doses of gardenia oil
(high dose group, 4.50 g/kg gardenia oil; middle dose group, 1.80
g/kg gardenia oil; low dose group, 0.72 g/kg gardenia oil). The
rats administered diethylstilbestrol or saline served as the
positive and the untreated controls, respectively. The sham group
served as the false-surgery control, since the rats in this group
received the same surgery, with the exception of the ovariectomy.
Fat of the same volume as the ovaries was removed from the sham
group. The rats in the sham group were also medicated with
saline.
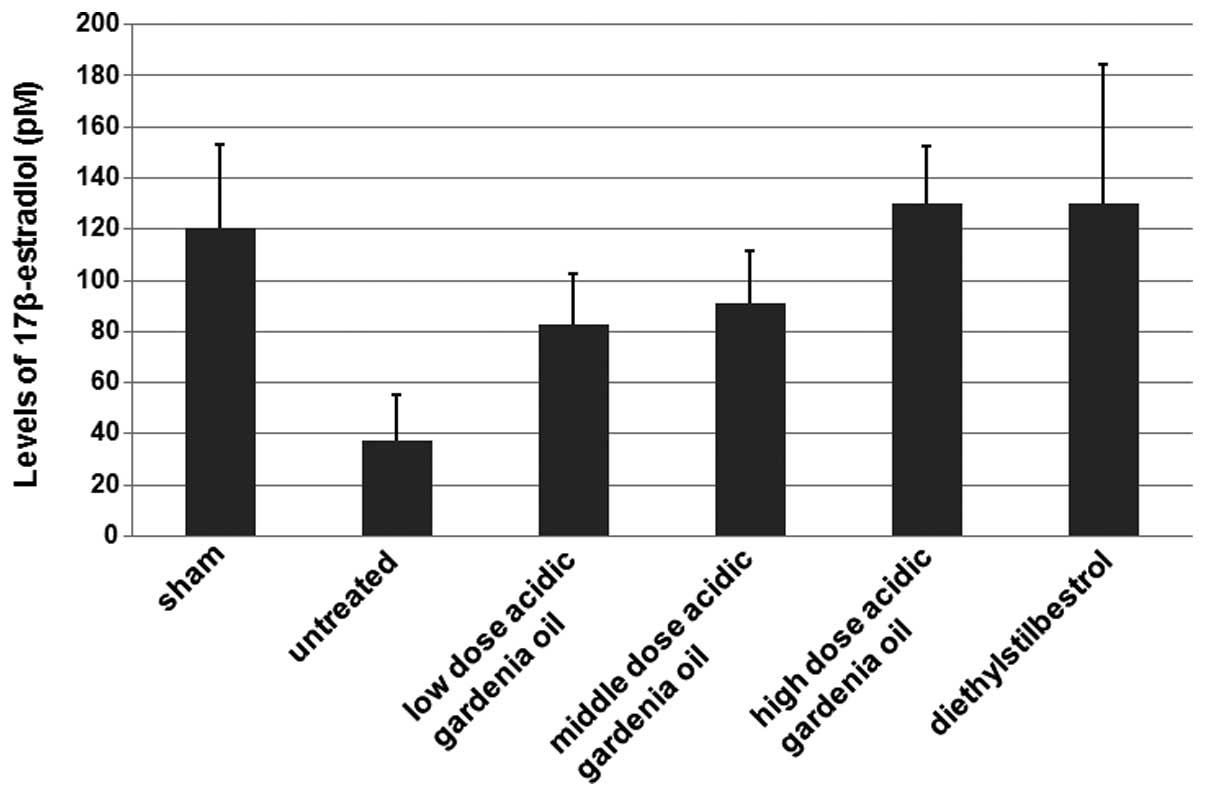
As shown in Fig. 1,
the 17β-estradiol levels in the untreated group were decreased in
comparison with those in the sham group where the rats did not
receive the ovariectomy. When compared with the untreated control,
the ovariectomized female rats in the gardenia oil groups had
increased levels of 17β-estradiol. The 17β-estradiol levels in the
high dose gardenia oil group were similar to the 17β-estradiol
levels in the diethylstilbestrol group (positive control). These
results suggest that gardenia oil increases the levels of
17β-estradiol in the ovariectomized female rats.
Follicle-stimulating hormone and luteinizing hormone
are two hormones associated with 17β-estradiol. As listed in
Table I, the gardenia oil
significantly reduced the follicle-stimulating hormone and
luteinizing hormone levels in ovariectomized female rats compared
with the levels in the untreated group. These results suggest that
gardenia oil has similar effects on follicle-stimulating hormone
and luteinizing hormone levels as diethylstilbestrol.
 | Table I.Effects of gardenia oil on sex
hormones of ovariectomized female rats (mean ± SEM). |
Table I.
Effects of gardenia oil on sex
hormones of ovariectomized female rats (mean ± SEM).
| Group | Number of rats | Follicle-stimulating
hormone (U/l) | Luteinizing hormone
(U/l) |
|---|
| Sham | 10 | 0.25±0.07b | 0.24±0.06a |
| Untreated | 12 | 0.35±0.16 | 0.44±0.17 |
|
Diethylstilbestrol | 9 | 0.06±0.04a | 0.20±0.07a |
| High dose | 7 | 0.22±0.07b | 0.27±0.08a |
| Middle dose | 7 | 0.26±0.07 | 0.32±0.07b |
| Low dose | 8 | 0.27±0.06 | 0.34±0.10 |
In the ovariectomized rats, the gardenia oil reduced
the levels of the serum ALP and elevated the levels of calcium and
phosphorus compared with those in the untreated control group
(Table II). The levels of the
serum ALP were also reduced and the levels of calcium and
phosphorus were also increased in the diethylstilbestrol group
(Table II). These pieces of
evidence also indicated that gardenia oil has a similar effect to
diethylstilbestrol.
 | Table II.Effects of gardenia oil on the serum
alkaline phosphatase, calcium and phosphorus levels of
ovariectomized female rats (mean ± SEM). |
Table II.
Effects of gardenia oil on the serum
alkaline phosphatase, calcium and phosphorus levels of
ovariectomized female rats (mean ± SEM).
| Group | Number of rats | ALP (pg/ml) | Ca (mM) | P (mmol/l) |
|---|
| Sham | 10 | 143.50±47.29 | 2.44±0.13 | 2.34±0.21 |
| Untreated | 12 | 147.17±15.11 | 2.40±0.16 | 2.29±0.19 |
|
Diethylstilbestrol | 9 | 91.80±22.15a | 2.56±0.16b | 2.48±0.19b |
| High dose | 7 | 104.57±26.55a | 2.66±0.17a | 2.49±0.28b |
| Middle dose | 7 | 116.33±24.71b | 2.48±0.12 | 2.35±0.22 |
| Low dose | 8 | 127.83±29.41a | 2.45±0.15 | 2.30±0.20 |
Gardenia oil significantly increases BMD
and the maximum stress and maximum strain of bone
In the untreated ovariectomized female rats, the BMD
and the bone maximum stress and maximum strain were decreased when
compared with those of the sham group (Table III). Diethylstilbestrol increased
the BMD and the bone maximum stress and maximum strain compared
with those in the untreated control group (Table III). Gardenia oil also increased
the BMD and bone maximum stress and maximum strain compared with
those in the untreated control group. These results indicate that
gardenia oil has a similar effect to diethylstilbestrol on BMD and
the maximum stress and the maximum strain of bone.
 | Table III.Effects of acid parker gardenia oil on
bone material density and bone biomechanics of ovariectomized
female rats (mean ± SEM). |
Table III.
Effects of acid parker gardenia oil on
bone material density and bone biomechanics of ovariectomized
female rats (mean ± SEM).
| Group | Number of rats | Tibiofibular BMD
(g/cm2) | Thigh BMD
(g/cm2) | Thigh maximum stress
(N) | Thigh maximum strain
(mm) |
|---|
| Sham | 10 | 0.145±0.015 | 0.146±0.026 | 132.4±23.7 | 1.32±0.43 |
| Untreated | 12 | 0.142±0.014 | 0.145±0.021 | 130.4±23.1 | 1.08±0.12 |
|
Diethylstilbestrol | 9 | 0.171±0.043b | 0.186±0.051b | 155.8±16.2a | 1.77±0.27a |
| High dose | 7 | 0.163±0.031b | 0.157±0.021b | 143.0±15.8b | 1.48±0.25a |
| Middle dose | 7 | 0.146±0.016 | 0.170±0.040b | 147.3±37.0 | 1.45±0.29a |
| Low dose | 8 | 0.152±0.024 | 0.149±0.025 | 142.5±21.7 | 1.14±0.35 |
Levels of COX-2 protein are significantly
increased in the high dose gardenia oil group
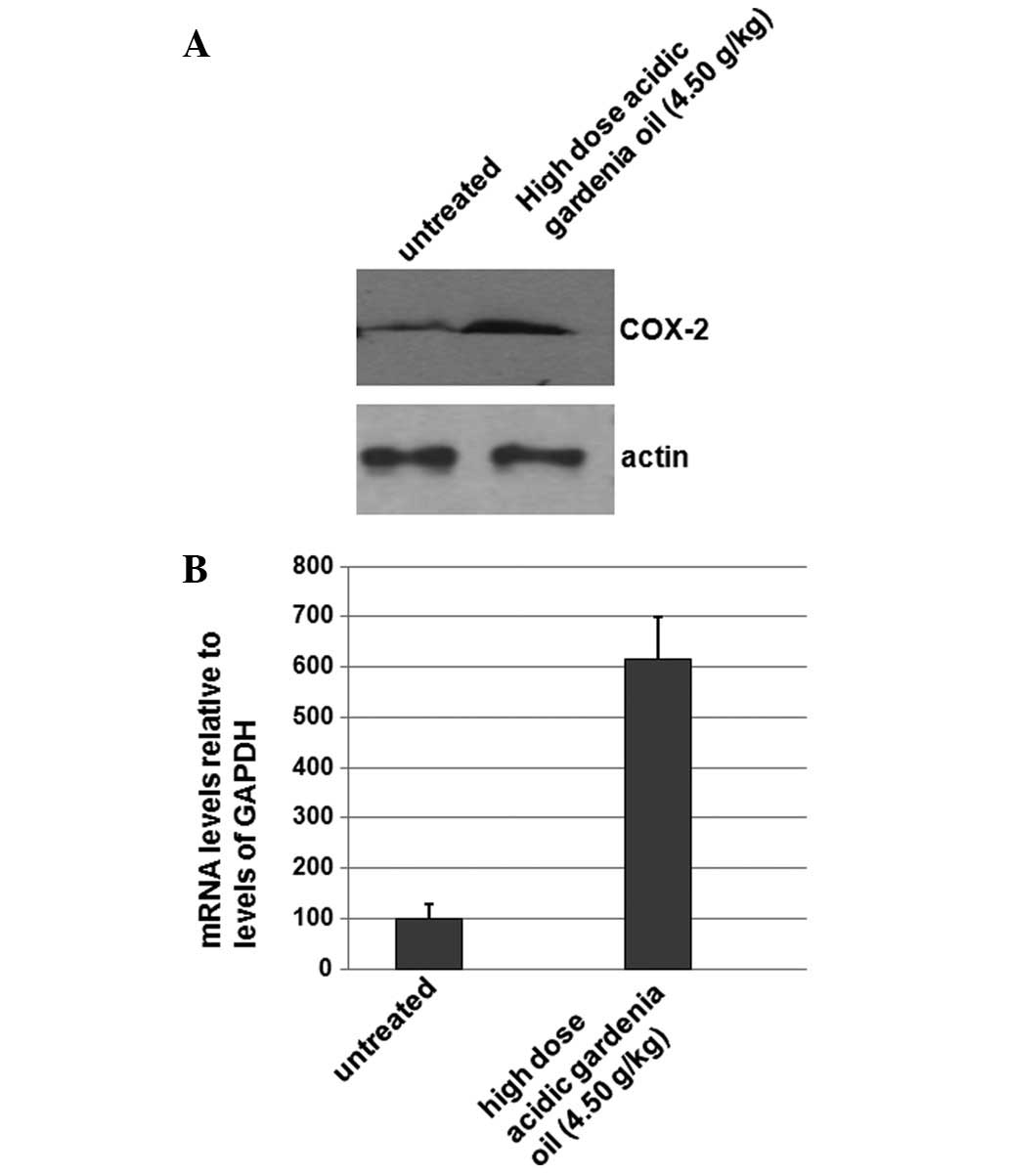
To determine if gardenia oil affects the expression
levels of COX-2, the total proteins were isolated from the
untreated control group and the gardenia oil group. Western
blotting was performed; as shown in Fig. 2A, the expression levels of COX-2
were significantly increased in the gardenia oil group when
compared with the levels in the untreated control group. The levels
of β-actin were used as a loading control. The results shown in
Fig. 2A indicate that the
expression levels of COX-2 were increased in the gardenia oil
group, which may be associated with its effects on BMD.
Levels of COX-2 mRNA are significantly
increased in the high dose gardenia oil treatment group
To determine if gardenia oil affects the mRNA levels
of COX-2 gene, the total RNAs were isolated from the
untreated control and the gardenia oil group. qPCR was performed to
detect the levels of COX-2 mRNA. As shown in Fig. 2B, the mRNA levels of COX-2
were significantly increased ∼6-fold in the gardenia oil group when
compared with the levels in the untreated control group. The
results shown in Fig. 2B suggest
that gardenia oil may increase levels of COX-2 mRNA.
Discussion
In this study, through the determination of bone
density and bone biomechanics, it was observed that gardenia oil
increases BMD and the maximum stress and maximum strain of bones in
ovariectomized female rats. BMD and the maximum stress and maximum
strain of bones are closely associated with the quality and
microstructural integrity of bones and osteoporosis.
In the prevention and treatment of osteoporosis,
medicines derived from plants have been widely used because of
their relatively minor side-effects. Gardenia oil contains oleic
acid, linoleic acid and linolenic acid (10). Oleic acid and linoleic acid may
generate γ-linolenic acid, arachidonic acid and prostaglandins. The
α-linolenic acid exists mainly as EPA and DHA in the body (15,16).
In the current study, we demonstrated that gardenia oil may
increase estrogen levels due to its effects on COX-2 expression.
Gardenia oil reduces the serum ALP level and increases levels of
calcium and phosphorus, thereby attenuating osteoporosis. It has
been reported that ω-3 polyunsaturated fatty acids derived from
oleic acids, linoleic acids and linolenic acids may reduce the rate
of postmenopausal bone loss (17–19).
The mechanism of action of gardenia oil in the
regulation of estrogen levels is unclear. However, in the current
study, we observed that the mRNA levels of COX-2 were
significantly increased ∼6-fold in the gardenia oil group when
compared with the levels in the untreated control group. The
results indicate that gardenia oil increases the levels of
COX-2 mRNA. The expression levels of COX-2 protein were
significantly increased in the high dose gardenia oil group when
compared with the levels in the untreated control group. These
results demonstrate that the expression level of COX-2 was
increased in the gardenia oil group, which may be associated with
its effects on BMD. By increasing the level of COX-2 expression,
gardenia oil may induce metabolic changes, such as promoting the
ovaries and other glands to secrete estrogen (20).
References
|
1.
|
Chiang SS and Pan TM: Beneficial effects
of phytoestrogens and their metabolites produced by intestinal
microflora on bone health. Appl Microbiol Biotechnol. 97:1489–1500.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tyagi AM, Srivastava K, Singh AK, Kumar A,
Changkija B, Pandey R, Lahiri S, Nagar GK, Yadav DK, Maurya R,
Trivedi R and Singh D: Formononetin reverses established osteopenia
in adult ovariectomized rats. Menopause. 19:856–863. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Schilling T, Ebert R, Raaijmakers N,
Schütze N and Jakob F: Effects of phytoestrogens and other
plant-derived compounds on mesenchymal stem cells, bone maintenance
and regeneration. J Steroid Biochem Mol Biol. Dec 20–2012.(Epub
ahead of print).
|
|
4.
|
Dagdemir A, Durif J, Ngollo M, Bignon YJ
and Bernard-Gallon D: Breast cancer: mechanisms involved in action
of phytoestrogens and epigenetic changes. In Vivo. 27:1–9.
2013.PubMed/NCBI
|
|
5.
|
Biggar RJ: Molecular pathways: digoxin use
and estrogen-sensitive cancers - risks and possible therapeutic
implications. Clin Cancer Res. 18:2133–2137. 2012. View Article : Google Scholar
|
|
6.
|
Di Vito C, Bertoni A, Nalin M, Sampietro
S, Zanfa M and Sinigaglia F: The phytoestrogen 8-prenylnaringenin
inhibits agonist-dependent activation of human platelets. Biochim
Biophys Acta. 1820:1724–1733. 2012.PubMed/NCBI
|
|
7.
|
Cao PC, Lei W and Gao YL: Effects of
Crocus sativus on bone mineral density and biochemistry
markers of bone metabolism in ovariectomized rats. Progress Modern
Biomed. 11:1009–1012. 2011.(In Chinese).
|
|
8.
|
Somjen D, Katzburg S, Sharon O, Knoll E
and Stern N: Estrogens and hyperglycemic modulation of mRNAs
expressions involved in bone metabolism: An overshadowed
association? Connect Tissue Res. 54:176–180. 2013. View Article : Google Scholar
|
|
9.
|
Walsh JS and Eastell R: Role of estrogen
in the age-related decline in bone microstructure. J Clin
Endocrinol Metab. 98:519–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Li BL, Fu ZY, Chen YH, Gao F and Zhang ZX:
Influence of soft capsule of compound oil of jujube, arboruitae and
gardenia on learning and memory, and brain NO, Ach content of
castrated rats. Chin J Appl Physiol. 28:403–404. 2012.PubMed/NCBI
|
|
11.
|
Lee SJ, Oh PS and Lim KT: Hepatoprotective
and hypolipidaemic effects of glycoprotein isolated from
Gardenia jasminoides ellis in mice. Clin Exp Pharmacol
Physiol. 33:925–933. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM,
Kim SJ, Kim BC, Jin C, Lim CJ and Park EH: Antiinflammatory effects
of genipin, an active principle of gardenia. Eur J Pharmacol.
495:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yuan L, Jiang R, Yang Y, Ding S and Deng
H: 1,25-Dihydroxyvitamin D3 inhibits growth of the
breast cancer cell line MCF-7 and downregulates cytochrome P4501B1
through the COX-2/PGE2 pathway. Oncol Rep. 28:2131–2137. 2012.
|
|
14.
|
Mukawa K, Fujii S, Tominaga K, et al:
Inhibitory effects of the cyclooxygenase-2 inhibitor, etodolac, on
colitis-associated tumorigenesis in p53-deficient mice treated with
dextran sulfate sodium. Oncol Rep. 19:393–399. 2008.PubMed/NCBI
|
|
15.
|
Gao F, Fu ZY and Li BL: A study on the
effects of compound oil of semen spinosa, semen orientalis, and
jasminoides on soothing nerves and improving mental capacities in
mice. Chin J Appl Physiol. 27:240–245. 2011.PubMed/NCBI
|
|
16.
|
Zhou TY, Ren F, Deng L, et al: Research
progress in physiological and biochemical function of γ-linolenic
acid. Guizhou Agricultural Sciences. 39(3): 53–58. 2011.(In
Chinese).
|
|
17.
|
Xu P, Ma R, Chang XX, et al: Effects of
ω-3 polyunsaturated fatty acids on bone biomechanics of
ovariectomized rats. Parenteral & Enteral Nutrition. 16:96–99.
2009.(In Chinese).
|
|
18.
|
Kettler DB: Can manipulation of the ratios
of essential fatty acids slow the rapid rate of postmenopausal bone
loss? Altern Med Rev. 6:61–77. 2001.PubMed/NCBI
|
|
19.
|
Song LL: Regulation of PUFA in the diet to
bone cell function. Progress in Modern Biomedicine. 6:55–57.
2006.(In Chinese).
|
|
20.
|
Fu ZY, Wang YP and Chen Y: Observation of
insulin exocytosis by a pancreatic β cell line with total internal
reflection fluorescence microscopy. Chin Med Sci J. 26:60–63.
2011.
|