Introduction
Adult-onset hypophosphatemic vitamin D-resistant
osteomalacia (AHVDRO) is a group of diseases characterized mainly
by poor bone mineralization, osteomalacia or rickets (caused by
hypophosphatemia) and insufficient active vitamin D production.
There are three forms of AHVDRO: X-linked hypophosphatemic
rickets/osteomalacia (XLH), autosomal dominant hypophosphatemic
rickets (ADHR) and tumor-induced osteomalacia (TIO). Previous
studies have investigated other factors that may contribute to the
development of hypophosphatemia-associated osteomalacia, such as
vitamin D receptor resistance. In the current study we present a
patient with bone pain associated with multiple fractures. Large
doses of neutral phosphate preparations, vitamin D3 and calcium
improved the patient's symptoms. For bone pain associated with
multiple fractures in elderly patients, calcium and phosphorus
metabolism disorders and active vitamin D deficiency should be
considered and an early diagnosis should be established. The
presetn study was approved by the Ethics Committee of Jinan
Military General Hospital, Jinan, China.
Case report
A 59-year-old female was admitted to Jinan Military
General Hospital (Jinan, China) on March 3, 2010 after experiencing
sternocostal, back and bilateral groin pain, and progressive
lower-extremity weakness for more than 2 years. The cause of the
pain was not evident initially. The patient described the pain as
being intermittent and dull. Gradually, both lower extremities
became weaker, weakness and pain were aggravated following exercise
and labor and relieved by rest, and the condition worsened.
However, the patient did not present morning stiffness, low fever
or night sweats, and the extent of pain was not affected by weather
changes. At 1 year prior to admission, magnetic resonance imaging
(MRI) of the lumbar vertebrae had been performed on the patient,
which revealed L3/4 and L4/5 disk herniation. MRI of the thoracic
vertebra showed ligamentum flavum hypertrophy and calcification at
the T6/7 vertebral level, resulting in spinal cord compression. A
conservative treatment regimen was initiated, but there was no
improvement in the severity of the symptoms. The patient had
increasing difficulty rolling over and getting up, and upon
hospitalization, the patient was not able to walk independently.
Thus, the patient was admitted to the Department of Osteopathy,
Jinan Military General Hospital for thoracic spinal stenosis.
Osteopathic surgeons considered that the cause of
the clinical manifestations was unlikely to be thoracic spinal
stenosis and tests on anti-neuron antibodies [anti-Hu, 70.080 ng/ml
(normal, <76.8 ng/ml); anti-Ri, 31.7412 ng/ml (normal, <26.5
ng/ml) and anti-Yo, 16.598 ng/ml (normal, <18.0 ng/ml)] showed
that the concentrations of anti-Hu and anti-Ri were significantly
increased above normal levels. Therefore, the patient was diagnosed
with suspected paraneoplastic syndrome and was transferred to the
Department of Neurology, Jinan Military General Hospital. Following
admission, heart, lung and abdominal examinations showed no
significant abnormities. Neurological examinations showed that the
patient had full consciousness and fluent speech, cranial nerve
examination was normal, double upper-limb myodynamia classified as
Grade V, double lower-limb distal myodynamia classified as Grade
III- and proximal myodynamia classified at Grade V-. The muscle
tone of all four limbs was normal, the double upper-limb tendon
reflex was symmetrical and normal, the double lower-limb tendon
reflex was symmetrical and reduced, and the finger-to-nose test was
performed stably and accurately. However, the heel-knee-tibia test
on the two lower limbs could not be completed. Sensation
examination showed no significant abnormities, the test for
pathological reflexes was negative, the neck was soft and flexible,
and meningeal irritation signs were negative. The pressing pain at
the sternum, multiple ribs, bilateral hip joints and groin was
significant. Since the onset of the illness, drinking, eating,
urination and defecation were normal, and the body weight had not
reduced significantly. Past medical history and family history
revealed nothing of note. The results of blood examinations were
normal (red blood cells, 3.31×1012/l; hemoglobin, 95
g/l; and platelets, 568×109/l). The alkaline phosphatase
level was elevated [339 U/l (normal, 26–150 U/l)], the blood
phosphorus level was reduced [0.64 mmol/l (normal, 0.80–1.60
mmol/l)] and the calcium [2.22 mmol/l (normal, 2.10–2.60 mmol/l)]
and magnesium concentrations were normal. The levels of
tumor-marker antibodies [sialic acid: 82.1 mg/dl (normal, 45.6–75.4
mg/dl), carcinoembryonic antigen (CEA), α-fetoprotein (AFP),
carbohydrate antigen 72-4 (CA72-4), carbohydrate antigen 12-5
(CA12-5), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen
15-3 (CA15-3), cytokeratin 19 fragment (CY21-1) and neuron-specific
enolase (NSE)] were within the normal range, as were the
erythrocyte sedimentation rate (31 mm/h), serum protein
electrophoresis, blood parathormone level [21.55 ng/l (normal,
15–65 ng/l)] and albumin levels. Results from the 5-item thyroid
function test [thyroid stimulating hormone, (TSH); free
triiodothyronine (FT3); free thyroxine (FT4); thyroid peroxidase
antibodies (TPO); and thyroglobulin antibody (TGAb)], 6-item sex
hormone test (estradiol, progesterone, testosterone, prolactin,
luteinizing hormone and follicle stimulating hormone), 5-item
hepatitis B and C virus tests and HIV antibody test did not show
any anomalies, and the Bence Jones urinary protein test was
negative. Bone marrow puncture, gynecological ultrasonography,
abdominal ultrasonography, echocardiography and double hip-joint
computed tomography (CT) scan showed no obvious abnormalities.
Electromyography showed no characteristic changes and the
repetition-frequency stimulation test showed no significant
alternation. Pulmonary CT demonstrated an increase in double lung
markings, the presence of pleural thickening and calcification in
the right liver lobe. Thyroid color ultrasonography revealed
multiple thyroid nodules, localized neoplasia, locally coexisting
Hashimoto's disease, the nature of left nodule was undetermined,
parathyroid lesions could not be excluded and cervical lymph nodes
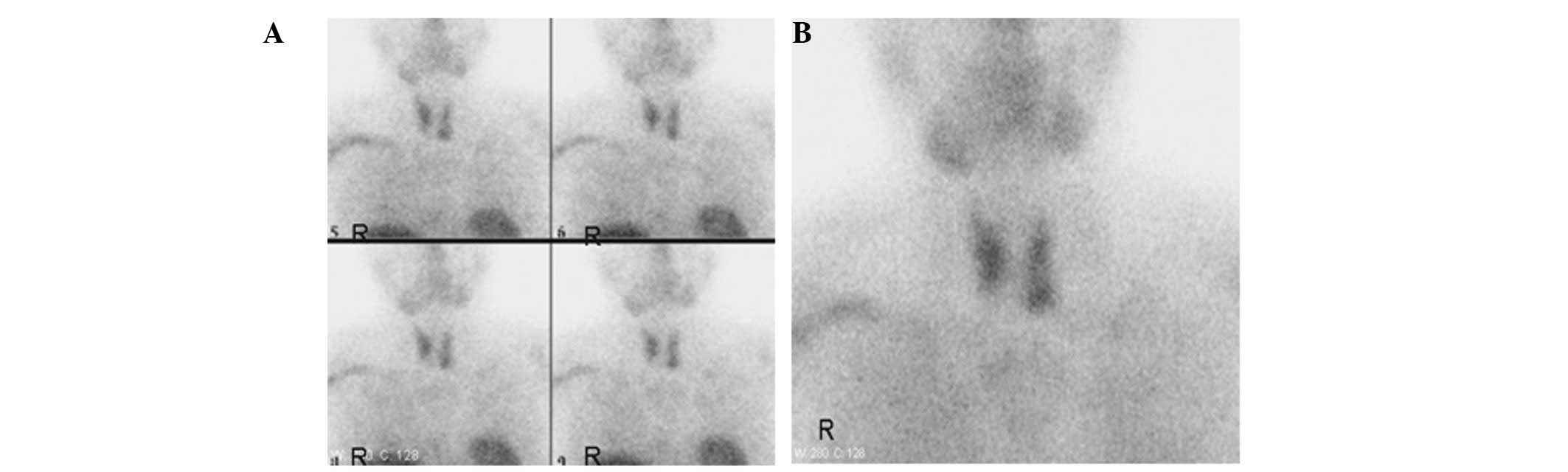
were swollen. No significant abnormal nuclide intake was
demonstrated following bilateral parathyroid ECT examination
(Fig. 1) and clinical tests
repeatedly showed that the parathormone and 24-h urine phosphorus
and urine calcium levels were normal. Therefore, a diagnosis of a
parathyroid disease was not considered.
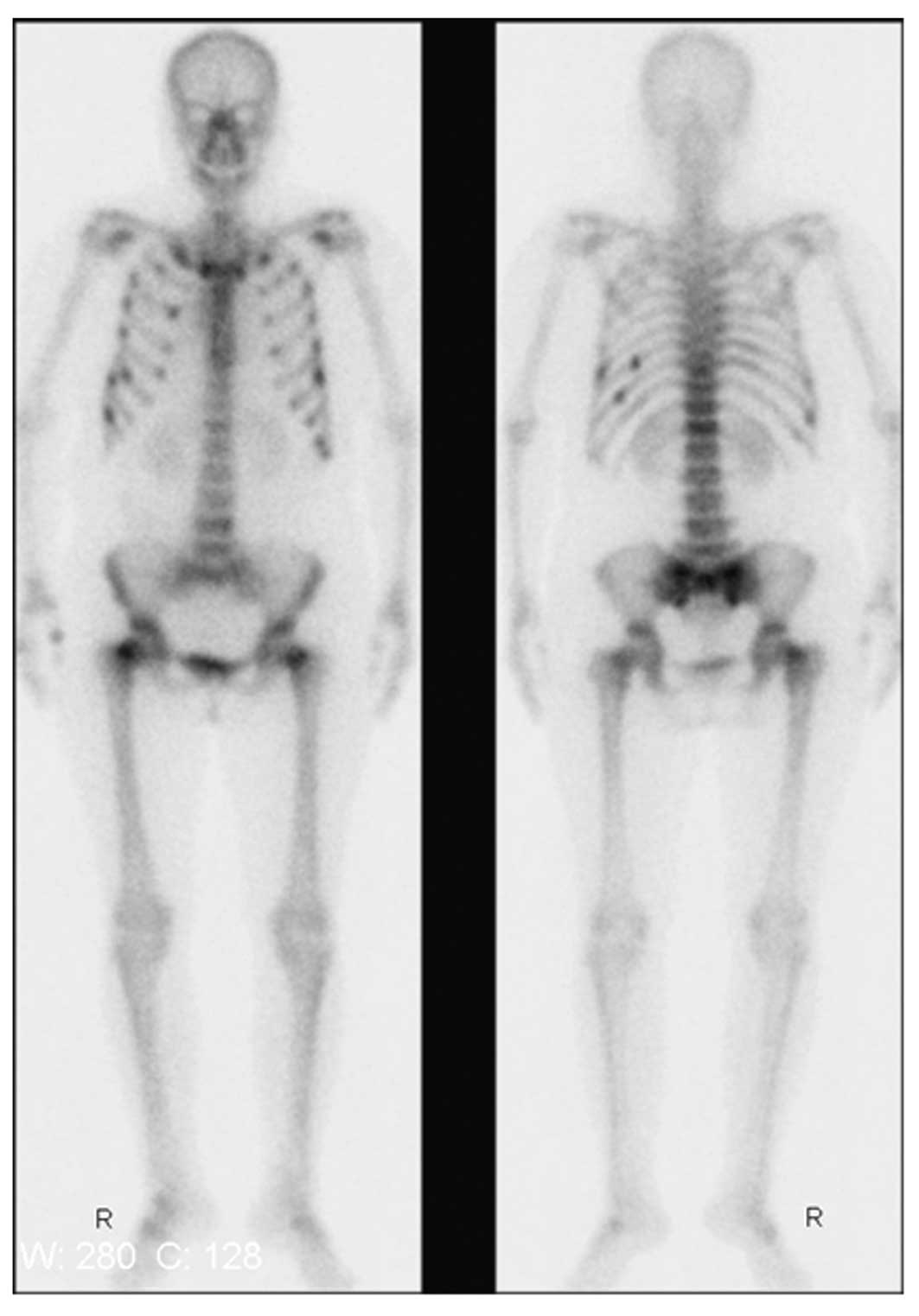
Whole-body bone emission computed tomography (ECT;
via the intravenous administration of 99 mTc - MDP)
showed that nuclide intake was increased in several locations
(Fig. 2), and the cause was
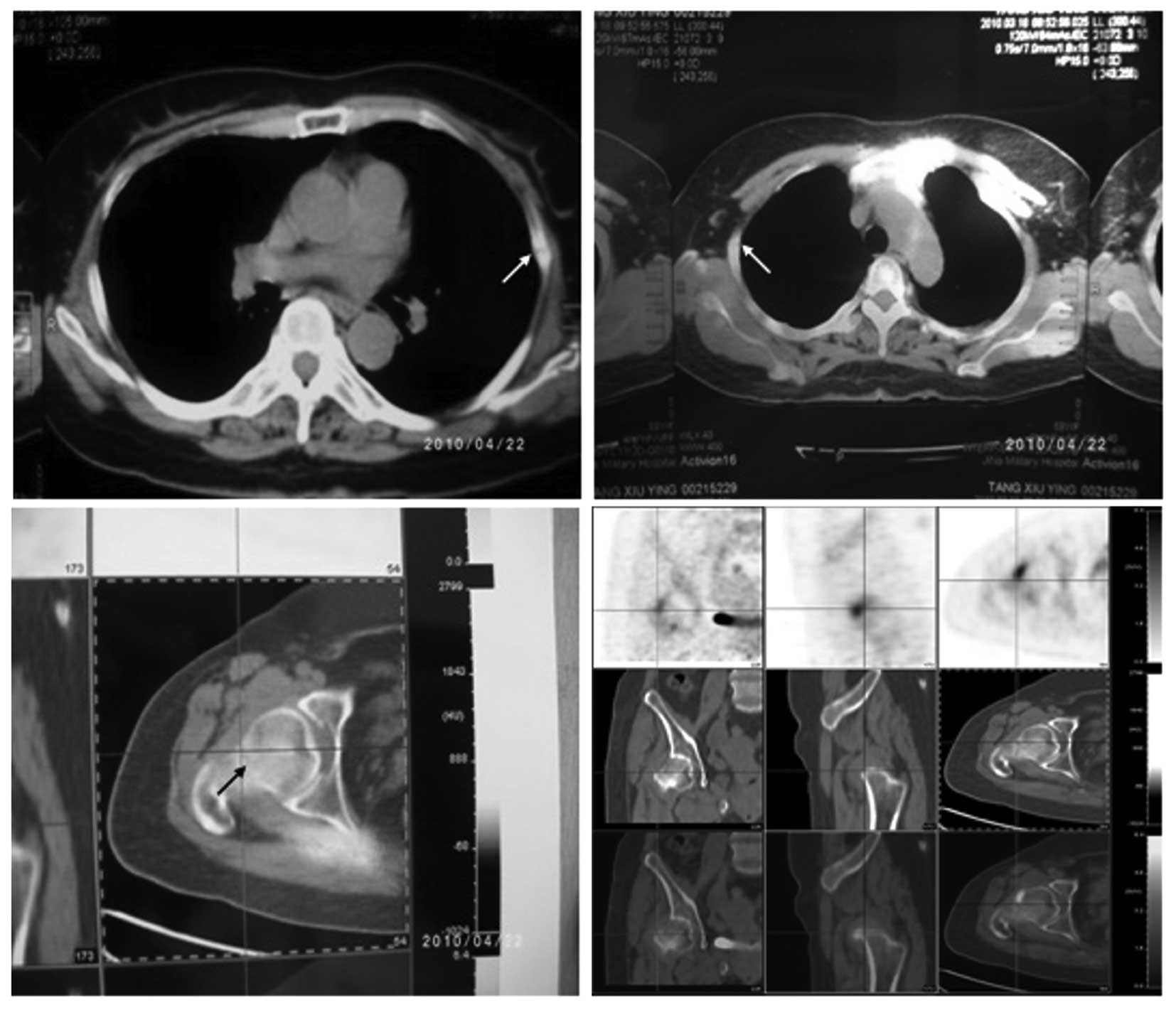
considered to be malignant. Whole-body positron-emission tomography
CT (PET-CT) examination (Fig. 3)
showed that several ribs and double femoral neck (incompletely) had
discontinuity and numerous old fracture lines (loose lines) were
present, as well as mild T12 compression changes. Local
fluorodeoxyglucose (FDG) metabolism increased in the cervical
vertebra-2 accessory bone, but did not increase in the regions of
reduced local density of the left thyroid gland, which were
considered to be thyroid nodules. Bilateral hip joint swelling was
accompanied by an increase in FDG metabolism and these regions were
classified as inflammatory hip lesions. Bone mineral density tests
showed that left forearm bone density had reduced by 17.96%
[T-score: −1.43 (−1>T-score >−2.5 is suggestive of bone
loss)]. The results of blood phosphorus tests (0.48–0.64 mmol/l)
led to the clinical consideration that the patient may be diagnosed
with hypophosphatemic osteomalacia. However, re-examination of the
patient following the administration of sodium glycerophosphate and
Rocaltrol® (Roche, Shanghai, China) demonstrated that
the treatment was ineffective (the blood phosphorus concentration
did not increase). Tests of blood from the right subclavian vein
indicated that the concentration of fibroblast growth factor
(FGF)-23 was 32.44 ng/l (normal, 40–90 ng/l).
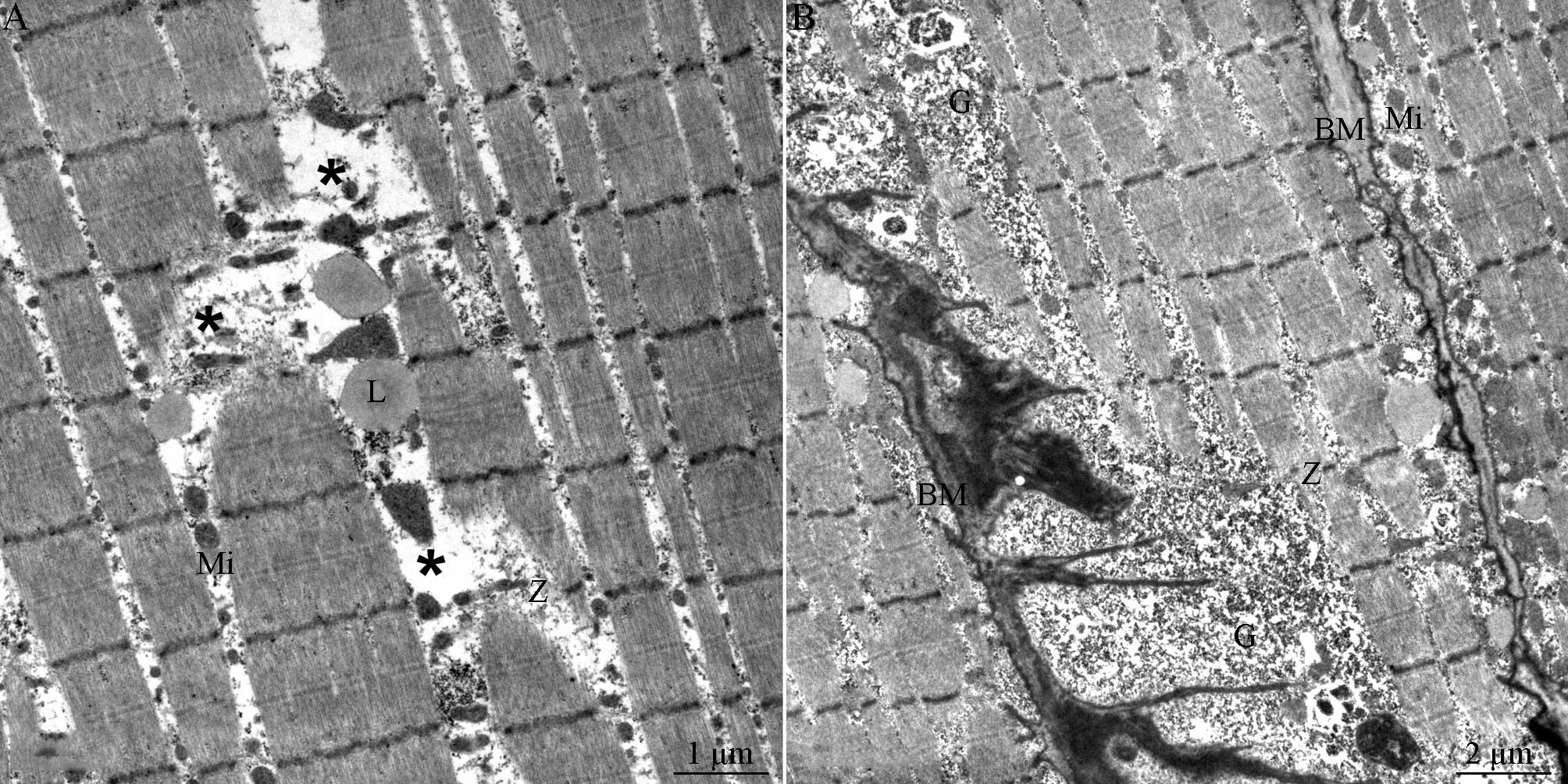
To ensure a definitive diagnosis, a tibialis
anterior muscle biopsy was conducted on March 27, 2010 (Fig. 4). Single or small angular atrophic
muscle fibers were apparent but no necrotic muscle fibers or
inflammatory cell infiltration were observed (Fig. 4A and B). The proportion of the two
types of muscle fibers was approximately normal, although there was
some clustering of the muscle fiber types and the atrophic muscle
mainly comprises type I fibers (Fig.
4C). Gomori staining (Fig. 4D and
E) and Oil Red O staining (Fig.
4F) showed no abnormalities. Electron microscopy results
(Fig. 5) showed fractured or
missing muscle fibers and the presence of lipid grains between the
fractured muscle fibers. The muscle fibers had a sparse transverse
arrangement and relatively few mitochondria. In addition, an
accumulation of glycogenosomes was observed beneath the muscle cell
membrane.
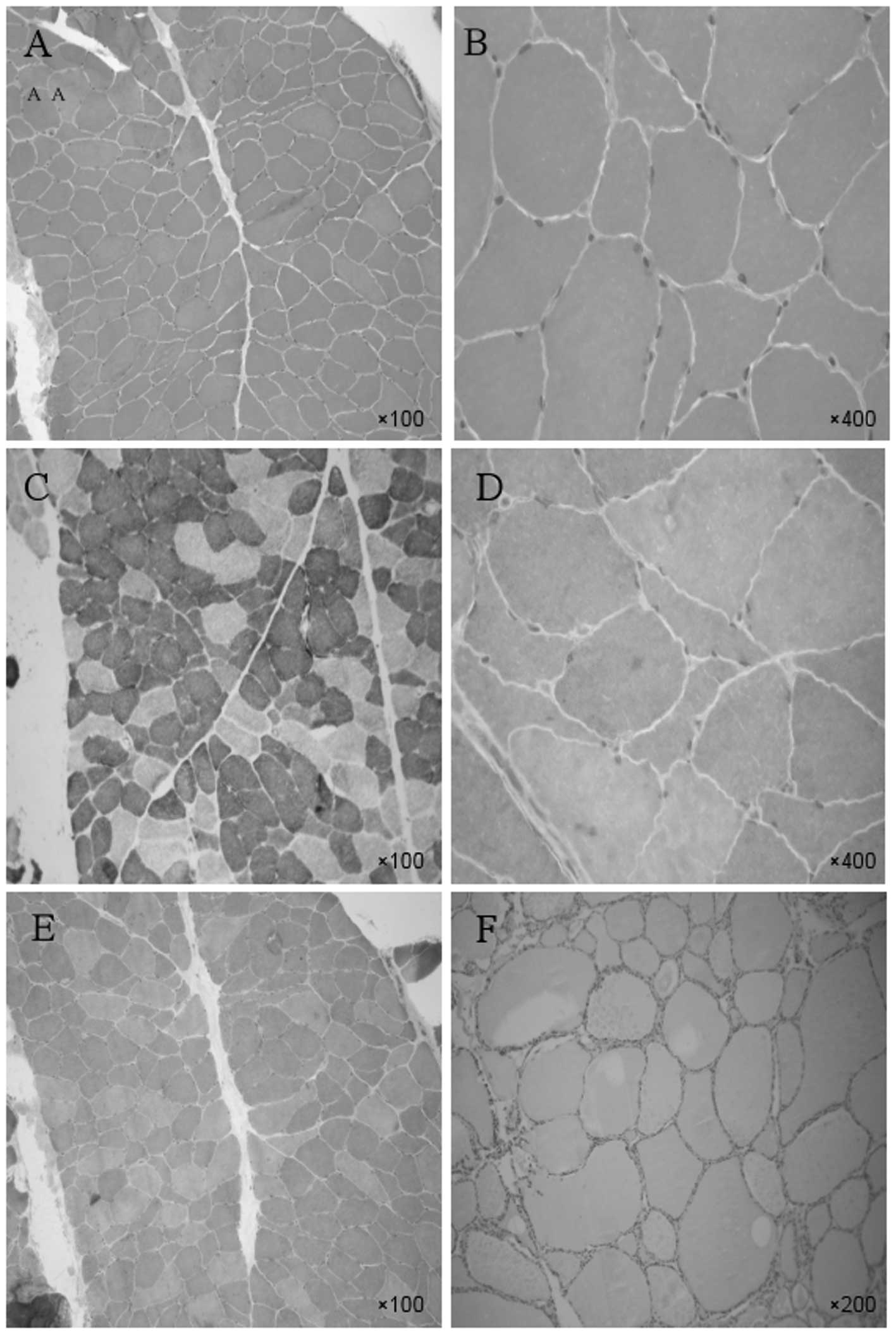 | Figure 4Tibialis anterior muscle biopsy.
H&E staining: Single or small angular atrophic muscle fibers
were apparent but no necrotic muscle fibers or inflammatory
cellular infiltrations were observed at (A) magnification, ×100 or
(B) magnification, ×400. (C) Nicotinamide adenine dinucleotide
tetrazolium oxidoreductase (NADH-TR) staining: The proportions of
the two types of muscle fibers were approximately normal, although
they were moderately distributed in groups according to muscle
fiber type, and the atrophic muscle mainly comprised type I fibers
(magnification, ×100). MGT staining (D) magnification, ×400 and (E)
magnification, ×100, and (F) Oil Red O staining, magnification,
×200, showed no abnormalities. H&E, hematoxyin and eosin. |
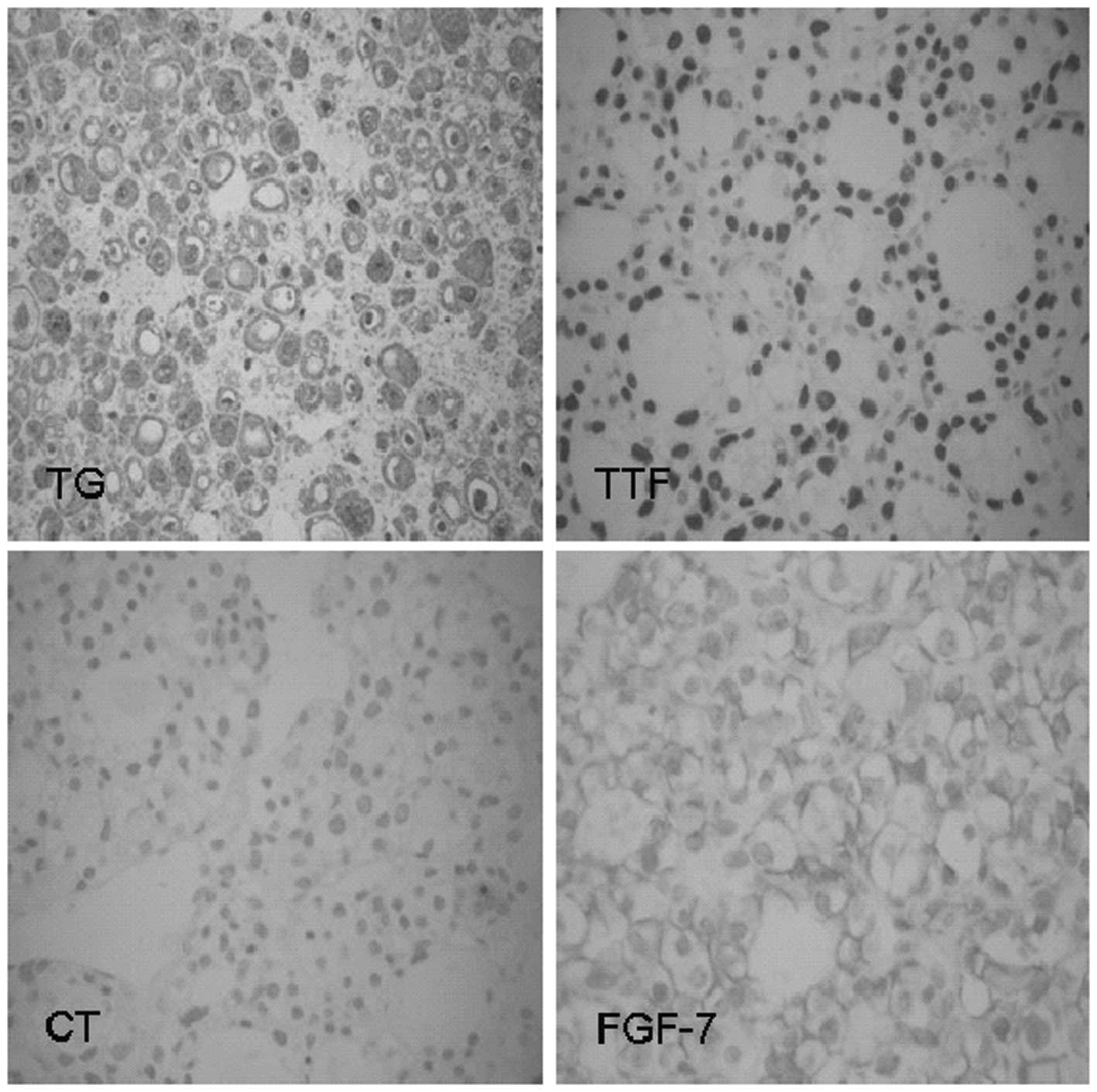
On April 16, 2010, a thyroid nodulectomy was
performed to exclude TIO as the cause of the symptoms. The
postoperative pathological results showed nodular goiter in the
left lobe. Partial follicular lesions had adenomatous hyperplasia,
with abundant cells and active growth. The results of
immunohistochemical staining (Fig.
6) were positive for thyroid transcription factor-1 (TTF-1),
CK8/18, FGF-7 and thyroglobulin (TG), and negative for calcitonin
(CT). At 1 week after the surgery, although the sternal pain had
been alleviated, the patient continued to experience significant
pain in the bilateral rib arch, back and groin, and was unable to
roll over, get up or walk. A re-examination was performed on May
20, 2010 and the whole-body bone imaging ECT results showed no
significant changes. The results of a postoperative re-examination
were as follows; anti-Hu, anti-Ri and anti-Yo antibody levels were
normal, the erythrocyte sedimentation rate was 25 mm/h,
post-operative continuous phosphorus supplementation was
ineffective (phosphorus: 0.48–0.56 mmol/l), calcium and magnesium
levels were normal, and the 24-h urine calcium and urine phosphorus
concentrations were normal. Following thyroid tumor resection, the
condition of the patient did not improve significantly, therefore,
a diagnosis of TIO was not considered. The vitamin
1,25-(OH)2D3 concentration was significantly lower than
normal [<4.00 ng/ml (normal concentration: ≥30.00 ng/ml)], which
was considered to be caused by hypophosphatemic vitamin D-resistant
osteomalacia which was subsequently treated with neutral phosphate
preparations (73.1 g disodium hydrogen phosphate and 6.4 g
potassium dihydrogen phosphate in an aqueous solution of final
volume 1,000 ml). Every 4 h, 20 ml of the preparation was
administered orally (5 times daily), in addition to a 1-ml vitamin
D3 intramuscular injection once every 2 weeks (or oral vitamin D3
tablets and calcium). The condition of the patient improved
gradually, and after 2 months, the sternocostal, back and groin
pain was relieved significantly. The phosphorus concentration at
the 6-month follow-up visit was 0.79 mmol/l, and had increased to
0.98 mmol/l at the 1-year follow-up. The whole-body pain had ceased
and the patient was able to roll over, get up alone and walk slowly
with assistance. When walking, the patient continued to experience
weakness in both legs, but to a lesser extent than previously.
Discussion
The patient had no relevant family history to aid in
diagnosis confirmation and therefore, it was considered a sporadic
case. ADHR and XLH were excluded as possible causes, and a
diagnosis of TIO was considered initially. TIO is a rare
paraneoplastic syndrome, an acquired form of hypophosphatemic
osteomalacia caused by the excessive secretion of
phosphorus-regulating factors and an increase in renal phosphorus
excretion. In 1980, China reported the first case of TIO caused by
groin mesenchymoma (1). To date,
fewer than 200 cases have been reported worldwide. TIO has several
typical clinical manifestations. First, bone pain develops
progressively, mainly in the arms and legs and at weight-bearing
joints. Second, the excretion of phosphorus in the urine increases
significantly and blood phosphorus levels are significantly
reduced, while blood calcium levels remain normal. Third, the
administration of conventional phosphorus and vitamin D supplements
has almost no effect; high-dose phosphorus and vitamin D
supplementation is usually required. Fourth, alkaline phosphatase
levels increase in the blood. Fifth, vitamin
1,25-(OH)2D3 levels decrease; in certain cases, this is
accompanied by secondary hyperparathyroidism. Finally, a relevant
tumor is usually present and the determinant of TIO, although it is
often hidden and of small size. Therefore, following tumor
resection, symptoms such as blood phosphorus levels may rapidly and
significantly improve. Radiographs of patients with TIO typically
show reduced bone densities, as well as vague deformations in the
bone trabecula, pelvis and vertebra, and bone fractures or
pseudo-fracture formations are present in numerous cases (2). In the current case, following thyroid
nodulectomy, no significant improvement of the clinical symptoms
was observed and blood phosphorus levels did not increase
significantly. Therefore, a diagnosis of TIO was not made.
Studies have shown that FGF-23 is associated with
the onset of hypophosphatemic osteomalacia; it is a known
phosphorus-regulating factor (3,4),
with a normal level of ~10–50 ng/l. The tumor in TIO patients may
express and secrete large quantities of FGF-23 (5), which reduces renal phosphate
reabsorption and increases the amount of phosphorus excreted in the
urine (6). In addition, an
increase in FGF-23 concentrations may inhibit the production and
activity of 1-α hydroxylase, thereby reducing the production of
1,25-(OH)2D3 and phosphorus. Following tumor resection,
FGF-23 levels may quickly decrease (7). As test results showed that FGF-23
concentrations were normal in this patient, a diagnosis of TIO was
not supported. Therefore, since calcium levels were normal,
phosphorus levels were reduced, alkaline phosphatase levels were
increased, urine phosphorus concentrations were normal or
increased, and there was no medical history of deficiency in
vitamin D, azotemia or other renal tubular function insufficiency,
a diagnosis of hypophosphatemic vitamin D-resistant osteomalacia
(AHVDRO) was considered. The treatment of this disease should
initially target the cause and if a tumor is discovered to be the
cause, resection may reduce the severity of the disease and
biochemical indicators may be completely restored to normal levels
(8). If it is not possible to
discover or eliminate the cause, symptomatic treatment should be
adopted, including the administration of high-dose active vitamin D
and phosphorus supplements, as well as calcium supplements.
Notably, lifelong treatment is required. Phosphorus should be
supplemented every 4–6 h since it is rapidly excreted. During
treatment, it is important to re-examine calcium and phosphorus
levels regularly to avoid vitamin D intoxication. The prognosis is
closely associated with the degree of bone density change (9). In the current study, following
treatment, the pain of the patient was alleviated. However,
considering the long course of the disease and the severe
destruction of the bones, the clinical symptoms are unlikely to be
eased completely. Therefore, the early detection, diagnosis and
treatment of AHVDRO are likely to significantly improve its
prognosis.
References
|
1
|
Zhang XQ: A case report: mesenchymoma
accompanied by vitamin D-resistant osteomalacia. Chin Med J.
60:150–152. 1980.
|
|
2
|
Zura RD, Minasi JS and Kahler DM:
Tumor-induced osteomalacia and symptomatic looser zones secondary
to mesenchymal chondrosarcoma. J Surg Oncol. 71:58–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murer H, Hernando N, Forster I and Biber
J: Proximal tubular phosphate reabsorption: molecular mechanisms.
Physiol Rev. 80:1373–1409. 2000.PubMed/NCBI
|
|
4
|
Schiavi SC and Kumar R: The phosphatonin
pathway: New insights in phosphate homeostasis. Kidney Int.
65:1–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimada T, Mizutani S, Muto T, et al:
Cloning and characterization of FGF23 as a causative factor of
tumor-induced osteomalacia. Proc Natl Acad Sci USA. 98:6500–6505.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Müller M, Biedermann M and Strecker W: A
complication during kyphoplasty. Cement penetration through the
azygos vein into the superior vena cava. Orthopade. 35:1183–1186.
2006.(In German).
|
|
7
|
Kobayashi K, Nakao K, Kawai K, et al:
Tumor-induced osteomalacia originating from the temporal bone: a
case report. Head Neck. 33:1072–1075. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harbeck B, Schöcklmann H, Seekamp A, Czech
N and Mönig H: Tumor-induced osteomalacia: successful treatment by
radio-guided tumor surgery. J Clin Rheumatol. 15:31–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Lu CY and Wang T: A case of
hypophosphatemic osteomalacia and literature view. Chin J
Osteoporosis Bone Miner Res. 3:203–206. 2009.
|