Introduction
Wound problems commonly occur in clinical practice;
∼l% of the global population suffers from persistent wound
problems, and 5% of total medical costs are spent on wound
treatments (1,2). Accurate wound treatment is required
to solve this problem effectively, and such treatment relies on a
better understanding of the wound healing mechanism. However, wound
healing is a complex biological process. Different types of wounds
have different healing processes and the mechanisms have not yet
been fully elucidated. The exchange of signals among cells,
transmembrane conductance and intracellular signal transduction are
the potential physical mechanisms controlling the process. As a
result, signal transduction mechanisms in wound healing have been
the focus of the majority of studies. Witherden et al
(3) observed an increased
coxsackievirus and adenovirus receptor (CAR) expression near the
keratinised cells in a mouse skin wound biopsy. It was demonstrated
that CAR was involved in a cell-to-cell contact signalling pathway
that resulted in an increase in the number of local cell growth
factors and inflammatory mediators, thereby promoting wound
healing. These results indicated that CAR functions as a signalling
molecule in skin wound biopsies.
CAR is a 46 kDa type I transmembrane glycoprotein
belonging to the immunoglobulin superfamily (4). In mice, CAR is mainly distributed in
the heart, liver, brain, kidneys and lungs, with the highest
expression level observed in the liver (5–7).
Numerous studies have shown that CAR significantly functions as an
important adhesion protein and molecule in viral infection
(8–11) and the tumour development process
(12–16). The correlation between CAR and
wound healing was first described by Witherden et al
(3). However, only the changes in
CAR expression in the keratinocytes of skin wound biopsies have
been observed; no other similar observations have been described
for burn wounds, a different form of wound from a skin wound
biopsy. To determine whether CAR exhibited similar expression
changes in burn wounds, in vitro mouse and cellular
experiments were performed in the present study. Such experiments
were also conducted to observe the effects of the changes in CAR
expression in thermally stimulated mouse skin keratinocytes. In
addition, the function of CAR in the burn wound healing process was
elucidated.
Materials and methods
Establishment of animal models
Twenty BALB/c mice (age, 6–8 weeks; 10 males/10
females; weight, 20–25 g; Academy of Military Medical Sciences,
Beijing, China) were randomly divided into two groups. The mice
remained awake while their backs were shaved. Following this, the
depilated areas of one group (the scald group) were treated with
hot water gauzes heated at 100°C for 1 to 3 sec. The other group
(the sham heat or control group) was treated with humidity gauzes
heated at room temperature. Six hours later, all the mice were
sacrificed, and specimens of back skin were obtained for the
subsequent experiment. This study was performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of the 309th Hospital of the Chinese
PLA (Beijing, China).
Keratinocyte culture and thermal
stimulation model
The dorsal skin from BALB/c foetal mice (gestational
age, 20 days) was washed with D-Hanks solution and cut into strips
(0.3×1 cm). The skin was added to 1.25 U/ml dispase II solution
(Roche, Basel Switzerland) and digested at 4°C for 24 h. Following
this, the epidermis and dermis were separated, and the epidermis
was cut in pieces, added to 1.25 μ/ml dispase II solution at
37°C and digested for 30 min. All of the tissue fragments were
removed via a mesh filter, while the remaining cells were collected
by centrifugation and cultured in Epilife medium (Invitrogen Life
Technologies, Carlsbad, CA, USA). After 3 to 4 days, the fused
cells were digested and passaged. The third generation of cells was
used and divided into two groups for the subsequent experiments.
The first group (the normal control group) was cultured at 37°C in
5% CO2 for 1 h; the other group (the heat stress group)
was cultured at 42°C in 5% CO2 for 1 h. The two groups
of cells were subsequently cultured under normal conditions for 6 h
and then harvested.
Immunohistochemistry
Following the dewaxing and hydration of the paraffin
sections of the back skin samples, the antigens were retrieved by
citric acid heating for 10 min. Endogenous catalases were blocked
using freshly prepared 2% ddH2O at room temperature for
20 min, and non-specific binding sites were blocked by horse serum
for 30 min. All the sections were incubated with a rabbit
anti-mouse CAR antibody (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) at 4°C overnight and incubated with a secondary
antibody (Santa Cruz Biotechnology, Inc.) at 37°C for 30 min.
Avidin/Biotin Complex (ABC) reagents (Vector Laboratories,
Burlingame, CA, USA) were added under the previous conditions.
Diaminobenzidine (DAB) colour reactions were viewed under a
microscope (5–10 min). The samples were dehydrated and sealed using
graded ethanol, dimethylbenzene and resin. In the blank control
group, the primary antibody was replaced with phosphate-buffered
saline (PBS). Under the microscope, a granular appearance on the
cell surface or a homogeneous brown reaction product was considered
to be a positive result.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from fresh skin specimens
using RNeasy Plus Mini kit (Qiagen, Hilden, Germany) according to
the manufacturer’s instructions. Total RNA (4 μg) was
subjected to reverse transcription using a transcriptor high
fidelity cDNA synthesis kit (Roche). Following this, total RNA,
random hexamer primer (2.0 μl) and ddH2O were
added to obtain a final volume of 11.4 μl. The samples
subsequently reacted at 65°C for 10 min and were placed immediately
in an ice bath. Following this, 4.0 μl 5X efficient reverse
transcription buffer, 0.5 μl RNase inhibitor, 2.0 μl
deoxynucleic acid mix, 1.0 μl dithiothreitol (DTT) and 1.1
μl efficient reverse transcription enzyme were added to
obtain a final volume of 20 μl. The following two reaction
phases were conducted at 50°C for 30 min and 85°C for 10 min. The
primer sequences were designed based on a GenBank (National Centre
for Biotechnology Information, Bethesda, MD, USA) query: for CAR,
forward primer, 5′-TACGAGTAACGATGTCAAGT-3′; reverse primer
5′-CCTGAAGGCTTAACAAGAAC-3′; for glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward primer 5′-TGAGTATGTCGTGGAGTC-3′;
reverse primer 5′-CAATCTTGAGTGAGTTGTCAT-3′. The standard curve was
then prepared. To detect the amplification efficiency of CAR and
GAPDH by qPCR using RealmasterMix (SYBR-Green; Tiangen
Biotechnology Co., Ltd., Beijing, China), the reverse transcription
product cDNA was diluted five times by a gradient (50,
51, 52, 53 and 54). The
reaction system comprised 1 μl cDNA, 9 μl 5X PCR
buffer, 0.4 μl forward/reverse primer and 9.2 μl
ddH2O. The reaction conditions were as follows: one
cycle of 95°C for 2 min; 94°C for 15 sec and 58°C for 10 sec; and
40 cycles of 68°C for 18 sec. The melting curve was analysed based
on the following parameters: one cycle of 95°C for 1 min; one cycle
of 55°C for 1 min; 57 cycles of 65–93°C, read board for 0.06 sec
once a 0.5°C increase in temperature occurred, and read board for
0.05 sec once a 20°C increase in temperature occurred. Three
duplicated wells and a negative control treatment with no template
were set.
Western blot analysis
Approximately 50 mg fresh skin specimens were
homogenised with radio-immunoprecipitation assay (RIPA) lysis
solution [10 mmol/l Tris HCl (pH 7.4), 150 mmol/l NaCl, 5 mmol/l
EDTA, 1% Triton-X, 50 mmol/l NaF, 0.2 mmol/l
Na3VO4, 1% sodium deoxycholate and complete
mini EDTA-free protease inhibitor cocktail (Roche)]. The samples
were completely homogenised for 30 min and allowed to crack.
Following this, the samples were transferred into an EP tube and
centrifuged at 8,000 × g for 20 min (4°C), prior to the
supernatants being stored. The protein samples were electrophoresed
by 15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE) and a transmembrane was formed at a constant voltage of
15 V for 1 h. The membranes were blocked by 5% skimmed milk at room
temperature for 1.5 h and then incubated with anti-CAR (Santa Cruz
Biotechnology, Inc.) and anti-GAPDH (Sigma, St. Louis, MO, USA) at
4°C overnight, as well as with goat anti-mouse secondary antibody
(Earthox, LLC, San Francisco, CA, USA) at room temperature for 2 h.
Enhanced chemiluminescence (ECL) fluorescence was determined using
X-ray film in the dark. Grey-scale analysis was performed using
Glyko Bandscan software (Glyko, Hayward, CA, USA).
Flow cytometry
A Vybrant apoptosis kit (Invitrogen Life
Technologies) was used to detect apoptosis. The cells were
incubated overnight (4°C) with CAR rabbit anti-mouse antibody
(Santa Cruz Biotechnology, Inc.) and then with goat anti-rabbit
immunoglobulin (Ig) G-fluorescein isothiocyanate (FITC; Santa Cruz
Biotechnology Inc.) secondary antibodies at room temperature for 2
h. Following this, the samples were washed with PBS to detect
apoptosis.
Statistical analysis
Experimental data were analysed using SPSS 12.0
statistical software (SPSS, Inc., Chicago, IL, USA). The CAR
expression in two independent samples were compared using one way
analysis of variance (ANOVA) and the Student’s t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Histological observation
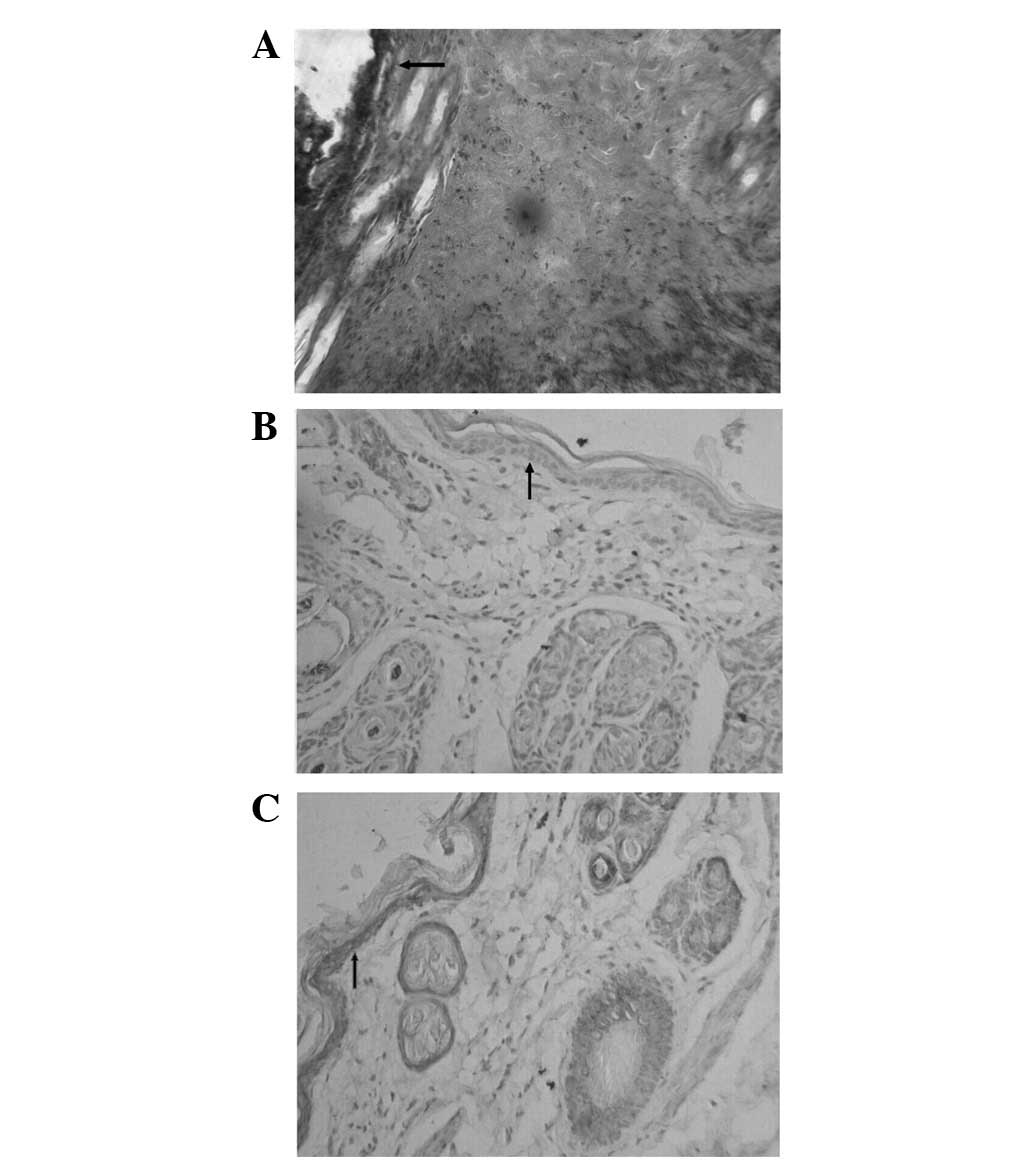
No staining was observed in the blank control
specimen (Fig. 1A). In the
epidermal keratinocytes of the normal mouse skin (Fig. 1B), a mild positive staining was
detected, suggesting that in normal circumstances mouse skin
epidermal keratinocytes express a low level of CAR. Following
thermal stimulation, the positive staining of the skin epidermis
(Fig. 1C) was intensified. This
result indicated that thermal stimulation may lead to the
upregulation of CAR expression in mouse skin keratinocytes. CAR was
also expressed in hair follicles, sweat glands, and epithelial
cells. By contrast, no evident expression of CAR was observed in
the skin fibroblasts.
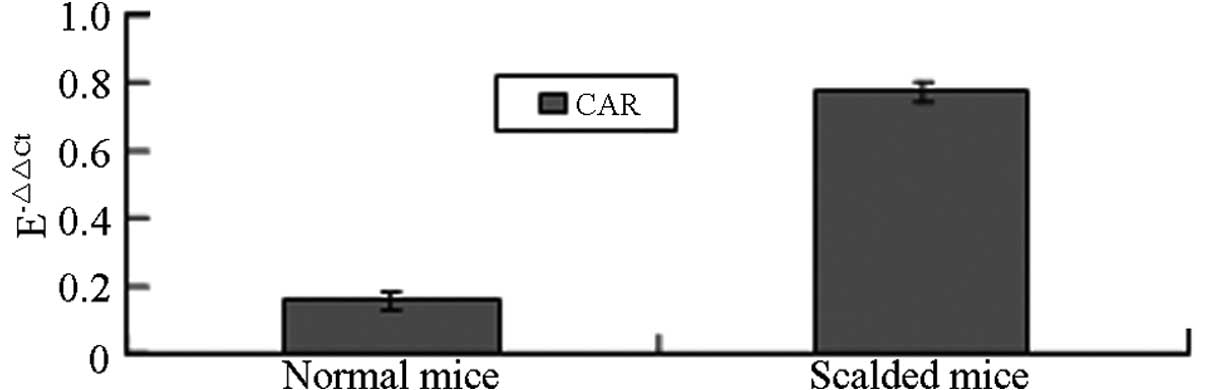
qPCR
In this study, the Pfaffl method (17) was used to evaluate the relative
quantification of the CAR gene. The epidermal keratinocytes of the
mouse skin were thermally stimulated and the results revealed that
the CAR mRNA expression level of the thermally stimulated
keratinocytes was significantly higher than that of the normal
mouse keratinocytes (P<0.05; Table
I and Fig. 2).
 | Table I.Relative quantitative expression of
CAR gene. |
Table I.
Relative quantitative expression of
CAR gene.
| Group | CAR Ct | GAPDH Ct |
E-ΔΔCt |
|---|
| Normal mice | 20.69±0.106 | 15.60±0.220 | 0.157±0.027 |
| Scalded mice | 19.89±0.018 | 16.98±0.048 | 0.773±0.029a |
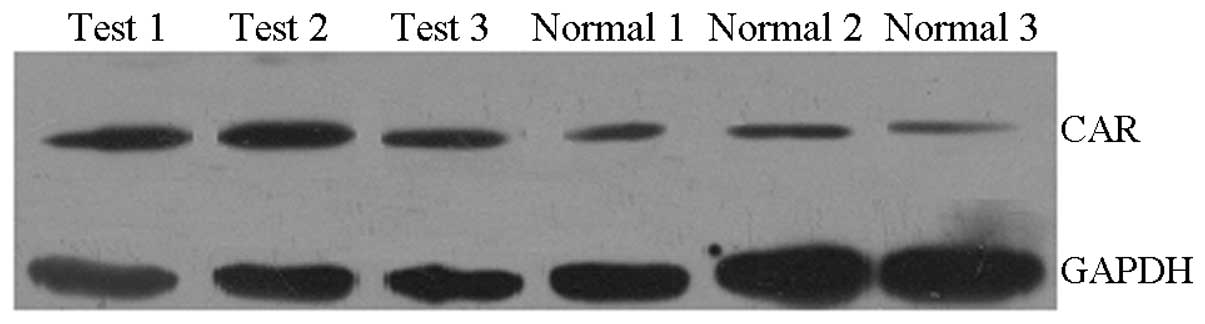
Western blot
The grey-scale analysis was performed using Glyko
Bandscan software and the grey-scale value of GAPDH was used as a
reference. The ratio of the grey-scale value of the reference and
the corresponding CAR value indicated the relative CAR expression.
The results showed that there was a slight CAR protein expression
in normal mouse skin keratinocytes and that this expression was
increased in local skin keratinocytes following thermal stimulation
(P<0.05; Table II and Fig. 3).
 | Table II.Relative grey value of CAR
protein. |
Table II.
Relative grey value of CAR
protein.
| Group | n | CAR/GAPDH |
|---|
| Normal mice | 10 | 0.227±0.093 |
| Scalded mice | 10 | 0.891±0.144a |
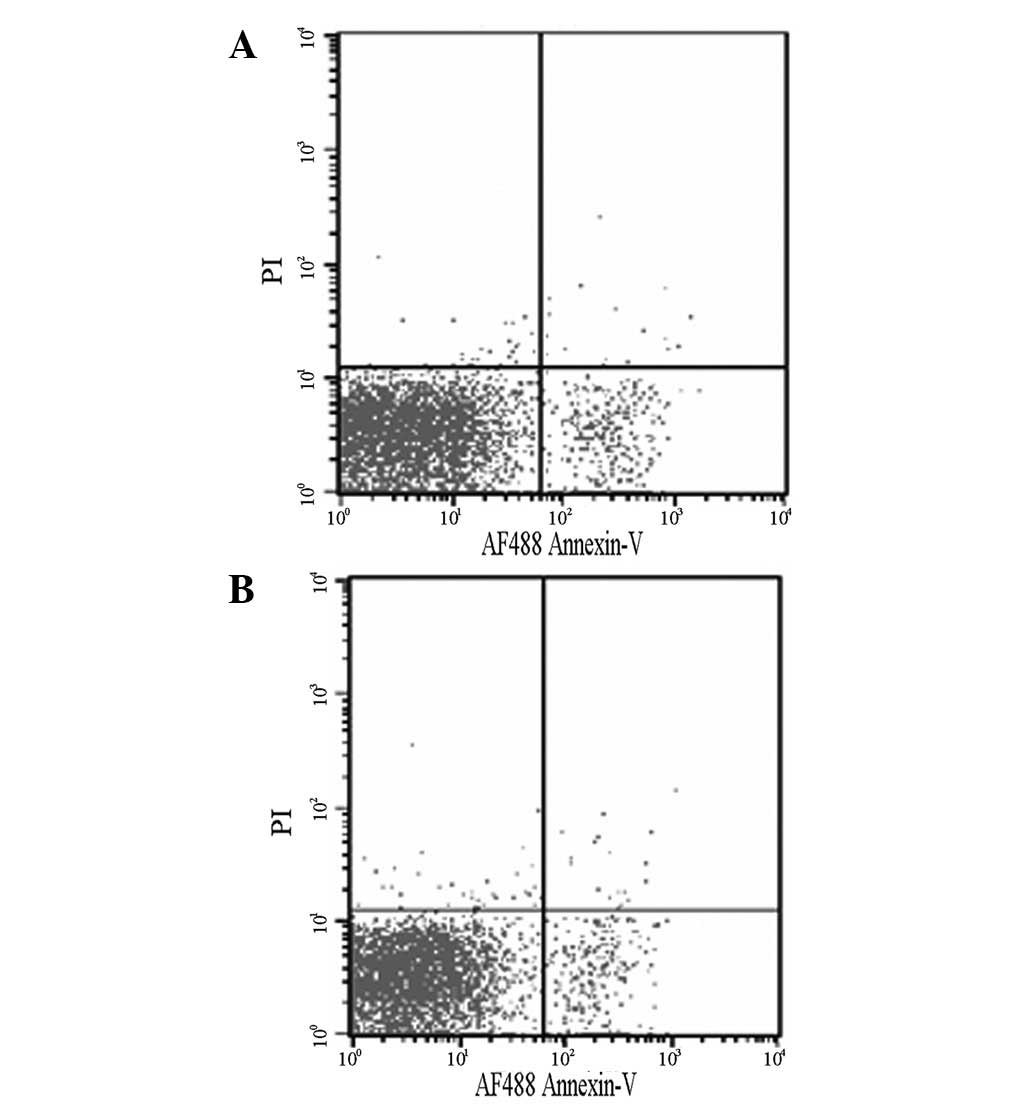
Changes in apoptosis following heat
stress
Normal keratinocytes and thermally stimulated
keratinocytes were subjected to apoptosis analysis. The apoptotic
rates of the normal keratinocytes and thermally stimulated
keratinocytes were 5.72±1.30 and 7.35±1.66%, respectively; however,
these apoptotic rates were not significantly different (P>0.05;
Fig. 4).
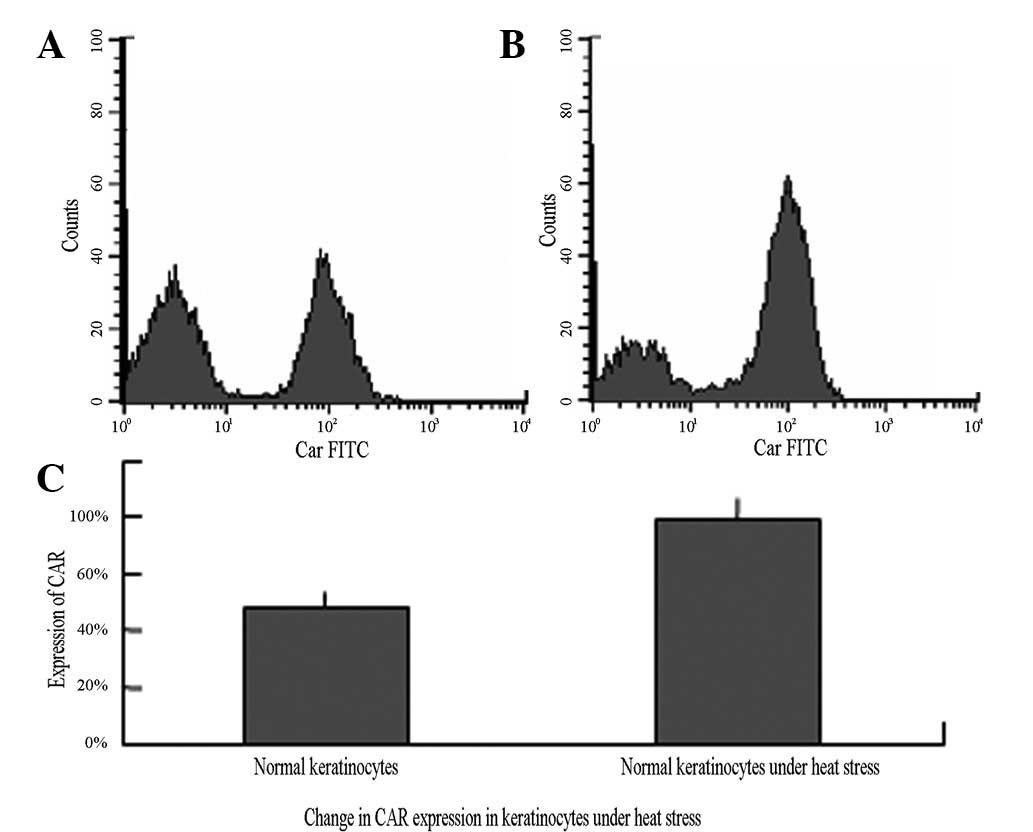
CAR expression in keratinocytes following
heat stress
In normally cultured keratinocytes, the expression
rate of CAR was 48.36±5.07%; however, this rate significantly
increased to 78.64±7.96% following the cells being subjected to
heat stress (P<0.05; Table III
and Fig. 5).
 | Table III.Expression of CAR in keratinocytes
detected using flow cytometry (mean ± SD)%. |
Table III.
Expression of CAR in keratinocytes
detected using flow cytometry (mean ± SD)%.
| Group | n | CAR expression |
|---|
| Normal
keratinocytes | 12 | 48.36±5.07 |
| Keratinocytes after
thermal stimulation | 12 | 78.64±7.96a |
Discussion
Wound healing occurs via different mechanisms. In
contrast to skin wound biopsies, burn wounds do not undergo partial
blood vessel rupture with bleeding and platelet aggregation at the
vascular stump or the release of biologically active
platelet-associated substances. Unlike burn wounds, skin wound
biopsies exhibit few residual necrotic tissues, which may aggravate
local inflammation. However, these wounds are acute; thus, their
healing processes are similar (18). For keratinised epithelial cells,
the increase in CAR expression in mouse skin wound biopsies was
consistent with the upregulation in burn wounds. The experimental
results confirmed this hypothesis, as the immunohistochemistry and
western blot analyses of the skin tissues showed a significantly
increased expression of CAR protein following thermal stimulation.
qPCR further demonstrated that the expression of CAR mRNA in skin
keratinocyte epithelial cells increased following heat stimulation,
which suggests that the increase in the level of CAR expression may
resulted from an enhancement of CAR gene transcription.
In vitro cell experiments were performed to
determine whether the changes in CAR expression in keratinocytes
following heat stress required signal exchanges with other cells.
The primary keratinocytes underwent a strong thermal stimulation
without the apoptotic rate being significantly affected; however,
the CAR expression was significantly elevated. This result
suggested that the changes in CAR expression following heat stress
may be attributed to the self-stress response of cells, and that
the thermally stimulated cells did not require signals from other
cells to alter CAR expression.
The increased CAR expression level in the burn
wounds of mice has a crucial function in wound healing. This result
indicated that dendritic epidermal T cells (γδT cells, DETCs) are
important in mouse skin wound healing (19); such cells may adjust to multiple
factors of the process (20–23),
particularly at the initiation of skin wound healing (23–27).
However, mice with DETC dysfunction have an impaired wound healing
response. A previous study (28)
revealed that CAR in keratinocytes is able to combine with the
junctional adhesion molecule-like protein (JAML) on the surface of
DETCs and thereby enhance the activation level of the DETCs. This
occurs via a co-stimulatory signal that passes via the
phosphatidylinositol 3-kinase (PI3K)/Akt signalling pathway,
thereby promoting the synthesis and secretion of interleukin
(IL)-2, tumour necrosis factor (TNF) α, keratinocyte growth factor
(KGF)-1 and interferon (IFN) γ, as well as DETC proliferation for
wound healing.
At present, the effect of increased CAR expression
in keratinocytes remains unclear. The increased CAR expression in
different cells may be a result of different self-feedback systems.
Okegawa et al (29)
revealed that CAR was a potential growth inhibitory factor, and
that increased CAR expression in bladder cancer cells induced the
upregulation of p21. Subsequently, retinoblastoma (Rb)
phosphorylation and accumulation led to cell cycle arrest at the G1
phase and/or apoptosis. Bagheri et al (30) also suggested that CAR was a growth
inhibitory factor, due to the fact that bladder carcinoma cells
with high CAR expression levels demonstrated a potent binding
ability to oncolytic viruses, thus showing a good oncolytic
therapeutic effect. Vindrieux et al (31) also described CAR as a potential
type of unknown cytokine. In breast cancer patients, CAR expression
is upregulated due to the effect of oestrogen, and this phenomenon
is associated with cancer cell proliferation. Since the increased
CAR expression in burn wound keratinocytes is a response to injury
factors, self-feedback may be inhibitory. However, this requires
further investigation.
Based on the positive effect of CAR on wound
healing, Verdino et al (32) studied a monoclonal antibody
HL4E10a, with a molecular structure similar to that of CAR, which
was able to combine with JAMLs on DETCs. Although their binding
sites were not identical, this antibody was able to effectively
improve the level of activated DETCs. In the same study (32), the skin wounds of mice were treated
with HL4E10 and a positive effect on wound healing was
observed.
CAR is widely distributed in human tissues, and an
80% homology between human and mouse CAR nucleotide sequences has
been identified (33). Similar to
DETCs in mice, γδT cells are localised in the human skin (34,35).
Therefore, there is a requirement for studies to be conducted to
determine whether CAR and γδT cells in humans have the same
functions as CAR and DETCs in mice. A better understanding of the
function of CAR in wound healing may provide a new strategy for the
treatment of wound problems.
References
|
1.
|
Birkballe S, Karlsmark T, Noerregaard S
and Gottrup F: A new concept of a multidisciplinary lymphoedema
centre: established in connection to a department of dermatology
and the Copenhagen Wound Healing Center. Br J Dermatol.
167:116–122. 2012. View Article : Google Scholar
|
|
2.
|
Hobizal KB and Wukich DK: Diabetic foot
infections: current concept review. Diabet Foot Ankle.
3:2012.PubMed/NCBI
|
|
3.
|
Witherden DA, Verdino P, Rieder SE, et al:
The junctional adhesion molecule JAML is a costimulatory receptor
for epithelial γδ T cell activation. Science. 329:1205–1210.
2010.PubMed/NCBI
|
|
4.
|
Philipson L and Pettersson RF: The
coxsackie-adenovirus receptor - a new receptor in the
immunoglobulin family involved in cell adhesion. Curr Top Microbiol
Immunol. 273:87–111. 2004.PubMed/NCBI
|
|
5.
|
Gye MC, Oh YS, Lee JE, Shim S, Choi KJ and
Ahn HS: Expression of coxsackievirus and adenovirus receptor
isoforms in developing mouse bladder uroepithelium. Urology.
77:1009.e9–1009.e18. 2011.PubMed/NCBI
|
|
6.
|
Pazirandeh A, Sultana T, Mirza M, et al:
Multiple phenotypes in adult mice following inactivation of the
Coxsackievirus and Adenovirus Receptor (Car) gene. Plos One.
6:e202032011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mirza M, Pang MF, Zaini MA, et al:
Essential role of the coxsackie- and adenovirus receptor (CAR) in
development of the lymphatic system in mice. Plos One.
7:e375232012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cifuente JO, Ferrer MF, de Giusti CJ, et
al: Molecular determinants of disease in coxsackievirus B1 murine
infection. J Med Virol. 83:1571–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Burckhardt CJ, Suomalainen M,
Schoenenberger P, Boucke K, Hemmi S and Greber UF: Drifting motions
of the adenovirus receptor CAR and immobile integrins initiate
virus uncoating and membrane lytic protein exposure. Cell Host
Microbe. 10:105–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Adamson RE, Frazier AA, Evans H, et al: In
vitro primary cell culture as a physiologically relevant method for
preclinical testing of human oncolytic adenovirus. Hum Gene Ther.
23:218–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Carson SD, Chapman NM, Hafenstein S and
Tracy S: Variations of coxsackievirus B3 capsid primary structure,
ligands, and stability are selected for in a coxsackievirus and
adenovirus receptor-limited environment. J Virol. 85:3306–3314.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Martin TA, Mason MD and Jiang WG: Tight
junctions in cancer metastasis. Front Biosci. 16:898–936. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Majhen D, Stojanović N, Špeljko T, et al:
Increased expression of the coxsackie and adenovirus receptor
downregulates αvβ3 and αvβ5 integrin expression and reduces cell
adhesion and migration. Life Sci. 89:241–249. 2011.PubMed/NCBI
|
|
14.
|
Zhang X, Fang B, Mohan R and Chang JY:
Coxsackie-adenovirus receptor as a novel marker of stem cells in
treatment-resistant non-small cell lung cancer. Radiother Oncol.
105:250–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wunder T, Schmid K, Wicklein D, et al:
Expression of the coxsackie adenovirus receptor in neuroendocrine
lung cancers and its implications for oncolytic adenoviral
infection. Cancer Gene Ther. 20:25–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shan L, Cui S, Du C, et al: A
paclitaxel-conjugated adenovirus vector for targeted drug delivery
for tumor therapy. Biomaterials. 33:146–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Werner S: A novel enhancer of the wound
healing process: the fibroblast growth factor-binding protein. Am J
Pathol. 179:2144–2147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Jameson J, Ugarte K, Chen N, et al: A role
for skin γδ T cells in wound repair. Science. 296:747–749.
2002.
|
|
20.
|
Toulon A, Breton L, Taylor KR, et al: A
role for human skin-resident T cells in wound healing. J Exp Med.
206:743–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Colburn NT, Zaal KJ, Wang F and Tuan RS: A
role for γ/δ T cells in a mouse model of fracture healing.
Arthritis Rheum. 60:1694–1703. 2009.
|
|
22.
|
Byeseda SE, Burns AR, Dieffenbaugher S,
Rumbaut RE, Smith CW and Li Z: ICAM-1 is necessary for epithelial
recruitment of γδ T cells and efficient corneal wound healing. Am J
Pathol. 175:571–579. 2009.
|
|
23.
|
Havran WL and Jameson JM: Epidermal T
cells and wound healing. J Immunol. 184:5423–5428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Komori HK, Witherden DA, Kelly R, et al:
Cutting edge: dendritic epidermal γδ T cell ligands are rapidly and
locally expressed by keratinocytes following cutaneous wounding. J
Immunol. 188:2972–2676. 2012.
|
|
25.
|
Witherden DA and Havran WL: EPCR: a stress
trigger for γδ T cells. Nat Immunol. 13:812–814. 2012.PubMed/NCBI
|
|
26.
|
Macleod AS and Havran WL: Functions of
skin-resident γδ T cells. Cell Mol Life Sci. 68:2399–2408.
2011.
|
|
27.
|
Chodaczek G, Papanna V, Zal MA and Zal T:
Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol.
13:272–282. 2012.PubMed/NCBI
|
|
28.
|
Verdino P, Witherden DA, Havran WL and
Wilson IA: The molecular interaction of CAR and JAML recruits the
central cell signal transducer PI3K. Science. 329:1210–1214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Okegawa T, Pong RC, Li Y, Bergelson JM,
Sagalowsky AI and Hsieh JT: The mechanism of the growth-inhibitory
effect of coxsackie and adenovirus receptor (CAR) on human bladder
cancer: a functional analysis of car protein structure. Cancer Res.
61:6592–6600. 2001.
|
|
30.
|
Bagheri N, Shiina M, Lauffenburger DA and
Korn WM: A dynamical systems model for combinatorial cancer therapy
enhances oncolytic adenovirus efficacy by MEK-inhibition. PLoS
Comput Biol. 7:e10010852011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Vindrieux D, Le CL, Hsieh JT, et al:
Coxsackie and adenovirus receptor is a target and a mediator of
estrogen action in breast cancer. Endocr Relat Cancer. 18:311–321.
2011. View Article : Google Scholar
|
|
32.
|
Verdino P, Witherden DA, Ferguson MS, et
al: Molecular insights into γδ T cell costimulation by an anti-JAML
antibody. Structure. 19:80–89. 2011.
|
|
33.
|
Tomko RP, Xu R and Philipson L: HCAR and
MCAR: the human and mouse cellular receptors for subgroup C
adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA.
94:3352–3356. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Nestle FO, Di MP, Qin JZ and Nickoloff BJ:
Skin immune sentinels in health and disease. Nat Rev Immunol.
9:679–691. 2009.PubMed/NCBI
|
|
35.
|
Toulon A, Breton L, Taylor KR, et al: A
role for human skin-resident T cells in wound healing. J Exp Med.
206:743–750. 2009. View Article : Google Scholar : PubMed/NCBI
|