Introduction
FK506 (tacrolimus) is a type of macrolide
immunosuppressant drug that has been widely used in organ
transplantation. In addition to exhibiting an immunosuppressive
effect with a high efficiency and low toxicity, FK506 also promotes
neural regeneration. Gold et al (1) first reported the application of FK506
in the treatment of rats with sciatic nerve crush injury and
observed that FK506 accelerated the injured nerve regeneration and
promoted the recovery of neural function (1,2). A
number of studies investigating the role of FK506 in different
models of spinal cord injury have also demonstrated the
neurotrophic and neuroprotective effects of FK506 and its
contribution to functional recovery following spinal cord injury
(3–5), thereby indicating a potential novel
pathway for drug therapy of spinal cord injury. Nerve growth factor
(NGF) is an important member of the neurotrophic factor family,
which is widely located in peripheral tissues, the peripheral and
central nervous systems and is a critical factor in neuronal
development and survival, axonal remodeling and function, as well
as in the repair process following spinal cord injury (6,7).
When spinal cord injury occurs, NGF is expressed in the injured
tissues, which has a positive effect on the prevention of secondary
injury caused by microenvironmental changes. However, the
expression level is low and, therefore, exogenous NGF may be
considered as a means of treating spinal cord injury. At present,
in vivo and in vitro studies have demonstrated the
synergistic effect of FK506 and NGF in the treatment of peripheral
nerve injuries (8–11); however, it remains unclear whether
this effect is present in the treatment of spinal cord injury. The
aim of this study was to observe whether FK506 and NGF exhibited a
synergistic effect on the recovery of spinal cord functions
following acute spinal cord injury in rats.
Materials and methods
Materials
A total of 120 Sprague-Dawley female clean rats,
weighing 180–220 g, were provided by the Animal Experimental Center
of Dalian Medical University, China [license No. SCXK (Liao)
2008–0002]. The study was approved by the Animal Research Ethics
Committee of Dalian Medical University. All experimental procedures
were in accordance with the Guidance Suggestions for the Care and
Use of Laboratory Animals, published by the Ministry of Science and
Technology of the People’s Republic of China (2006-09-30).
Grouping and establishment of models
The 120 female rats were randomly divided into five
groups: control, FK506 treatment, NGF treatment, FK506 plus NGF
treatment and sham surgery, with 24 rats in each group. After being
weighed, the rats were anesthetized with 10% chloral hydrate (300
mg/kg) via intraperitoneal injection and fixed in the prone
position. Following this, a dorsal midline incision was made under
sterile conditions and models of spinal cord injury were
established using the modified Allen’s method (12). In brief, T9–T10 spinous processes
and lamina were excised, exposing the spinal dura mater. A 5-g iron
hammer was then allowed to fall freely from a 5-cm height on to the
dural sac, at a strength of 5 g × 5 cm and a damage diameter of 2
mm. The rats exhibited tail flicking immediately following the
attack, retraction of the hind limbs and the body and then hind
limb paralysis. These manifestations indicated the success of the
modeling. In the sham surgery group, only the T9–T10 segment
laminectomy was performed, with no attack on the spinal cord. At 30
min subsequent to injury, the rats in the three treatment groups
were treated with 0.3 mg/kg FK506 (Sigma-Aldrich, St. Louis, MO,
USA), 40 μg/ kg NGF (Staidson Biopharmaceuticals Co., Ltd,
Beijing, China) and 0.3 mg/kg FK506 plus 40 μg/kg NGF,
respectively, via intraperitoneal injection, once a day, for one
week.
Specimens from the injury area
Three rats were randomly selected from each group at
each time-point and the NF200 protein expression was determined
using immunohistochemical methods. Rats were anesthetized with 10%
chloral hydrate via intraperitoneal injection, the chest was opened
and rats were fixed in 4% paraformaldehyde for cardiac perfusion
until body stiffness was present. This took 20–30 min. Following
the completion of the perfusion, the injured spinal cord tissues
were harvested and 1.0-cm-long specimens were stored in 4%
paraformaldehyde for 24 h and embedded in paraffin.
A further three rats were randomly selected from
each group at each-time point for the detection of NF200 mRNA
expression, using the reverse transcription-polymerase chain
reaction method. Rats were anesthetized with 10% chloral hydrate
via intraperitoneal injection, prior to the injured spinal cord
tissues being harvested (∼100 mg). The specimens were stored at
−80°C.
Immunohistochemical detection of NF200
protein expression
Paraffin specimens of 4-μm thickness were
hydrated and rinsed with phosphate-buffered saline (PBS; pH 7.4)
three times, for 3 min each, prior to being incubated with 3%
hydrogen peroxide solution at room temperature for 10 min and
rinsed with PBS a further three times for 3 min each. Specimens
were then microwave repaired with 0.01 M carbonate buffer (CB) (pH
6.0) at 100°C for 15 min and naturally cooled to room temperature,
prior to being rinsed with PBS three times for 3 min each.
Specimens were blocked with 5% goat serum at room temperature for
10 min and were subsequently incubated with NF200 antibody (1:100)
at 4°C overnight and rinsed with PBS three times for 3 min each.
Each section was then incubated with goat anti-rabbit antibody at
37°C for 30 min and rinsed with PBS three times for 3 min each.
Following this, 3,3’-diaminobenzidine (DAB) coloration was
performed for 10 min and the specimens were rinsed with tap water
for 10 min, prior to being counterstained with hematoxylin for 10
sec, dehydrated and mounted.
Five visual fields randomly selected from the
injured spinal cord sections were observed using the Image-Pro Plus
6.0 medical image analysis system (Media Cybernetics, Inc., Silver
Spring, MD, USA) and the average intracellular optical density (OD)
value for the NF200 staining was calculated.
Reverse transcription-polymerase chain
reaction detection of NF200 mRNA expression
Total RNA extraction from the specimens was
performed according to the instructions of the RNAiso Plus kit
[Takara Biotechnology (Dalian) Co., Ltd., Dalian, China] and the
reverse transcription-polymerase chain reaction primers were
designed using Primer 5.0 software (NF200: 5’-GCA GAC ATT GCC TAC
C-3’ and 5’-TCA CTC CTT CCG TCA CCC-3’; and β-actin: 5’-GTA AAG ACC
TCT ATG CCA ACA-3’ and 5’-CCT TCA CCG TTC CAG TTT-3’, forward and
reverse, respectively). The polymerase chain reaction was performed
according to the instructions of the PrimeScript® One Step RT-PCR
kit version 2 [Dye Plus; TaKaRa Biotechnology (Dalian) Co., Ltd].
In brief, the cycle conditions for NF200 were: 50°C for 30 min and
94°C for 2 min, followed by 30 cycles at 94°C for 30 sec, 57°C for
30 sec and at 72°C for 1 min. The conditions for β-actin were: 50°C
for 30 min and 94°C for 2 min, followed by 30 cycles at 94°C for 30
sec, 52°C for 30 sec and at 72°C for 1 min. Polymerase chain
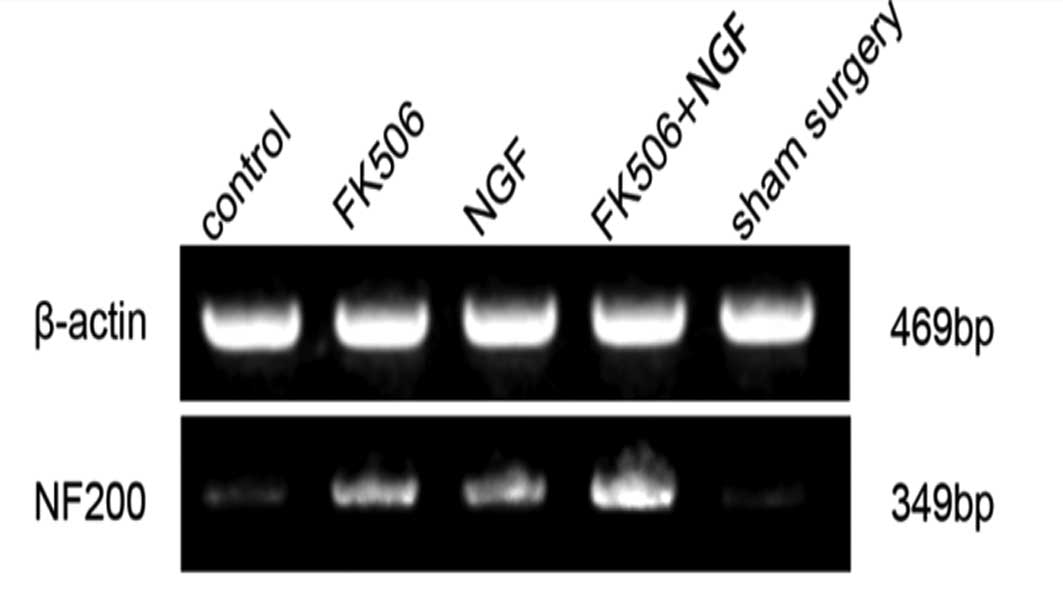
reaction products were detected with 2% agarose gel electrophoresis
and analyzed with a gel imaging analyzer. The ratio of the target
gene (NF200) OD to the reference gene (β-actin) OD in the same
specimen was calculated and considered as the target gene mRNA
relative content.
Assessment of spinal motor function
The spinal cord functions were assessed by the
Basso, Beattie and Bresnahan (BBB) scale (13). Five rats from each group were
randomly selected at 3, 7, 14 and 21 days post-injury and their
lower limb functions were tested using BBB scores. Hind limb
paralysis was scored as 0 points, while a completely normal spinal
cord was scored as 21 points. The spinal cord functions were scored
according to the number and motion range of the joints as well as
limb and tail activities. The assessment was performed by two
physicians independently within 5 min, using a double-blind method,
and the average values of the two test results were taken as the
recording values.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA) and data are
expressed as the mean ± standard deviation. Multiple groups were
compared using one-way analysis of variance and differences between
two groups were compared using the Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
NF200 protein expression following spinal
cord injury
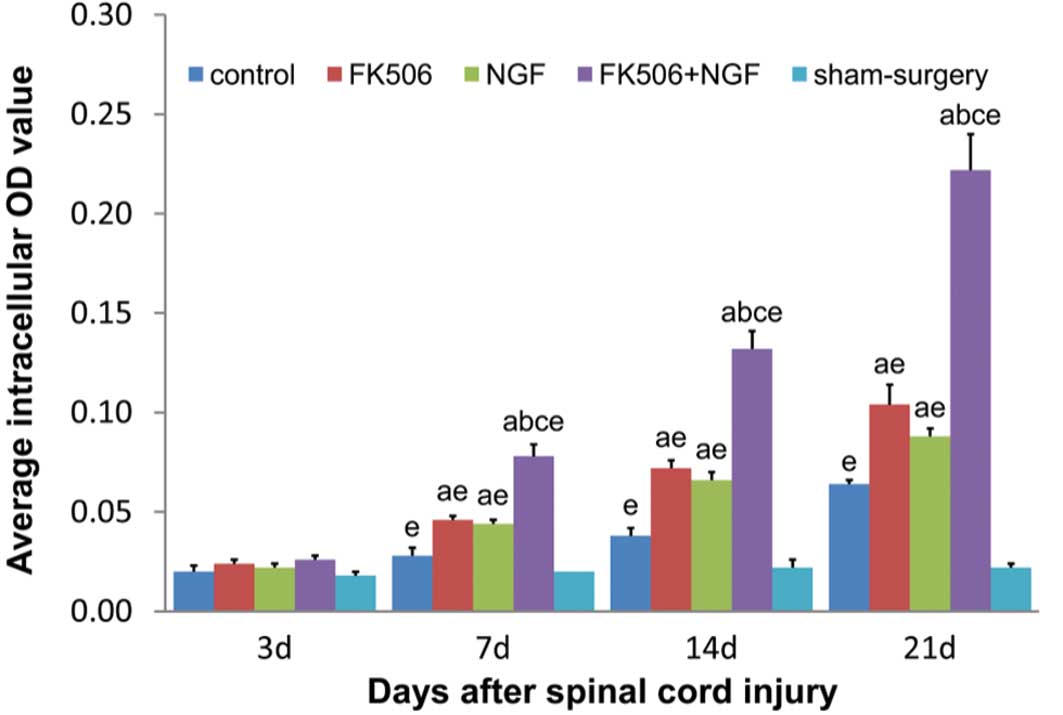
The NF200 expression is shown in Figs. 1 and 2. There were no significant differences
in the average intracellular NF200 staining OD values among the
groups 3 days after the injury (P>0.05). At 7, 14 and 21 days
post-injury, the average intracellular NF200 staining OD values
were shown to have gradually increased in all the treatment groups
and the control group, with the treatment groups showing
significantly higher expression levels than the control and sham
surgery groups (P<0.05). In the FK506 plus NGF treatment group,
the average intracellular NF200 staining OD value was significantly
higher than the ODs in the FK506 alone and NGF alone groups
(P<0.05), with no significant difference between the FK506 and
NGF groups.
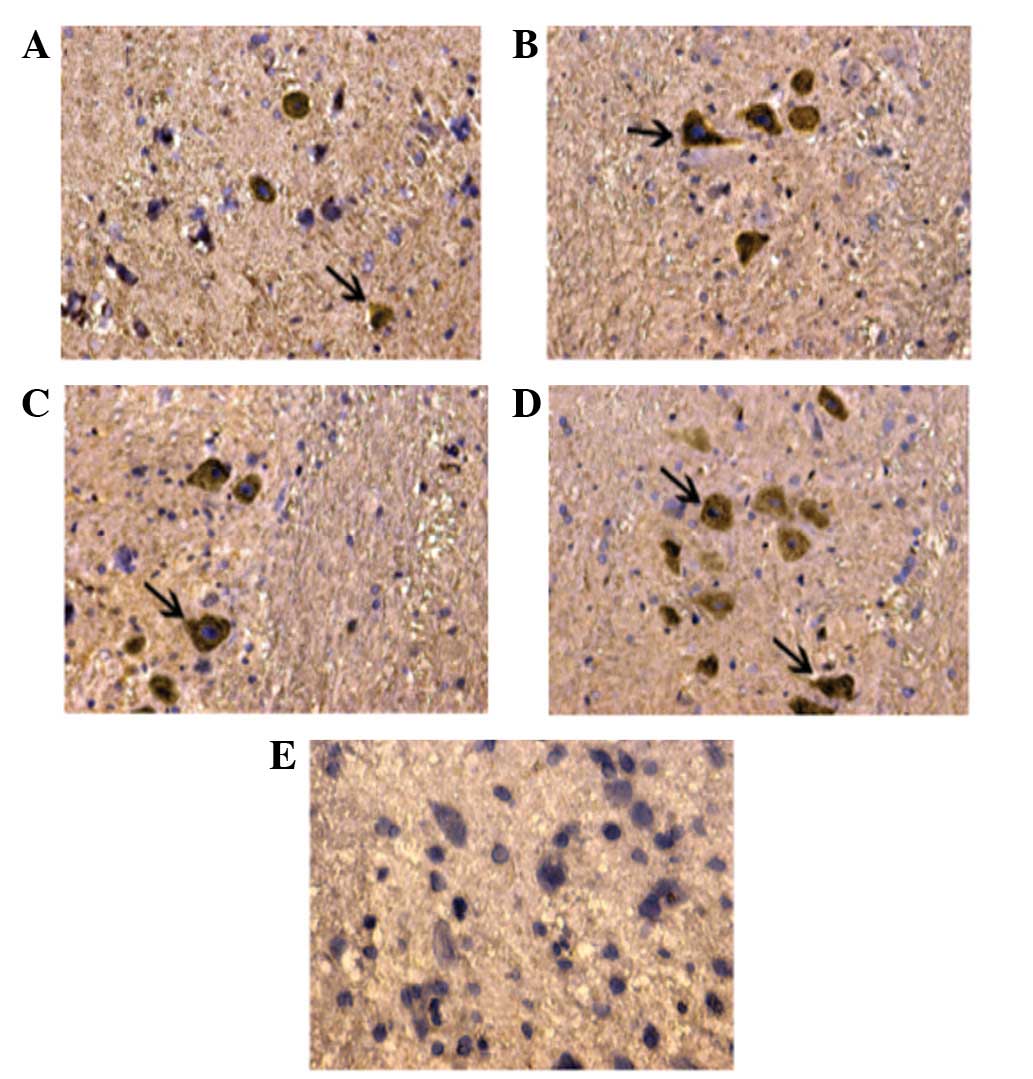
Detection of cells positive for NF200
staining
There were few cells positive for NF200 staining in
the control group. However, there were significantly increased
numbers of cells positive for NF200 staining in all the treatment
groups, with a higher number in the FK506 plus NGF treatment group
than in the groups treated with FK506 or NGF alone.
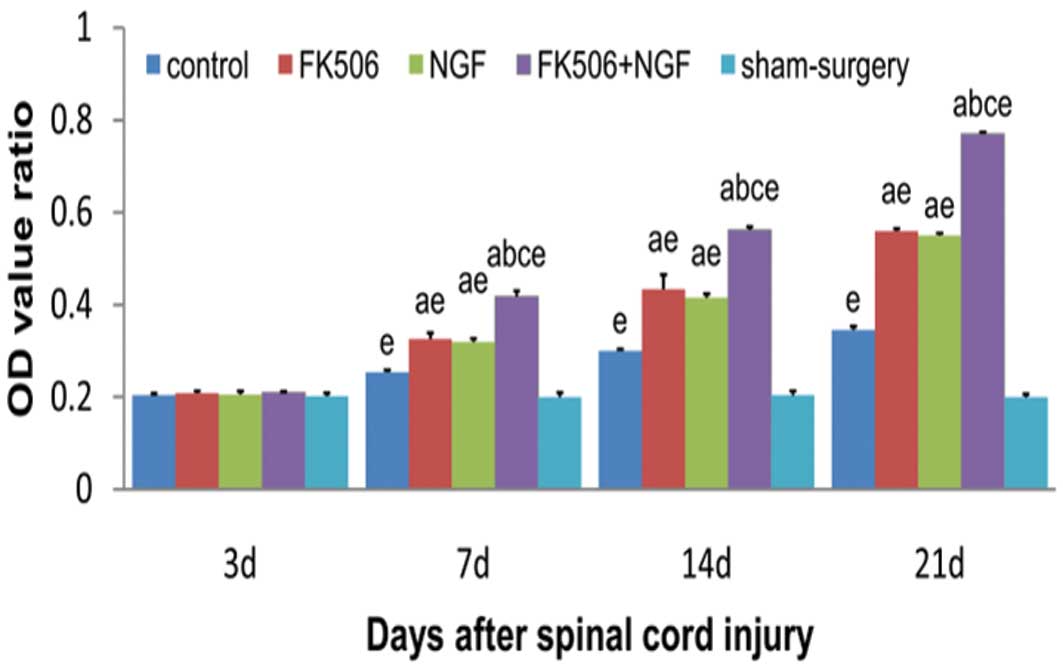
The reverse transcription-polymerase chain reaction
was performed to further confirm the results of the
immunohisto-chemistry (Figs. 3 and
4). There were no significant
differences in the OD value ratios of NF200 mRNA at 3 days after
injury (P>0.05). At 7, 14 and 21 days post-injury, the OD value
ratios of NF200 mRNA were shown to have gradually increased in all
treatment groups and the control group, with the treatment groups
showing significantly higher expression levels than the control and
sham surgery groups (P<0.05). In the FK506 plus NGF treatment
group, the OD value ratio of NF200 mRNA was significantly higher
than those in groups treated with FK506 or NGF alone (P<0.05),
with no significant difference between the FK506 and NGF
groups.
Lower limb function recovery following
spinal cord injury
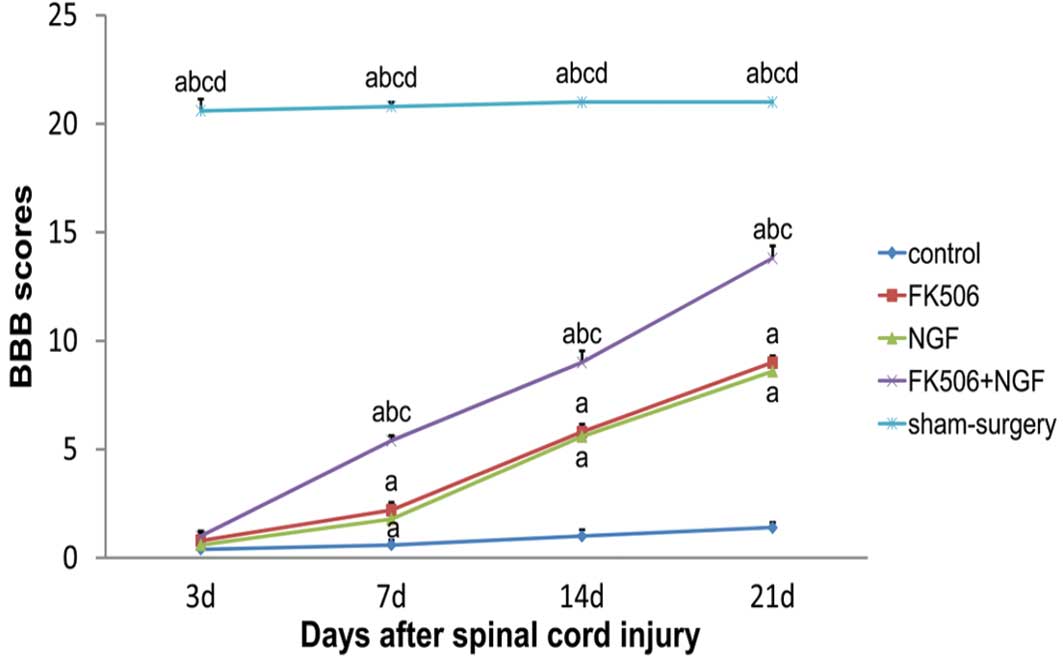
The BBB scores at each time-point are shown in
Fig. 5. There were no significant
differences in the BBB scores among the groups, with the exception
of the sham surgery group, at 3 days subsequent to injury.
Furthermore, the BBB scores in the treatment groups were
significantly higher than those in the control group at 7, 14 and
21 days post-injury (P<0.05), and were significantly increased
in the FK506 plus NGF treatment group compared with those in the
groups treated with FK506 or NGF alone (P<0.05). There was no
significant difference in the BBB scores between the FK506 and NGF
groups (P>0.05).
Discussion
Neurofilament protein NF200 is the main component of
the neuronal and axonal cytoskeleton and is of particular
significant in the maintenance of neuronal functions, axoplasmic
transport and a series of pathophysiological changes associated
with the repair process following spinal cord injury (14). In normal circumstances, although
NF200 exists in neurites, it is scarcely seen in cell bodies;
therefore, the normal NF200 staining result is negative. Following
spinal cord injury, cells at the area of the injury degenerate and
become necrotic, while the adjacent neurons may synthesize a large
quantity of NF200 under the stimulation of the insult. Accordingly,
these adjacent neurons may show cell body staining (15). A previous study observed a close
correlation between the number of NF200-positive neurons/the degree
of neuronal cell body staining and lower limb functional recovery
following incomplete spinal cord injury (16). The aim of the current study was to
observe NF200 staining, in a broader attempt to investigate the
morphology and functions of neurons following injury.
NGF is a type of polypeptide growth factor that
exerts biological effects on the development, repair and
regeneration of the central nervous system through selective
binding with the high affinity receptor, TrkA (10,17,18),
and is very important in the repair process following spinal cord
injury (19). In a previous study
(20), the NGF receptor mRNA
levels were shown to be significantly increased at day 4 subsequent
to spinal cord injury and were observed to peak at day 7, at levels
five-seven-sfold as high as those in the control group. Even 14–28
days subsequent to injury, the level remained four-fold that of the
control group. In the current study, the BBB scores and NF200
protein and mRNA expression levels in the NGF treatment group were
higher than those of the control group at 7, 14 and 21 days
post-injury, with statistically significant differences
(P<0.05). These experimental observations were consistent with
those regarding the NGF receptor expression; therefore, it was
suggested that exogenous NGF may promote neural regeneration and
functional recovery in rats with spinal cord injury, with 7 days
post-injury as the optimal treatment-point. The drug delivery
method in the present study was intraperitoneal injection; although
this was convenient, the biological utilization rate was low.
Therefore, the requirement for a synergistic drug for NGF treatment
is increasing in the field of spinal cord injury.
FK506 serves as an immunosuppressive agent and also
promotes neural regeneration (21,22).
A number of studies (23,24) have shown that FK506 upregulates the
expression of growth associated protein (GAP)-43 in neuronal cells
by inhibiting the CaN activity and promotes neurite extension and
neuronal recovery following spinal cord injury. In addition FK506
has been demonstrated to suppress cysteine proteinase-3 activation
in oligodendrocytes and reduce neuronal apoptosis following spinal
cord injury (25,26). With regard to the correlation
between FK506 and NGF, Lyons et al (8) observed that NGF upregulated the FK506
binding protein levels in PC12 cells, while FK506 enhanced the PC12
cell sensitivity to NGF and reduced the NGF concentration by
20-50-fold. Jifeng et al (11) applied the combined treatment of an
FK506 and NGF composite membrane to repair the injured sciatic
nerve in rats and showed that the combined treatment was more
effective than single applications and was able to reduce the
dosage of the immunosuppressant, FK506. In a study by Price et
al (10), FK506 was shown to
promote NGF expression and induce axonal outgrowth. Furthermore,
Gold et al (27)
demonstrated that, whereas the immunosuppressive effect of FK506
was dependent on the immunophilin FKBP12, the FK506 pro-nerve
growth effect was mediated by binding to the immunophilin FKBP-52,
through the FKBP52/HSP90/steroid receptor (SR) complex (27). FK506 binding with FKBP-52 was shown
to separate heat shock protein 90 and FKBP52 from the complexes,
with heat shock protein 90 acting with mitogen-activated protein
kinase/extracellular signal-regulated kinase 2 (28), in a pathway that may be
cross-linked to NGF signaling pathways (29,30).
This is indicative of the synergistic mechanism.
In the current study, animal models of acute spinal
cord injury were established using the modified Allen’s method and
the successful models were treated with FK506 and/or NGF
intraperitoneal injection 30 min after modeling, once a day for
seven days. At day 7 subsequent to injury, the BBB scores and NF200
expression in all treatment groups were significantly higher than
those in the control group, and the BBB scores and NF200 expression
of the combined treatment group were higher than those of the
single treatment group. Our experimental results showed the
synergistic effects of FK506 and NGF in the treatment of spinal
cord injury, and demonstrated that the combined treatment was able
to effectively promote neural regeneration and functional recovery
in rats following spinal cord injury. We consider that combined
treatment with FK506 and NGF is able to increase the biological
utilization efficiency of FK506 and exogenous NGF in the treatment
of spinal cord injury and that the neurotrophic and neuroprotective
mechanisms of the combined treatment exhibited a synergistic effect
on NF200 expression, effectively promoting neural regeneration and
functional recovery following spinal cord injury. This may provide
a new treatment means for acute spinal cord injury.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (grant nos.
30901950 and 81270052) and the Program for Liaoning Excellent
Talents in University.
References
|
1.
|
Gold BG, Storm-Dickerson T and Austin DR:
The immunosuppressant FK506 increases functional recovery and nerve
regeneration following peripheral nerve injury. Restor Neurol
Neurosci. 6:287–296. 1994.PubMed/NCBI
|
|
2.
|
Gold BG, Katoh K and Storm-Dickerson T:
The immunosuppressant FK506 increases the rate of axonal
regeneration in rat sciatic nerve. J Neurosci. 15:7509–7516.
1995.PubMed/NCBI
|
|
3.
|
López-Vales R, García-Alías G, Forés J,
Udina E, Gold BG, Navarro X and Verdú E: FK 506 reduces tissue
damage and prevents functional deficit after spinal cord injury in
the rat. J Neurosci Res. 81:827–836. 2005.
|
|
4.
|
Saganová K, Orendácová J, Sulla I Jr,
Filipcík P, Cízková D and Vanický I: Effects of long-term FK506
administration on functional and histopathological outcome after
spinal cord injury in adult rat. Cell Mol Neurobiol. 29:1045–1051.
2009.PubMed/NCBI
|
|
5.
|
Lü DC, Yuan XH, Li HJ and Wei XL: An
experimental study of the neuroprotective effect of FK506 on acute
spinal cord injury in dogs. Zhonghua Wai Ke Za Zhi. 43:1088–1090.
2005.(In Chinese).
|
|
6.
|
Brown A, Ricci MJ and Weaver LC: NGF mRNA
is expressed in the dorsal root ganglia after spinal cord injury in
the rat. Exp Neurol. 205:283–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kim DH, Gutin PH, Noble LJ, Nathan D, Yu
JS and Nockels RP: Treatment with genetically engineered
fibroblasts producing NGF or BDNF can accelerate recovery from
traumatic spinal cord injury in the adult rat. Neuroreport.
7:2221–2225. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lyons WE, George EB, Dawson TM, Steiner JP
and Snyder SH: Immunosuppressant FK506 promotes neurite outgrowth
in cultures of PC12 cells and sensory ganglia. Proc Natl Acad Sci
USA. 91:3191–3195. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Takadera T, Sakamoto Y, Hizume Y and
Ohyashiki T: Cyclosporine A- and FK506-induced apoptosis in PC12
cells. Cell Biol Toxicol. 23:355–360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Price RD, Yamaji T and Matsuoka N: FK506
potentiates NGF-induced neurite outgrowth via the Ras/Raf/MAP
kinase pathway. Br J Pharmacol. 140:825–829. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jifeng H, Dezhong L, Qiongjiao Y, Huayong
Z and Shipu L: Evaluation of PRGD/FK506/NGF conduits for peripheral
nerve regeneration in rats. Neurol India. 58:384–391. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Allen AR: Surgery of experimental lesion
of spinal cord equivalent to crush injury of fracture dislocation
of spinal column: a preliminary report. JAMA. 57:878–880. 1911.
View Article : Google Scholar
|
|
13.
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kimura N, Kumamoto T, Ueyama H, Horinouchi
H and Ohama E: Role of proteasomes in the formation of
neurofilamentous inclusions in spinal motor neurons of
aluminum-treated rabbits. Neuropathology. 27:522–530. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Millecamps S, Gowing G, Corti O, Mallet J
and Julien JP: Conditional NF-L transgene expression in mice for in
vivo analysis of turnover and transport rate of neurofilaments. J
Neurosci. 27:4947–4956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Schumacher PA, Eubanks JH and Fehlings MG:
Increased calpain I-mediated proteolysis, and preferential loss of
dephosphorylated NF200, following traumatic spinal cord injury.
Neuroscience. 91:733–744. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Michael GJ, Kaya E, Averill S, Rattray M,
Clary DO and Priestley JV: TrkA immunoreactive neurones in the rat
spinal cord. J Comp Neurol. 385:441–455. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Josephson A, Widenfalk J, Trifunovski A,
Widmer HR, Olson L and Spenger C: GDNF and NGF family members and
receptors in human fetal and adult spinal cord and dorsal root
ganglia. J Comp Neurol. 440:204–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bowes M, Tuszynski MH, Conner J and Zivin
JA: Continuous intrathecal fluid infusions elevate nerve growth
factor levels and prevent functional deficits after spinal cord
ischemia. Brain Res. 883:178–183. 2000. View Article : Google Scholar
|
|
20.
|
Brunello N, Reynolds M, Wrathall JR and
Mocchetti I: Increased nerve growth factor receptor mRNA in
contused rat spinal cord. Neurosci Lett. 118:238–240. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Saganová K, Gálik J, Blaško J, Korimová A,
Račeková E and Vanický I: Immunosuppressant FK506: focusing on
neuroprotective effects following brain and spinal cord injury.
Life Sci. 91:77–82. 2012.PubMed/NCBI
|
|
22.
|
Toll EC, Seifalian AM and Birchall MA: The
role of immunophilin ligands in nerve regeneration. Regen Med.
6:635–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Keswani SC, Rosenberg B and Hoke A: The
use of GAP-43 mRNA quantification in high throughput screening of
putative neuroprotective agents in dorsal root ganglion cultures. J
Neurosci Methods. 136:193–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Madsen JR, MacDonald P, Irwin N, et al:
Tacrolimus (FK506) increases neuronal expression of GAP-43 and
improves functional recovery after spinal cord injury in rats. Exp
Neurol. 154:673–683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Asai A, Qiu J, Narita Y, et al: High level
calcineurin activity predisposes neuronal cells to apoptosis. J
Biol Chem. 274:34450–34458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Nottingham S, Knapp P and Springer J:
FK506 treatment inhibits caspase-3 activation and promotes
oligodendroglial survival following traumatic spinal cord injury.
Exp Neurol. 177:242–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Gold BG, Densmore V, Shou W, Matzuk MM and
Gordon HS: Immunophilin FK506-binding protein 52 (not FK506-binding
protein 12) mediates the neurotrophic action of FK506. J Pharmacol
Exp Ther. 289:1202–1210. 1999.PubMed/NCBI
|
|
28.
|
Pratt WB and Toft DO: Steroid receptor
interactions with heat shock protein and immunophilin chaperones.
Endocr Rev. 18:306–360. 1997.
|
|
29.
|
Volonté C, Angelastro JM and Greene LA:
Association of protein kinases ERK1 and ERK2 with p75 nerve growth
factor receptors. J Biol Chem. 268:21410–21415. 1993.PubMed/NCBI
|
|
30.
|
York RD, Yao H, Dillon T, Ellig CL, Eckert
SP, McCleskey EW and Stork PJ: Rap1 mediates sustained MAP kinase
activation induced by nerve growth factor. Nature. 392:622–626.
1998. View Article : Google Scholar : PubMed/NCBI
|