Introduction
Aging is known to increase the propensity for the
occurrence of atrial arrhythmias, particularly atrial fibrillation
(AF) (1,2). AF usually occurs in conjunction with
other cardiovascular diseases; however, not all patients with AF
have an underlying disease (3).
This suggests that age-associated changes in the atrium may be
important in the development of AF (4). However, the electrophysiological
changes that cause the atria of elderly individuals to be more
susceptible to AF than those of younger adults remain poorly
understood.
The role of the left atrium in the development of AF
has previously been identified (5), and previous studies have shown that
in left atrial (LA) tissue, the action potential (AP) duration
(APD) is prolonged and the AP plateau becomes increasingly negative
with age (6,7). These alterations in the AP may
provide a substrate for reentry, which facilitates the occurrence
and maintenance of reentrant arrhythmias, including AF (8). The L-type Ca2+ current
(ICa.L) is the major current determining the plateau
level of the AP; thus, variations in ICa.L may lead to
changes in the AP plateau level. Previous studies have reported
that ICa.L is reduced in the right atrial (RA) cells of
aged canines compared with that in cells from younger adult canines
(9,10). However, to the best of our
knowledge, there are few published data on the effects of age on LA
ICa.L(7).
The ICa.L is mediated by the L-type
Ca2+ channel. The a1C (Cav1.2) subunit is considered to
be the most important polypeptide of the Ca2+
channel-forming proteins, since it forms the channel pore for ion
flow. However, published data concerning the effects of age on LA
Cav1.2 expression levels are lacking.
In the present study, we tested the hypothesis that
ICa.L and Cav1.2 expression levels in the left atrium
change with age, creating a substrate that favors the initiation of
AF. This was achieved by investigating the differences in
ICa.L and Cav1.2 expression in LA myocardia between
adult and aged dogs.
Materials and methods
Ethics
All experiments conformed to the Guide for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health (11). All
animal studies were approved by the Animal Use and Care Committee
of the First Teaching Hospital, Xinjiang Medical University
(Urumqi, China).
Animal preparation
Seven adult (2–2.5 years old) and ten aged (>8
years old) mongrels of either gender, weighing 18–26 kg, were used
in this study. The ages of the dogs were estimated by a
veterinarian based on standard measures for age, including
dentition, coat, eyes and musculoskeletal and conformational
descriptors. All dogs were anesthetized with sodium pentobarbital
(30 mg/kg) and ventilated with atmospheric air using a positive
pressure respirator. Core body temperature was maintained at
36.5±1.5°C. The dogs were subjected to twelve-lead
electrocardiograms (ECGs) to confirm sinus rhythm and
echocardiograms were performed to exclude structural heart disease.
Subsequently, continuous recording of standard ECG leads was
carried out to determine the heart rate and rhythm. Blood pressure
(BP) was continuously monitored via a pressure transducer
positioned in the right femoral artery.
The chest was entered via a left thoracotomy at the
4th intercostal space. Multi-electrode catheters (Biosense-Webster,
Diamond Bar, CA, USA) were secured to allow recording at the LA
appendage (LAA), left superior pulmonary vein (LSPV) and left
inferior pulmonary vein (LIPV). Similar electrode catheters were
attached to the RA appendage (RAA), right superior pulmonary vein
(RSPV) and right inferior pulmonary vein (RIPV) via a right
thoracotomy at the 4th intercostal space. All traces from the
electrode catheters were amplified and digitally recorded using a
computer-based Lab System (GE 2000; General Electric Company,
Fairfield, CT, USA). Bipolar electrograms were filtered at 30–500
Hz. ECG filter settings were 0.1–250 Hz.
Induction of AF
Rapid atrial pacing was delivered (1,000 bpm; 2X
threshold; duration, 1 msec) at the RAA. After 30 min, rapid atrial
pacing was terminated in order to measure AF inducibility.
Programmed stimulation at atrial myocardial sites or pulmonary vein
(PV) sleeves was performed using a programmable stimulator (DF-4A;
Suzhou Dongfang Electronic Instrument Factory, Suzhou, China).
Programmed pacing consisted of eight consecutive stimuli (S1–S1,
cycle length=330 msec) followed by a premature stimulus (S1–S2)
that was progressively decremented until refractoriness. Pacing was
performed at 2X diastolic threshold (TH) and at 4X TH. AF was
defined as irregular atrial rates (>500 bpm) associated with
irregular atrioventricular conduction (lasting >5 sec). The
window of vulnerability (WOV) was used as a quantitative measure of
AF inducibility. AF inducibility was quantitated as the longest
S1–S2 minus the shortest S1–S2 that induced AF at each pacing TH.
The cumulative WOV was the sum of the individual WOVs.
Atrial myocyte preparation
Following intravenous (i.v.) administration of
pentobarbital (30 mg/kg) and thoracotomy, the heart was rinsed in
oxygenated Ca2+-free Tyrode’s solution [137 mmol/l NaCl,
5.4 mmol/l KCl, 1.0 mmol/l MgCl2, 0.33 mmol/l
NaH2PO4,, 10 mmol/l HEPES and 10 mmol/l
glucose (adjusted to pH 7.4 with NaOH)]. The aorta was cannulated
and the heart was retrogradely perfused on a Langendorff apparatus
(ADInstruments, Inc., New South Wales, Australia) at 37°C. A
perfusion with Ca2+-free Tyrode’s solution for 5 min was
followed by perfusion with Ca2+-free Tyrode’s solution
containing 0.03% collagenase-II (Worthington Biochemical, Lakewood,
NJ, USA) and 1% BSA for 35 min. The left atria were dissected,
minced and gently triturated with a pipette in the
low-Ca2+ Tyrode’s solution containing 1% BSA at 37°C for
10 min. The cells were filtered through 200-μm nylon mesh
and resuspended in the Tyrode’s solution, in which the
Ca2+ concentration was gradually increased to 1.0
mmol/l. Only cells with a rod-shaped morphology and clear
cross-striation were used for subsequent experiments.
Cellular electrophysiology
LA cells were continuously superfused (2–3 ml/min)
in a 1-ml bath with normal Tyrode’s solution [137 mmol/l NaCl, 5.4
mmol/l KCl, 1.0 mmol/l MgCl2, 1.8 mmol/l
CaCl2, 0.33 mmol/l NaH2PO4,, 10
mmol/l HEPES and 10 mmol/l glucose (adjusted to pH 7.4 with NaOH)].
The solution was bubbled with 100% O2. Membrane currents
and APs were recorded using whole-cell patch-clamp techniques with
an EPC 10 Double amplifier and Patchmaster software (HEKA
Elektronik Dr. Schulze GmbH, Lambrecht/ Pfalz, Germany). Patch
pipette resistances ranged from 2.0–3.0 MΩ when filled with an
internal solution. APs were recorded in current-clamp mode. The
solution for AP recordings contained 137 mmol/l NaCl, 5.4 mmol/l
KCl, 1.0 mmol/l MgCl2, 1.8 mmol/l CaCl2, 10
mmol/l HEPES and 20 mmol/l glucose (adjusted to pH 7.4 with KOH).
The internal electrode solution for AP recordings contained 140
mmol/l KCl, 2.0 mmol/l MgCl2, 2.0 mmol/l egtazic acid,
5.0 mmol/l HEPES, 5 mmol/l EGTA and 4.0 mmol/l Na2 ATP
(adjusted to pH 7.4 with KOH). The Ca2+ currents were
recorded in voltage-clamp mode. The external solution for
ICa-L recording contained 137 mmol/l choline-Cl, 2.0
mmol/l CaCl2, 1.0 mmol/l MgCl2, 5 mmol/l
HEPES, 10 mmol/l glucose, 4.6 mmol/l CsCl, 10 mmol/l TEA-Cl and 5
mmol/l 4-aminopyridine (4-AP) (adjusted to pH 7.30 with CsOH). The
internal solution for ICa.L recording contained 120
mmol/l CsCl, 1.0 mmol/l MgCl2, 5.0 mmol/l MgATP, 10
mmol/l BAPTA, 10 mmol/l HEPES and 10 mmol/l TEA-Cl (adjusted to pH
7.3 with CsOH). Data acquisition was initiated 10 min after
membrane rupture. ICa.L magnitudes were normalized by
the membrane capacitance (pF) of each cell and expressed as current
density (pA/pF). Recordings were filtered using low-pass (2 Hz) and
high-pass (30 Hz) filters.
Detection of Cav1.2 gene expression
Total RNA was extracted from LAA samples using
TRIzol (Gibco-BRL, Carlsbad, CA, USA). Total RNA was reverse
transcribed using ReverTra Ace (Toyobo Biotech Co., Ltd., Osaka,
Japan). The expression levels of target genes were measured by
quantitative PCR (qPCR) using Sybr-Green qPCR Master Mix (Bio-Rad,
Hercules, CA, USA). In each assay, β-actin (used as an endogenous
control) and Cav1.2 genes from the same samples were amplified in
triplicate in separate tubes. The mRNA levels of Cav1.2 were
determined using the relative standard curve method, normalized
against the corresponding β-actin mRNA levels and then expressed as
the relative change from the control ± SD. The expected sizes of
amplicons were confirmed by gel electro-phoreses. The sequences of
the genes studied were obtained from GenBank and the primers were
designed using PRIMER 5.0 software (Applied Biosystems, Carlsbad,
CA, USA). The amplicon size and primer sequences for the genes are
shown in Table I.
 | Table I.Amplicon size and primer sequences of
genes. |
Table I.
Amplicon size and primer sequences of
genes.
| Gene | Primer
sequence | Amplicon size
(bp) |
|---|
| β-actin | F:
5′-AAGGACCTGTATGCCAACACA-3′ | 152 |
| R:
5′-ATCCACACAGAATACTTGCGTT-3′ |
| Cav1.2 | F:
5′-GACGCTATGGGCTATGAGTTAC-3′ | 199 |
| R:
5′-AGTCCAGGTAGCCCTTTAGGT-3′ |
Assessment of Cav1.2 protein
expression
For western blotting, 50 μg protein was
solubilized for 5 min at 95°C in one volume of loading buffer,
loaded onto 10% SDS-PAGE gels and then transferred to
nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The
membranes were blocked with 5% nonfat dry milk in PBST (containing
0.05% Tween 20), incubated overnight at 4°C with the primary
antibody (Cav1.2, 1:2,000; goat IgG, polyclone; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA), washed in PBST, incubated
with horseradish peroxidase-conjugated secondary antibody and
revealed using Immun-Star HRP Substrate (Bio-Rad). For
normalization of gel loading, the same western blots were reprobed
with anti-β-actin (dilution, 1:200; Santa Cruz Biotechnology Inc.).
The densities of the bands on the western blots were quantified
using an automatic gel imaging and analysis system (Bio-Rad).
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). All values are expressed
as the mean ± SD. Comparisons between the two groups were made
using the Student’s t test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ECG data
ECG data concerning the sinus rhythm for the adult
and aged groups are shown in Table
II. The ECGs of the aged group exhibited prolonged P wave
durations and increased P wave dispersion (PWD) compared with those
of the adult group. Other variables were not observed to differ
between the two groups.
 | Table II.ECG data of adult and aged dogs (mean
± SD). |
Table II.
ECG data of adult and aged dogs (mean
± SD).
| Group | P wave (msec) | PWD interval
(msec) | PR interval
(msec) | QRS interval
(msec) | QT interval
(msec) |
|---|
| Adult | 66.1±6.4 | 19.1±4.1 | 123.9±7.2 | 63.1±4.3 | 248.9±11.7 |
| Aged | 75.9±5.3a | 26.7±3.1a | 130.0±7.7 | 64.7±5.4 | 246.5±17.3 |
Induced AF
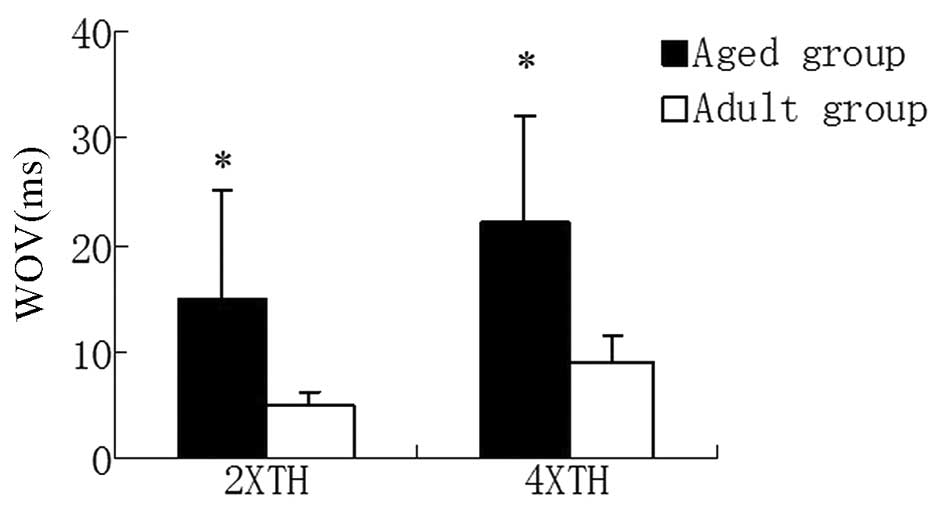
As shown in Fig. 1,
programmed electrical stimulation at the RAA in the aged group
induced a larger WOV (15±7.5 ms) compared with that of the adult
group (5±2.5 msec) during 2X TH. A similar result was observed
during 4X TH (aged group, 22±12 msec vs. adult group, 9±4.5
msec).
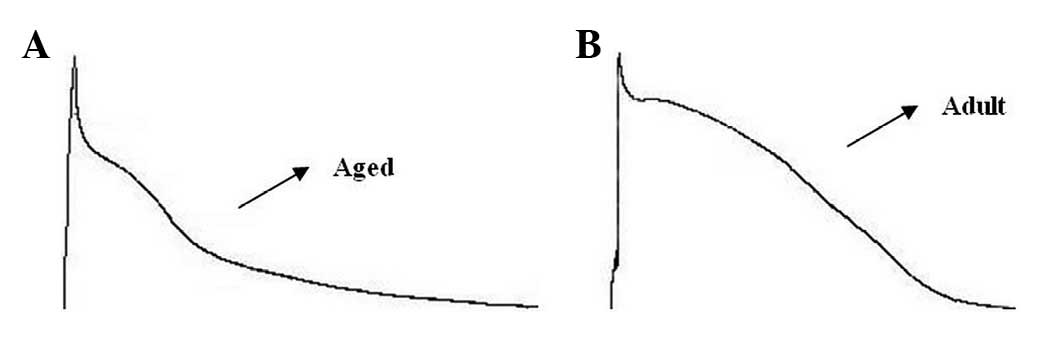
AP characteristics
LA cells from aged atria exhibited longer APDs and
lower plateau potentials compared with those from adult atria.
Representative AP recordings from the adult and aged groups are
shown in Fig. 2. AP
characteristics, at a cycle length of 2,000 msec, are shown in
Table III. While there were no
significant differences in the maximum diastolic potential (MDP) or
action potential amplitude (APA), action potential duration to 90%
repolarization (APD90) was longer in the aged dogs,
indicating that the slope of phase 3 repolarization was more
gradual in the aged than in the adult cells.
 | Table III.Action potential characteristics
recorded from adult and aged canine atria at a cycle length of 2000
msec (mean ± SD). |
Table III.
Action potential characteristics
recorded from adult and aged canine atria at a cycle length of 2000
msec (mean ± SD).
| Group | MDP (mv) | APA (mv) | Plateau (mv) | APD90
(msec) |
|---|
| Adult | −78.8±0.8 | 109.8±1.4 | −6.4±1.1 | 320.0±7.9 |
| Aged | −79.2±1.4 | 110.5±4.9 | −9.5±1.7 | 340.5±10.1a |
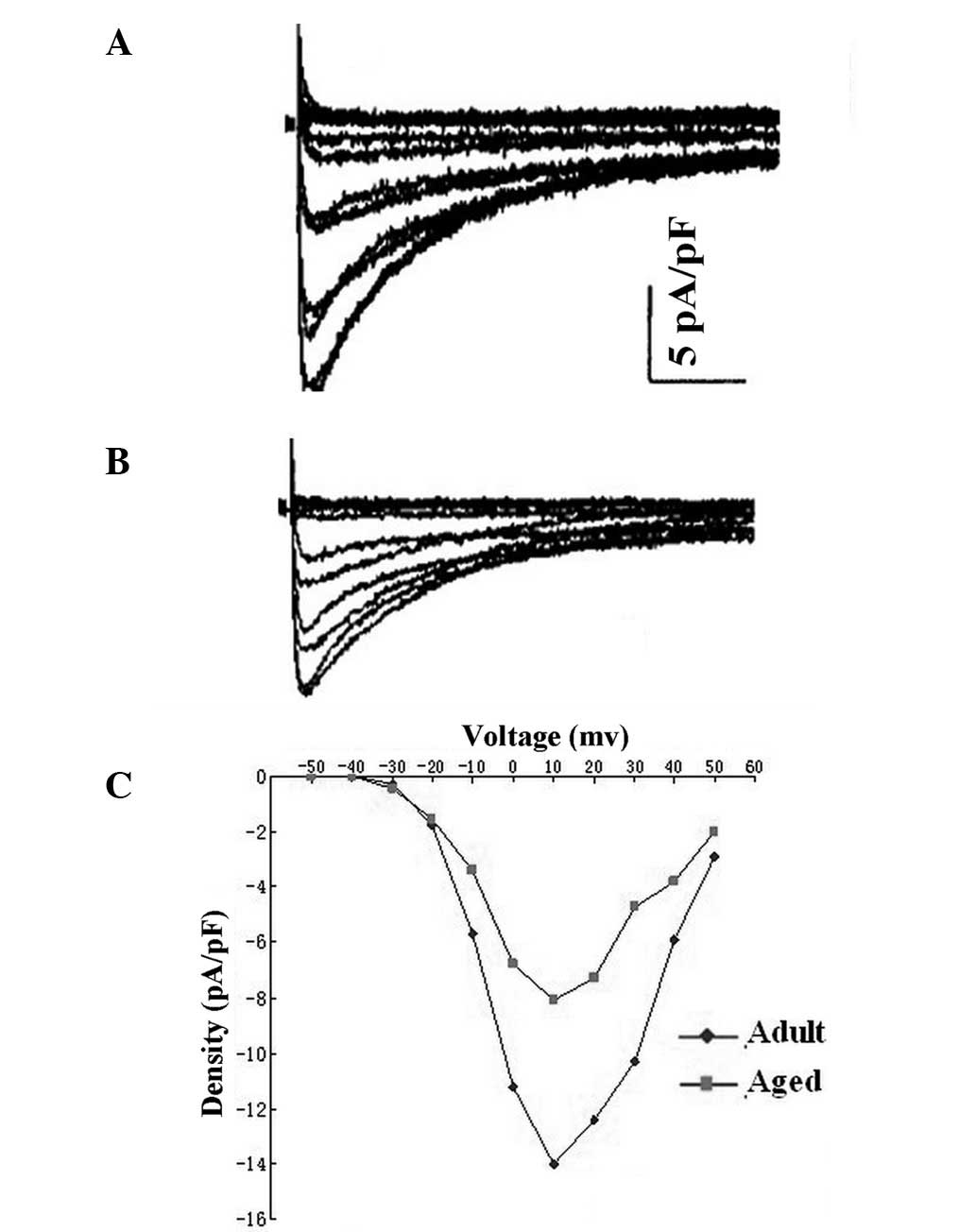
ICa.L
Typical ICa.L recordings from LA cells of
the adult and aged dogs are shown in Fig. 3. Aged LA cells had lower peak
ICa.L densities than adult LA cells (−8.1±0.5 vs.
−14.1±0.8, respectively, P<0.05; measured at +10 mV). Activation
voltage dependence was assessed from depolarization-induced
currents and the driving force was corrected with driving force
corrected by membrane potential-reversal potential, where reversal
potential is the voltage axis intercept of the ascending limb of
the current-voltage relation. There were no significant differences
in half-activation voltage or slope factor between the two groups
(Table IV). Inactivation was
assessed with 1-sec prepulses of −60, −50, −40, −30, −20, −10, 0,
10, 20, 30 and 40 mV, followed by 250-msec test pulses to +10 mV.
Furthermore, the current reduction in the aged cells was not
accompanied by a significant change in the refractory period
(Table IV).
 | Table IV.Electrophysiological characteristics
of the L-type calcium current (ICa.L) in adult and aged
canine LA cells (mean ± SD). |
Table IV.
Electrophysiological characteristics
of the L-type calcium current (ICa.L) in adult and aged
canine LA cells (mean ± SD).
| Group | n | ICa.L
density (pA/pF) | Steady-state
activation | Steady-state
inactivation | Monoexponential
recovery time constants (msec) |
|---|
|
|
|---|
| V0.5
(mV) | K (mV) | V0.5
(mV) | K (mV) |
|---|
| Adult | 14 | −8.1±0.5 | −7.1±1.5 | 5.7±0.4 | −23.1±2.1 | 6.2±0.3 | 51.9±3.3 |
| Aged | 16 | −14.1±0.8a | −6.7±2.8 | 5.5±0.5 | −22.9±3.3 | 6.4±0.5 | 53.1±3.1 |
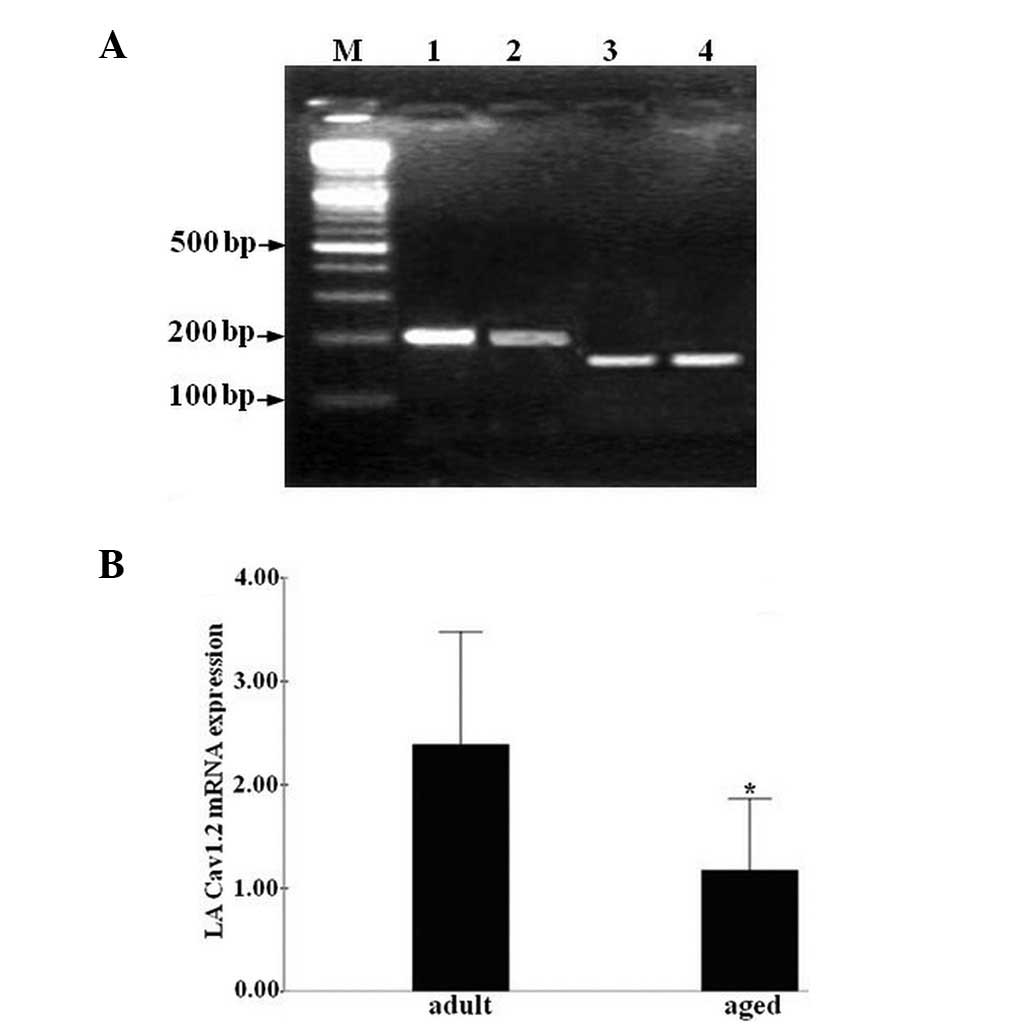
Cav1.2 gene expression in LA cells
To study Cav1.2 expression in LA cells, Cav1.2 mRNA
levels were analyzed using qPCR. The specificity of the amplified
PCR product was verified using agarose gel electrophoresis and
non-specific DNA fragments were not detected (Fig. 4A). Cav1.2 mRNA levels were
decreased in the aged group compared with those in the adult group
(P<0.05, Fig. 4B).
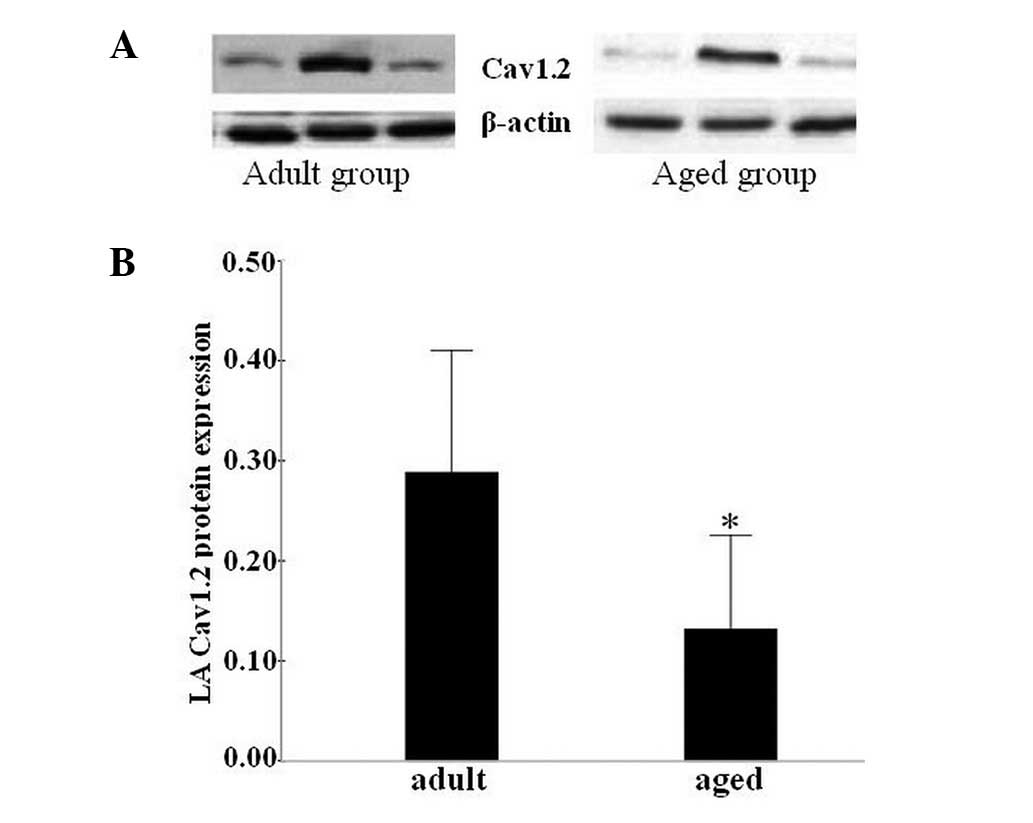
Cav1.2 protein expression in LA
cells
To confirm the qPCR results, western blotting was
performed with Cav1.2 antibodies. Fig.
5A presents the bands from a gel on which the Cav1.2 protein
levels from LA cells were studied. The β-actin bands were used to
confirm that the loading was equal. Densitometric data demonstrated
that the Cav1.2 protein levels were significantly lower in the aged
group compared with those in the adult group (P<0.05, Fig. 5B).
Discussion
In the present study, the susceptibility to AF was
greater in the aged dog group than in the adult group. We
hypothesized that certain electrophysiological changes in the atria
were responsible for this difference. We demonstrated that certain
characteristics of the APs in LA cells change with increasing age.
The most marked alteration was a significant lowering of the
plateau potential in aged LA cells. Previous studies have shown
that negative plateau potentials have a lower driving force in the
conduction of early premature beats (12). Therefore, our results imply that
alterations in the APs of aged atrial cells are likely to lead to a
decreased conduction of premature beats in aged atria. The slow
conduction of early premature impulses may further facilitate the
onset of AF. The currents that determine the plateau level of APs
in the atrium are IKur, Ito and
ICa.L(13). Therefore,
a reduction in the depolarizing current (ICa.L) or an
increase in the repolarizing currents (IKur and/or
Ito) may lead to a lower AP plateau (14). Moreover, the APD90 value
in LA cells was prolonged with age; this may be the result of
age-induced changes in the delayed rectifier potassium current
(IK) or may simply be a consequence of the low AP
plateau in aged canines.
In addition to age-associated changes in the
ICa.L of LA cells, previous studies have also reported
that ICa.L is reduced in the RA cells of aged canines
compared with those of adult canines. However, no previous studies
have reported on the effects of age on LA cell ICa.L. In
the current study, we showed that there was a significant reduction
in the peak ICa.L in aged canine LA cells compared with
that in adult canine LA cells. The current reduction in aged cells
was not accompanied by a significant change in Ca2+
channel availability or recovery from inactivation. These results
suggest that a reduction in the ICa.L is a major
mechanism underlying the low AP plateau in aged canine LA
cells.
The PWD was significantly longer in the aged dogs
than in the adult canines. This may be a result of the degree of
age-associated reduction in the conduction of the atria (15). Age-related changes in the content
and distribution of connective tissue in the atria reduce the
degree of cellular coupling and lead to discontinuous propagation,
thus slowing conduction (16).
Under normal conditions, sufficient depolarizing current is
transferred across such discontinuities to maintain normal
propagation. However, when the depolarizing current is reduced,
conduction slows (17). The
driving force of the discontinuous conduction is determined by the
plateau potential, and the ICa.L is important in the
maintenance of conduction (18,19).
Therefore, the present results imply that the reductions in
ICaL and AP plateau potential in aged LA cells may lead
to the occurrence of discontinuous conduction. These findings may
constitute a mechanism via which aged atria become more susceptible
to AF.
In cardiac myocytes, Ca2+ currents
through L-type Ca2+ channels are the main mechanism for
Ca2+ influx from the extracellular space into the
cytoplasm (20). Cardiac L-type
Ca2+ channels are composed of four polypeptide subunits
(α1, β, α2 and δ) (21). The α1 subunit is the most important
polypeptide of the Ca2+ channel-forming proteins; it
forms the channel pore for ion flow and is responsible for
voltage-dependent Ca2+ channel opening and channel
selectivity for Ca2+ ions (22,23).
To date, at least 10 different α1 subunit genes have been
identified, but only the a1C (Cav1.2) isoform is expressed at high
levels in cardiac muscle (24).
The current study demonstrated that Cav1.2 mRNA and protein
expression levels in LA cells were significantly lower in the aged
group compared with those in the adult group. This may be the main
cause of the reduction in ICa.L in aged canines. Jones
et al (25) obtained
similar findings, demonstrating that within the sinoatrial (SA)
node, an age-related decline in the expression levels of the Cav1.2
protein caused the suppression of AP formation and propagation,
leading to failure of the SA node as a pacemaker. Therefore, atria
Cav1.2 protein levels may decrease with age. However, research has
been limited to studies using Cav1.2 protein from only one region
of the left atrium and SA node (one region of RA). The mechanisms
underlying these changes are unknown. We hypothesized that aging
may result in the progressive deterioration of physiological
functions and metabolic processes, which alters the density and
distribution of ion channels.
Previous studies have attached particular importance
to the left atrium in the initiation and maintenance of AF
(26,27). The present study demonstrated that
there were age-associated changes in the electrophysiological
properties and ion channels of the LA myocardium. A lower AP
plateau potential and decreased ICa.L in the LA cells of
aged canines may contribute to the slow and discontinuous
conduction in the left atrium. These changes increase the
susceptibility of aged atria to AF. Furthermore, the decreased
expression of Cav1.2 with age may be the cause of the reduction in
ICa.L with increasing age. However, further studies of
the mechanisms of alteration in Cav1.2 expression are required.
Although the present study demonstrated
age-associated electrophysiological and molecular changes in the LA
cells of aged canines, the extent to which these effects are
clinically applicable remains to be determined.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (no. 308660299), the
Natural Science Foundation of the Xinjiang Uygur Autonomous Region
(no. 200821143) and the Doctoral Fund of the Ministry of Education
(no. 200807600004).
References
|
1.
|
Chen LY and Shen WK: Epidemiology of
atrial fibrillation: a current perspective. Heart Rhythm. 4(Suppl):
S1–S6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Go AS: The epidemiology of atrial
fibrillation in elderly persons: the tip of the iceberg. Am J
Geriatr Cardiol. 14:56–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Murgatroyd FD and Camm AJ: Atrial
arrhythmias. Lancet. 341:1317–1322. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Allessie MA, Boyden PA, Camm AJ, et al:
Pathophysiology and prevention of atrial fibrillation. Circulation.
103:769–777. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Corradi D, Callegari S, Maestri R, Benussi
S and Alfieri O: Structural remodeling in atrial fibrillation. Nat
Clin Pract Cardiovasc Med. 5:782–796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Anyukhovsky EP, Sosunov EA, Chandra P, et
al: Age-associated changes in electrophysiologic remodeling: a
potential contributor to initiation of atrial fibrillation.
Cardiovasc Res. 66:353–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dun W and Boyden PA: Aged atria:
electrical remodeling conducive to atrial fibrillation. J Interv
Card Electrophysiol. 25:9–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nattel S: Atrial electrophysiological
remodeling caused by rapid atrial activation: underlying mechanisms
and clinical relevance to atrial fibrillation. Cardiovasc Res.
42:298–308. 1999. View Article : Google Scholar
|
|
9.
|
Dun W, Yagi T, Rosen MR and Boyden PA:
Calcium and potassium currents in cells from adult and aged canine
right atria. Cardiovasc Res. 58:526–534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tipparaju SM, Kumar R, Wang Y, Joyner RW
and Wagner MB: Developmental differences in L-type calcium current
of human atrial myocytes. Am J Physiol Heart Circ Physiol.
286:H1963–H1969. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bayne K: Revised Guide for the Care and
Use of Laboratory Animals available. American Physiological Society
Physiologist. 39:199208–211. 1996.PubMed/NCBI
|
|
12.
|
Sugiura H and Joyner RW: Action potential
conduction between guinea pig ventricular cells can be modulated by
calcium current. Am J Physiol. 263:H1591–H1604. 1992.PubMed/NCBI
|
|
13.
|
Yue L, Feng J, Li GR and Nattel S:
Transient outward and delayed rectifier currents in canine atrium:
properties and role of isolation methods. Am J Physiol.
270:H2157–H2168. 1996.PubMed/NCBI
|
|
14.
|
Anyukhovsky EP, Sosunov EA, Plotnikov A,
et al: Cellular electrophysiologic properties of old canine atria
provide a substrate for arrhythmogenesis. Cardiovasc Res.
54:462–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Podrid PJ: Atrial fibrillation in the
elderly. Cardiol Clin. 17:173–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Spach MS and Dolber PC: Relating
extracellular potentials and their derivatives to anisotropic
propagation at a microscopic level in human cardiac muscle.
Evidence for electrical uncoupling of side-to-side fiber
connections with increasing age. Circ Res. 58:356–371. 1986.
View Article : Google Scholar
|
|
17.
|
Spach MS, Miller WT, Dolber PC, Kootsey
JM, Sommer JR and Mosher CE Jr: The functional role of structural
complexities in the propagation of depolarization in the atrium of
the dog. Cardiac conduction disturbances due to discontinuities of
effective axial resistivity. Circ Res. 50:175–191. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shaw RM and Rudy Y: Ionic mechanisms of
propagation in cardiac tissue. Roles of the sodium and L-type
calcium currents during reduced excitability and decreased gap
junction coupling. Circ Res. 81:727–741. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Rohr S and Kucera JP: Involvement of the
calcium inward current in cardiac impulse propagation: induction of
unidirectional conduction block by nifedipine and reversal by Bay K
8644. Biophys J. 72:754–766. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Richard S, Perrier E, Fauconnier J, et al:
‘Ca2+-induced Ca2+ entry’ or how the L-type
Ca2+ channel remodels its own signalling pathway in
cardiac cells. Prog Biophys Mol Biol. 90:118–135. 2006.
|
|
21.
|
Yamakage M and Namiki A: Calcium channels
- basic aspects of their structure, function and gene encoding;
anesthetic action on the channels - a review. Can J Anaesth.
49:151–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wang MC, Dolphin A and Kitmitto A: L-type
voltage-gated calcium channels: understanding function through
structure. FEBS Lett. 564:245–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bodi I, Mikala G, Koch SE, Akhter SA and
Schwartz A: The L-type calcium channel in the heart: the beat goes
on. J Clin Invest. 115:3306–3317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Treinys R and Jurevicius J: L-type
Ca2+ channels in the heart: structure and regulation.
Medicina (Kaunas). 44:491–499. 2008.
|
|
25.
|
Jones SA, Boyett MR and Lancaster MK:
Declining into failure: the age-dependent loss of the L-type
calcium channel within the sinoatrial node. Circulation.
115:1183–1190. 2007.PubMed/NCBI
|
|
26.
|
Tada H, Kurosaki K, Ito S, et al: Left
atrial and pulmonary vein ostial ablation as a new treatment for
curing persistent atrial fibrillation. Circ J. 69:1057–1063. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Roithinger FX, Steiner PR, Goseki Y,
Sparks PB and Lesh MD: Electrophysiologic effects of selective
right versus left atrial linear lesions in a canine model of
chronic atrial fibrillation. J Cardiovasc Electrophysiol.
10:1564–1574. 1999. View Article : Google Scholar : PubMed/NCBI
|