Introduction
The repair and regeneration of lost or damaged
alveolar bone presents significant problems in dentistry. Previous
studies have indicated that the chances of success are increased
when the induction of regeneration is induced with autologous
compounds, since the use of autologous compounds is associated with
an improved prognosis and the absence of the biocompatibility
problems that may occur with synthetic graft materials or grafts of
a heterologous nature (1,2).
Two platelet preparation products, platelet-rich
plasma (PRP) and plasma rich in growth factors (PRGF), have shown
positive effects in the regeneration process, as observed in
experimental studies (2–6). PRP has demonstrated an ability to
improve the regenerative processes (3), accelerate the regeneration of
periodontal defects and promote bone formation (4). Moreover, treatment with PRGF
following third molar exodontia has been shown to be successful in
stimulating bone regeneration. PRGF has also been shown to increase
the speed of wound epithelialization of the oral mucosa and improve
post-operative recovery (2,5,6).
PRP and PRGF act on already differentiated cells,
such as preosteoblasts and osteoblasts; however, they do not exert
any effects on the stem cells present in bone tissue, whose
differentiation is regulated by bone morphogenetic proteins
(BMPs).
It has been shown that drugs of the statins group
promote BMP expression, particularly BMP-2, acting indirectly in
the bone regeneration process (7,8). One
of these drugs, simvastatin (SIMV, also known as MK-733), has been
demonstrated to exhibit favorable effects in the treatment of bone
remodeling disorders and bone fractures through the promotion of
osteogenesis and the reduction of bone resorption (8,9).
Despite the numerous studies describing the benefits
of PRP, PRGF and SIMV separately, there has been a lack of
investigation into the simultaneous use of these agents. Therefore
the main objective of this study was to evaluate the effect of a
combined application on the repair of alveolar bone damaged by
thermal injury (caused by a rotary instrument) in an animal
model.
Materials and methods
Study design
This study used an experimental design of randomized
blocks. The independent variable was the induction of alveolar bone
damage, while the dependent variable was tissue regeneration. All
procedures used in the study were in accordance with the guidelines
in the Guide for the Care and Use of Laboratory Animals (10) and were approved by the Bioethics
Committee of the University of Talca (Talca, Chile).
Sample size
In order to determine the sample sizes required, a
recursive equation was used, as follows: E = N − T − B (10), where E is the degrees of freedom of
the error, N is the total degrees of freedom, T is the degrees of
freedom of the treatment (number of treatments minus 1) and B is
the degrees of freedom between the blocks (block number minus one)
(11).
Experimental groups
Eighteen male, non-consanguineous Sprague Dawley
rats, aged 12 weeks and weighing 330–430 g, were obtained with the
corresponding health certification from the Institute of Biomedical
Sciences (ICBM), University of Chile (Santiago, Chile). The rats
were housed at a controlled temperature (22±1°C) under a12-h
light/dark cycle (the light was turned on at 8.00 a.m.), with
freely available food and water. Each polycarbonate enclosure
(20×19×31 cm3) contained three rats and was enriched
with tissue paper and cardboard rolls (12). The rats were housed at the Animal
Facility of the University of Talca.
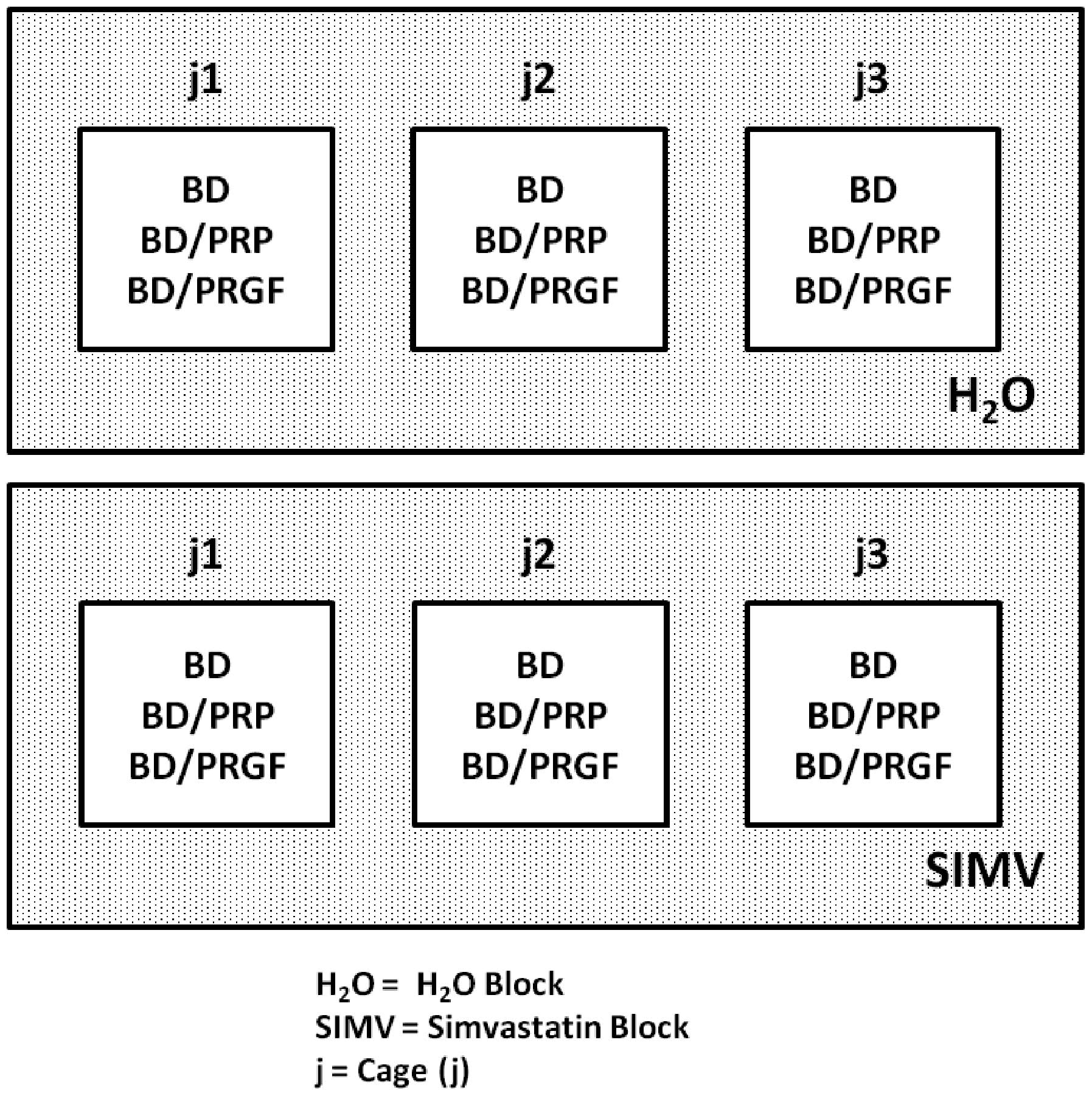
The rats were divided into six groups, in two blocks
(Fig. 1): the H2O block
(distilled water without the drug) and the SIMV block (water with
SIMV). The H2O block was subdivided into three groups,
as follows: BD/H2O (n=3), only alveolar bone damage;
BD/H2O/PRP (n=3), alveolar bone damage and PRP; and
BD/H2O/PRGF (n=3), alveolar bone damage and PRGF. The
SIMV block was also subdivided into three groups: BD/SIMV (n=3),
alveolar bone damage and SIMV; BD/SIMV/PRP (n=3), alveolar bone
damage, PRP and SIMV; and BD/SIMV/PRGF (n=3), alveolar bone damage,
PRGF and SIMV.
Procedures
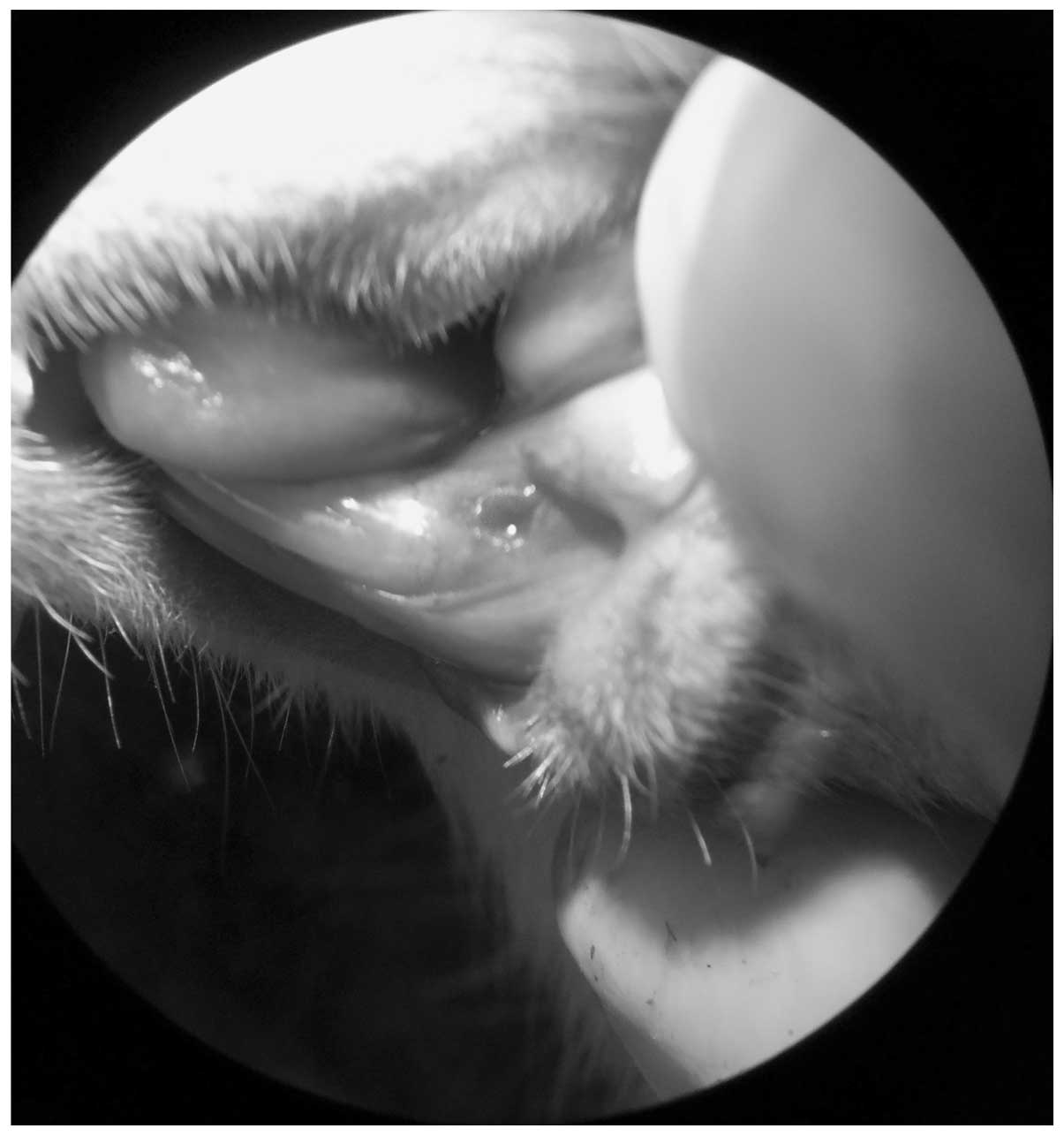
Alveolar bone damage induced by
thermal injury (BD)
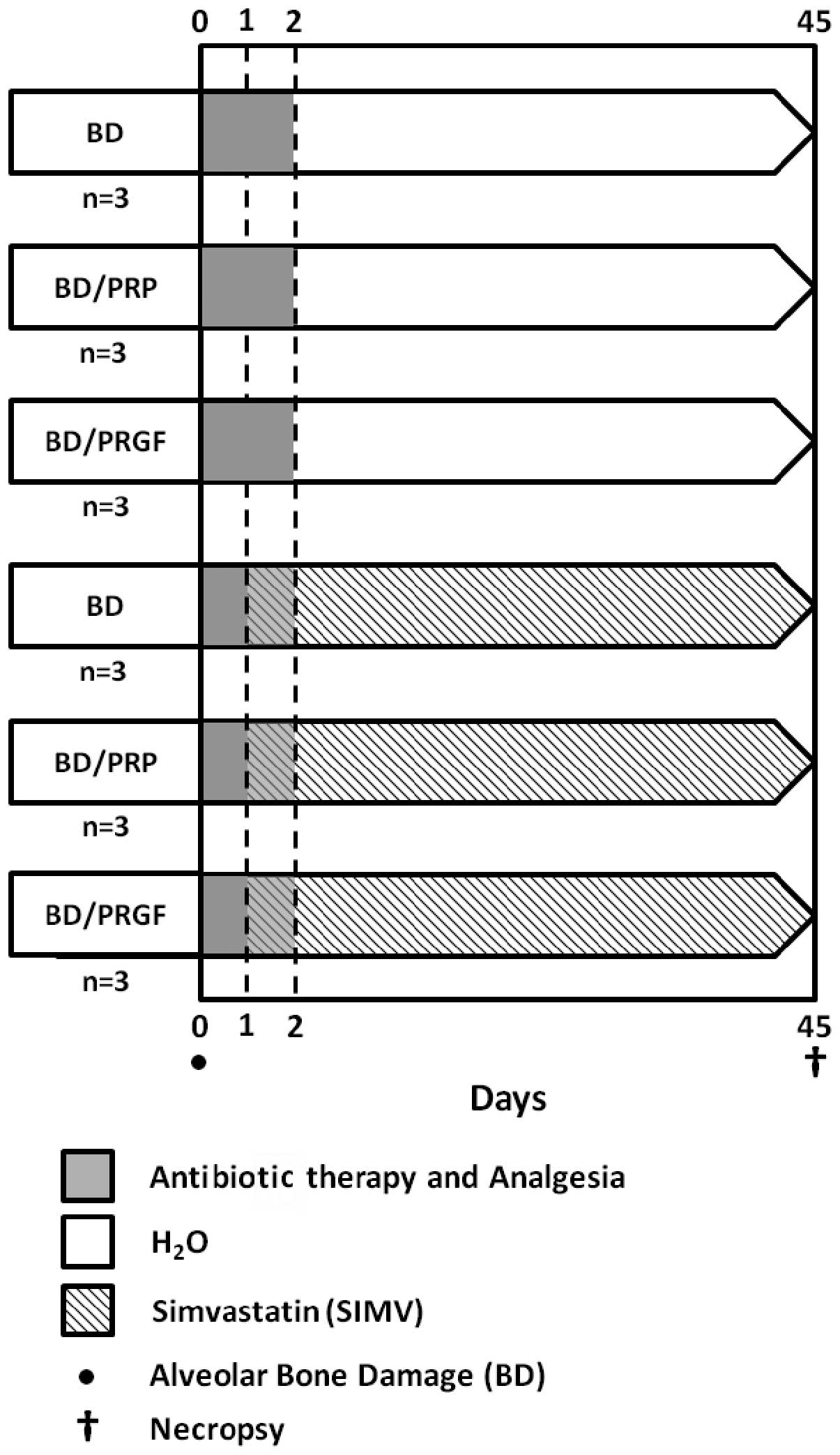
The principal procedures of the experimental phase
(total, 45 days) are shown in Fig.
2. Prior to the intraoral procedures, the rats were
anesthetized with 10% ketamine/2% xylazine/1% acepromazine (Drag
Pharma Chile Invetec S.A., Santiago, Chile) at a ratio of 50/5/1
mg/kg, intramuscularly (IM). Having established anesthesia, a
thermal injury was induced with a low-velocity 0.8-mm carbide drill
at 30,000 rpm, which was oriented in an axial direction, and with
constant physiological serum irrigation 2 mm distal to the left
lower incisor (Fig. 3). The damage
depth was determined with the active tip of the carbide drill.
PRP
PRP was obtained by the methods previously described
(13), with certain modifications.
Briefly, one Sprague Dawley rat was sacrificed in order to obtain
sufficient PRP for three surgical procedures. Using cardiac
puncture and a full bleed, 1.5 ml blood was obtained, which was
collected in a sterile syringe. The blood was deposited into
Eppendorf tubes with sodium citrate as an anticoagulant. The tubes
were then subjected to an initial centrifugation at 2,900 × g for
10 min. The platelet-rich fraction was separated and centrifuged
again at 600 × g for 15 min. For platelet activation, the plasma
was mixed for 60–90 sec using bovine thrombin (Thermo
Scientific®, Thermo Fisher Scientific, Inc., Waltham,
MA, USA) to produce a mixture with a gelatinous consistency that
was applied to the mandibular bone defects. The edges of the
incision were brought together with sutures, in order to prevent
the displacement of the gel.
PRGF
PRGF was obtained by the methods described
previously (14), with
modifications. One Sprague Dawley rat was sacrificed in order to
obtain sufficient PRGF for ten surgical procedures. Using cardiac
puncture, 1.5 ml blood was collected in a sterile syringe (13). The blood was then placed into
Eppendorf tubes with 3.8% sodium citrate solution as an
anticoagulant, prior to the tubes being exposed to centrifugation
at 300 × g for 8 min. With this process, the blood components were
separated into three phases. Phase I, the most superficial,
corresponded to the phase of platelet-poor plasma. This was
followed by Phase II, which had an intermediate concentration of
platelets, similar to that observed in circulating blood, and then
Phase III, which was located immediately above the white phase of
leukocytes and was the phase with the greatest concentration of
growth factors. Using a sterile pipette, the first two phases were
eliminated and the growth factor-rich Phase III was then
transferred to a new Eppendorf tube for activation with 10% calcium
chloride (ratio of 0.05 ml CaCl per 1 ml plasma). From this
procedure, a substance with a gelatinous consistency was produced,
which was applied to the bone defects of the left mandibular arch.
The edges of the incision were brought together with sutures to
prevent the displacement of the gel.
Post-surgical pharmacological
therapy
Following surgery, the animals received antibiotic
therapy, comprising 16,000 UI benzathine benzylpenicillin, IM, and
anti-inflammatory therapy, comprising 500 mg/500 ml acetaminophen,
which was administered via the common water during the first two
days subsequent to surgery, in accordance with the previously used
protocol (15).
SIMV
Following previously published guidelines and
methods (16,17), SIMV (Simvass; Laboratorios Rider
S.A., Santiago, Chile) was orally administered as a SIMV suspension
diluted in distilled water, at a dose of 20 mg/kg/day, for 6 weeks.
Free administration, using opaque bottles, commenced the day
subsequent to the induction of the bone damage by thermal
injury.
Sample collection
At the end of the experimental phase, the animals
were sacrificed by an overdose of anesthetics. The jaws of the rats
were hemisectioned and then fixed with 10% formalin, prior to
dentoalveolar segments, containing alveolar bone, the left lower
incisor and gingival tissue, being obtained. Tissue blocks from the
left sectioned jaw were dehydrated with 5% nitric acid for seven
days, in order to perform a subsequent conventional
histopathological analysis using hematoxylin and eosin (H&E)
staining.
Histological evaluation
The histological analysis was conducted by an
academic (CR) from the University of Talca. The observer was
unaware of the group to which the study samples belonged
(single-blind model). For the histological analysis of the
preparations, a Carl Zeiss Primo Star Trinocular (stereo)
microscope (Carl Zeiss MicroImaging Inc., Gottingen, Germany) was
used. Images were captured using a digital camera connected to the
microscope and then connected to a computer. A qualitative analysis
was performed for the histological assessment of the samples, in
order to determine the presence or absence (dichotomous variable)
of nine parameters for the regeneration/repair of bone, based on
the texts by Leeson et al(18) and Gartner and Hiatt (19) (Table
I).
 | Table IBone regeneration/reparation
parameters. |
Table I
Bone regeneration/reparation
parameters.
| Parameter | Description | Primary bone
tissue | Secondary bone
issue | Remodeled secondary
bone tissue |
|---|
| Mature osteon | Basic bone unit
(UBO) present in secondary bone tissue, with defined order | − | + | + |
| Immature
osteon | Basic bone unit
(UBO) present in primary bone tissue, undefined order | + | − | + |
| Central
channels | Central channel
observable in the osteon (both mature and immature) | + | + | + |
| Random collagen
fibers | Collagenous fibers
directed in all directions, not well-defined sheets | + | − | + |
| Organized collagen
fibers | Collagen fibers of
parallel course arranged on concentric lamellae | − | + | + |
| Globular
osteocytes | Young osteocytes
easily observable under the microscope, contained in rounded
lagoons between poorly defined lamellae | + | − | + |
| Ellipsoidal
osteocytes | Mature osteocytes
contained in ellipsoidal lagoons, visible between ordered
lamellae | − | + | + |
| Highly inorganic
matrix | Presence of
mineralized matrix | − | + | + |
| Osteoid tissue | Presence of
non-mineralized organic matrix | + | − | + |
Statistical analysis
A χ2 test was used for the statistical
analysis, with certain corrections according to the expected
frequencies in the contingency tables. P<0.05 was considered to
indicate a statistically significant difference.
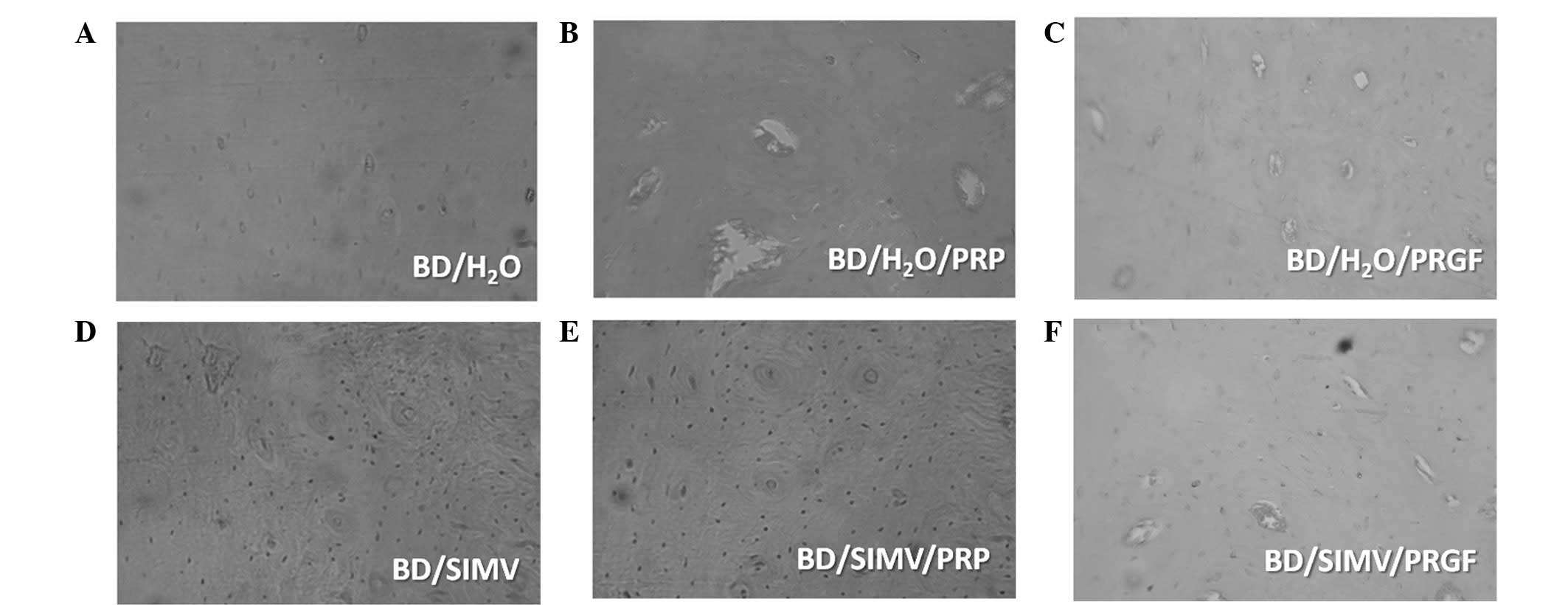
Results
Following the histological procedure, 36 plaques
were selected for analysis (six representatives for each study
group). The histological characteristics for each group are shown
in Fig. 4.
General histological characteristics
In group BD/H2O there was a dominance of
mature osteons with a well-defined structural order, characterized
by high mineralization and an absence of immature structures. Group
BD/PRP showed the presence of osteons in different phases of
maturation, in addition to globular (large) and ellipsoidal
osteocytes. In group BD/PRGF an equal presence of mature and
immature osteons was observed, in addition to large globular and
ellipsoidal osteocytes. Group BD/SIMV exhibited the presence of
mature osteons with a narrow central channel, ellipsoidal
osteocytes and a high inorganic component. Group BD/SIMV/PRP showed
the presence of osteons at different stages of maturation with
narrow central channels and ellipsoidal osteocytes. In group
BD/SIMV/PRGF, immature osteons with a central channel and blood
vessels were observed, in addition to ellipsoidal osteocytes.
Relevant comparisons
Table II shows the
features observed in the histological analysis. Mature osteons were
observed in the plates of all the groups; however, statistically
significant differences were apparent between groups
BD/H2O/PRP and BD/SIMV/PRP (67% and 100% presence,
respectively; χ2 test; P=0.035). In groups
BD/H2O/PRP, BD/H2O/PRGF and BD/SIMV/PRGF
there was a 100% presence of immature osteons, while the
BD/H2O and BD/SIMV/PRP groups were significantly
different with 17 and 33% presence, respectively (χ2
test; P≤0.05). In group BD/SIMV, no immature osteons were observed.
In group BD/H2O/PRP there was a 50% presence of random
collagen fibers, while in group BD/H2O random collagen
fibers were absent from all the plates examined. The latter result
was precisely the opposite of that observed for ordered collagen
fibers, which were present in all the examined plates
(χ2 test; P=0.046). The BD/H2O/PRP group
showed a 100% presence of large, globular osteocytes, which was
significantly different to the BD/H2O/PRGF group, in
which the presence was 50%, and these osteocytes were absent from
the BD/H2O group (χ2 test; P≤0.05). Group
BD/SIMV/PRP exhibited globular osteocytes in 67% of the plates,
which was statistically different from group BD/SIMV, in which no
globular osteocytes were present (χ2 test; P=0.038). No
statistically significant differences where observed between the
groups that used platelet preparations (BD/H2O/PRP and
BD/H2O/PRGF) and those treated with platelet
preparations plus SIMV (BD/SIMV/PRP and BD/SIMV/PRGF). Groups
BD/H2O/PRGF and BD/H2O/PRP exhibited a 50%
presence of osteoid tissue, while no osteoid tissue was observed in
group BD/H2O (χ2 test; P=0.046). With regard
to the characteristic of a highly inorganic matrix, the
BD/H2O group showed a 100% presence, while in group
BD/H2O/PRGF the presence had decreased to 50%
(χ2 test; P=0.046).
 | Table IICount of histological features in
each treatment. |
Table II
Count of histological features in
each treatment.
|
Treatments |
|---|
|
|
|---|
|
BD/H2O |
BD/H2O/PRP |
BD/H2O/PRGF | BD/SIMV | BD/SIMV/PRP | BD/SIMV/PRGF |
|---|
|
|
|
|
|
|
|
|---|
| Features | P | A | P | A | P | A | P | A | P | A | P | A |
|---|
| Mature osteon | 6 | 0 | 4 | 2 | 6 | 0 | 6 | 0 | 6 | 0 | 6 | 0 |
| Immature
osteon | 1 | 5 | 6 | 0 | 6 | 0 | 0 | 6 | 2 | 4 | 6 | 0 |
| Central
channels | 6 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 6 | 0 |
| Random collagen
fibers | 0 | 6 | 3 | 3 | 3 | 3 | 1 | 5 | 1 | 5 | 0 | 6 |
| Organized collagen
fibers | 6 | 0 | 5 | 1 | 3 | 3 | 5 | 1 | 6 | 0 | 6 | 0 |
| Globular
osteocytes | 0 | 6 | 6 | 0 | 3 | 3 | 0 | 6 | 4 | 2 | 1 | 5 |
| Ellipsoidal
osteocytes | 5 | 1 | 5 | 1 | 2 | 4 | 6 | 0 | 6 | 0 | 5 | 1 |
| Highly inorganic
matrix | 6 | 0 | 4 | 2 | 3 | 3 | 6 | 0 | 6 | 0 | 5 | 1 |
| Osteoid tissue | 0 | 6 | 3 | 3 | 3 | 3 | 0 | 6 | 0 | 6 | 0 | 6 |
Discussion
For the histological assessment, nine variables were
analyzed, corresponding with the morphological characteristics of
normal bone tissue (19–21) and the indicators for bone
regeneration. The groups treated with BD and PRP
(BD/H2O/PRP) and with BD and PRGF
(BD/H2O/PRGF) showed the presence of osteons with
central (Haversian) channels, random collagen fibers, large,
globular osteocytes and a lower degree of mineralization compared
with the control group (BD/H2O). The presence of these
variables, primarily the presence of osteons, whether mature or
immature, was indicative of bone tissue, since osteons are the
basic structural unit of this type of tissue (or BSU, Bone
Structural Unit, according to Geneser, 2008).
Defining the concept of repair as the restoration of
a lesion by a tissue that differs from the original in morphology
and function, with the existence of a greater presence of fibrous
tissue (22), it was proposed that
the BD/H2O/PRP and BD/H2O/PRGF groups
exhibited a healed bone tissue that was a result of regeneration
and not reparation. This was due to the fact that, in all the
groups, the new-formed tissue was indistinguishable from the
original tissue (23).
The results of this study were consistent with those
of previous studies (4,13,24),
in which it was concluded that platelet preparations comparable
with those used in the current study favor and accelerate bone
regeneration. When comparing the BD/PRR and BD/PRGF groups, the
results showed no statistically significant differences in
histological variables, and the features observed were similar in
the two groups. This suggested that there was not likely be any
difference between the use of either platelet preparation
(according to the analysis in the present study) and that
regeneration indicators were likely to remain essentially the same,
irrespective of the preparation used to treat the damage.
Furthermore, it was indicated that PRP or PRGF were likely to
result in regeneration based on a possible bone adaptable for
masticatory function.
In this study, a second block of groups was treated
with 10 mg SIMV, orally, for a period of 42 days. The results
suggested that SIMV exerted a favorable effect on bone
regeneration, since the histological parameters analyzed revealed
the formation of a mature compact bone, with the presence of
organized, mature osteons with a high mineral content.
SIMV, while acting on the mevalonate pathway,
generates increased bone formation and decreased bone resorption
(9,25). This may explain the results of the
histological analysis for the samples of the BD/SIMV group, in
which the bone tissue was observed to be mature, highly organized
(100% presence of mature osteons), with the presence of central
channels (100% presence), organized osteocytic lacunae (100%
presence of ellipsoidal osteocytes), a high mineral content (100%
presence of inorganic matrix) and ordered collagen fibers (83%
presence). These results were consistent with those of a previous
study, in which it was concluded that SIMV had the potential to
increase and maintain osteoblastic function, allowing the
regeneration of lost alveolar bone in rats with induced
periodontitis (26).
Another study evaluated the efficacy of the oral
administration of SIMV in the process of repairing defects in the
tibia of ovariectomized rats (17). SIMV had a favorable effect on bone
regeneration; this was observed in a group of rats treated with the
drug, where the bone formation was higher than that in the control
group, similar to other prior results (27). The results were indicative of high
osteoblastic activity and osteoclast scarcity, which showed the
action of the SIMV. By inhibiting the
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase enzyme
involved in the mevalonate pathway, SIMV stimulates osteoblastic
differentiation and promotes the expression of BMP-2, while
simultaneously decreasing the process of bone resorption by
blocking the prenylation of certain signaling molecules that are
essential for osteoclastic activity (28,29).
This was consistent with the favorable effect of SIMV on bone
tissue regeneration observed in the BD/SIMV group, since the tissue
exhibited features of mature or secondary-type bone tissue and was
highly organized with a significant mineral content.
A number of studies have investigated the effect of
SIMV on bone regeneration, experimentally and clinically, and have
revealed positive results (9,30,31),
while others have observed no beneficial effects (32–34).
These contradictory results may be associated with the different
doses used. A previous study suggested that the dose pattern
affects bone formation and resorption (16). This study, by Maritz et al,
measured the effect of different doses of orally administered SIMV
(1, 5, 10 and 20 mg/kg/day) in ovariectomized rats, and
demonstrated that high doses of the drug (20 mg/kg/day) equitably
increased bone formation and resorption, whereas a low dose (1
mg/kg/day) decreased formation and increased resorption (16). Therefore, the effects of SIMV on
bone tissue appeared to be directly dependent on the employed dose,
which may explain the contradictory results from other studies. A
further explanation may be that the intensity of the stimuli
(pressure and heat of carbide drill) used was different.
When comparing the results obtained in the present
study for the BD/SIMV and BD/SIMV/PRP groups, it was observed that
the combination of PRP and SIMV produced better indicators for bone
regeneration than SIMV alone, due to the coexistence of
characteristics of mature bone tissue and a bone remodeling
phase.
In group BD/SIMV/PRP, increases in the majority of
the histological evaluation parameters for bone regeneration were
observed. Consistent with the observations for the BD/SIMV group,
mature osteons, ellipsoidal osteocytes, organized collagen fibers
and inorganic matrix were present, which indicated that the
regenerated bone tissue in group BD/SIMV/PRP corresponded to a
mature or secondary-type bone tissue. Moreover, unlike the BD/SIMV
group, there was an increase in the presence of immature osteons,
ordered collagen fibers, large, globular osteocytes and central
channels (the latter two being statistically significant), which
suggested that a remodeling process was occurring in the bone
tissue, typical of a functional type of bone subjected to
mechanical loads. Therefore, due to the coexistence of the features
of mature and immature bone tissue, it was proposed that the
regenerated bone tissue in the BD/SIMV/PRP group corresponded to a
mature or secondary remodeled bone tissue (20).
Since the combination of these two platelet
preparations and SIMV in drinking water has not been studied
previously, it is not possible to perform an appropriate comparison
with other studies. However, as the therapeutic actions of the
platelet preparations have been demonstrated to be based on
processes for modulation and the acceleration of scarring through
the growth factors present in platelets (23), the results obtained in the
BD/SIMV/PRP group may be compared with studies using PRP.
Simman et al(13) evaluated the therapeutic role of PRP
in the repair of fractures induced in the tibias of Lewis rats. In
their study, it was determined that PRP accelerated fracture
repair, since four weeks subsequent to the administration of PRP
the histological analysis revealed that the group treated with PRP
showed greater bone formation. Furthermore, this result was
consistent with that of the radiographic analysis of the fractures,
which also showed higher callus formation in the PRP-treated group
than in the control group. It was observed that the fracture
healing in rats was dependent on the local expression of growth
factors and BMPs, which act on osteoblast differentiation and
promote osteogenesis. However, it has been suggested that growth
factors only act to promote the proliferation and differentiation
of preosteoblasts and osteoblasts, and are unlikely to act on
‘adult’ stem cells present in the bone tissue, whose
differentiation is regulated by BMPs (23). Based on this, the combination of
PRP with SIMV has been demonstrated to promote an increase in BMP
expression, particularly BMP-2 expression, leading to increased
osteoblast activity by inducing the differentiation of stem cells
into osteoblast cells (7,8). Consistent with this, the results
obtained in the BD/SIMV/PRP group indicated that PRP and SIMV
exerted a favorable effect on bone regeneration, based on the
greater presence of histological features of regeneration in the
BD/SIMV/PRP group than in the BD/SIMV group.
When comparing the histological parameters of bone
regeneration in the BD/SIMV and BD/SIMV/PRGF groups, it was
demonstrated that the combination of PRGF and SIMV produced the
best indicators of bone regeneration, with characteristic areas of
mature bone tissue coexisting with immature bone tissue. This
suggested a process of bone remodeling.
Consistent with the results for the BD/SIMV/PRP
group, in the BD/SIMV/PRGF group there was an increase in the
majority of the histological evaluation parameters for bone
regeneration. In comparison with the BD/SIMV group, it was observed
that there was mature bone tissue, which was organized with a high
mineral content, and a presence of mature osteons, ellipsoidal
osteocytes and an inorganic matrix. The increased presence of
immature osteons and central channels (statistically significant),
large, globular osteocytes, ordered collagen fibers and osteoid
tissue were indicative of the remodeling process in bone tissue and
characteristic of a functional-type bone. These results indicate
that the biologically regenerated bone, stimulated by the effects
of PRGF and SIMV, was a mature bone tissue or a bone of secondary
remodeling, comparable with that observed in the BD/SIMV/PRP
group.
A notable previous study used platelet-derived
growth factor (PDGF) and SIMV in microspheres, in order to overcome
the injury induced by the heat generated during the osteotomy while
placing dental implants in rats (35). In the absence of irrigation, a
significant reduction of cell viability and an increase in
inflammation and bone formation were observed, without evidence of
osteogenesis. SIMV and PDGF treatments facilitated cell viability
and the reduction of osteonecrosis, while the combination of the
two treatments further increased the signs of osteogenesis and bone
maturation in a sequential treatment modality, which showed the
positive effect of platelet products and this derivative of
lovastatin.
In the present study, in which qualitative variables
were used to analyze the results and compare the combination of PRP
and SIMV with PRGF and SIMV, it was not possible to determine which
platelet preparation produced superior effects in the process of
bone regeneration, due to the fact that no statistically
significant differences were observed between the two
treatments.
The essential limitation of this study was the lack
of quantitative data. Therefore, future interventions are required,
which consider similar variables to the present study, in addition
to discriminating the type of new bone and using a
histomorphometric analysis and/or cell count. It is further
recommended that serial sacrifices be performed, with an increased
number of experimental animals, in order to provide sufficient
temporal evidence of the regeneration process/alveolar bone
repair.
Acknowledgements
The authors would like to thank the Investigation
Directorate (DI) of the University of Talca for its cooperation. A
preliminary report was presented at the CONADEO, University of the
Frontier (Universidad de La Frontera, Temuco, Chile) in 2012.
References
|
1
|
Oporto Venegas G, Fuentes Fernández R,
Álvarez Cantoni H and Borie Echeverría E: Recuperación de la
morfología y fisiología maxilo mandibular: biomateriales en
regeneración ósea. Int J Morphol. 26:853–859. 2008.(In
Spanish).
|
|
2
|
Nazaroglou I, Stavrianos C, Kafas P, et
al: Radiographic evaluation of bone regeneration after the
application of plasma rich in growth factors in a lower third molar
socket: a case report. Cases J. 2:91342009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sommeling CE, Heyneman A, Hoeksema H,
Verbelen J, Stillaert FB and Monstrey S: The use of platelet-rich
plasma in plastic surgery: a systematic review. J Plast Reconstr
Aesthet Surg. 66:301–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sammartino G, Tia M, Marenzi G, di Lauro
AE, D’agostino E and Claudio PP: Use of autologous platelet-rich
plasma (PRP) in periodontal defect treatment after extraction of
impacted mandibular third molars. J Oral Maxillofac Surg.
63:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López-Jornet P, Camacho-Alonso F,
Molina-Miñano F and Vicente-Ortega V: Effects of plasma rich in
growth factors on wound healing of the tongue. Experimental study
on rabbits. Med Oral Patol Oral Cir Bucal. 14:e425–e428.
2009.PubMed/NCBI
|
|
6
|
Fierro Serna VM, Martínez-Rider R,
Hidalgo-Hurtado JA, Toranzo-Fernández JM and de Pozos-Guillén AJ:
Placement of plasma rich growth factors postsurgical extraction of
mandibular third molars. A case report. Revista Odontológica
Mexicana. 15:109–114. 2011.(In Spanish).
|
|
7
|
Mundy G, Garrett R, Harris S, et al:
Stimulation of bone formation in vitro and in rodents by statins.
Science. 286:1946–1949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bauer DC: HMG CoA reductase inhibitors and
the skeleton: a comprehensive review. Osteoporosis Int. 14:273–282.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JW, Xu SW, Yang DS and Lv RK: Locally
applied simvastatin promotes fracture healing in ovariectomized
rat. Osteoporosis Int. 18:1641–1650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Research Council, Institute of
Laboratory Animal Resources. Guide for the Care and Use of
Laboratory Animals. National Academy Press; Washington: 1996
|
|
11
|
Mead R, Gilmour SG and Mead A: Statistical
Principles for the Design of Experiments: Applications to Real
Experiments. Cambridge University Press; 2012, View Article : Google Scholar
|
|
12
|
Olsson IA and Dahlborn K: Improving
housing conditions for laboratory mice: a review of ‘environmental
enrichment’. Lab Anim. 36:243–270. 2002.
|
|
13
|
Simman R, Hoffmann A, Bohinc RJ, Peterson
WC and Russ AJ: Role of platelet-rich plasma in acceleration of
bone fracture healing. Ann Plast Surg. 61:337–344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anitua EA: Enhancement of osseointegration
by generating a dynamic implant surface. J Oral Implantol.
32:72–76. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gumieiro EH, Abrahão M, Jahn RS, et al:
Platelet-rich plasma in bone repair of irradiated tibiae of Wistar
rats. Acta Cir Bras. 25:257–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maritz FJ, Conradie MM, Hulley PA, Gopal R
and Hough S: Effect of statins on bone mineral density and bone
histomorphometry in rodents. Arterioscler Thromb Vasc Biol.
21:1636–1641. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hata S: Effect of oral administration of
simvastatin on bone regeneration process in ovariectomized rat. J
Hard Tissue Biology. 18:45–54. 2009. View Article : Google Scholar
|
|
18
|
Leeson TS, Leeson CR and Paparo AA:
Text/Atlas of Histology. 6th edition. Saunders; Philadelphia:
1988
|
|
19
|
Gartner LP and Hiatt JL: Colour Atlas of
Histology. Lippincott Williams & Wilkins; Philadelphia:
2006
|
|
20
|
Geneser F: Histología: sobre bases
biomoleculares. Médica Panamericana; Buenos Aires: 2000, (In
Spanish).
|
|
21
|
Gómez de Ferraris ME and Campos Muñoz A:
Histología y Embriología Bucodental: Bases Estructurales de la
Patología, el Diagnóstico, la Terapéutica y la Prevención
Odontológica. Madrid Médica Panamericana; Madrid: 1999, (In
Spanish).
|
|
22
|
Lindhe J, Karring T and Lang NP:
Periodontología Clínica e Implantología Odontológica. Madrid Médica
Panamericana; Madrid: 2001, (In Spanish).
|
|
23
|
García García V, Corral I and Bascones
Martínez A: Plasma rico en plaquetas y su utilización en
implantología dental. Av Periodoncia. 16:81–92. 2004.(In
Spanish).
|
|
24
|
Monteiro BS, Del Carlo RJ, Neto NMA, et
al: Platelet-rich plasma contribute to the process of bone repair
of critical defects created in the calvaria of mice. Cienc Rural.
40:1590–1596. 2010.(In Spanish).
|
|
25
|
Montagnani A, Gonnelli S, Cepollaro C, et
al: Effect of simvastatin treatment on bone mineral density and
bone turnover in hypercholesterolemic postmenopausal women: a
1-year longitudinal study. Bone. 32:427–433. 2003.PubMed/NCBI
|
|
26
|
Seto H, Ohba H, Tokunaga K, Hama H, Horibe
M and Nagata T: Topical administration of simvastatin recovers
alveolar bone loss in rats. J Periodontal Res. 43:261–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Junqueira JC, Mancini M, Carvalho YR,
Anbinder AL, Balducci I and Rocha RF: Effects of simvastatin on
bone regeneration in the mandibles of ovariectomized rats and on
blood cholesterol levels. J Oral Sci. 44:117–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Staal A, Frith JC, French MH, et al: The
ability of statins to inhibit bone resorption is directly related
to their inhibitory effect on HMG-CoA reductase activity. J Bone
Miner Res. 18:88–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maciel-Oliveira N, Bradaschia-Correa V and
Arana-Chavez VE: Early alveolar bone regeneration in rats after
topical administration of simvastatin. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 112:170–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu Z, Liu C, Zang G and Sun H: The effect
of simvastatin on remodelling of the alveolar bone following tooth
extraction. Int J Oral Maxillofac Surg. 37:170–176. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee Y, Schmid MJ, Marx DB, et al: The
effect of local simvastatin delivery strategies on mandibular bone
formation in vivo. Biomaterials. 29:1940–1949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anbinder AL, de Prado FA, de Prado MA,
Balducci I and Rocha RF: The influence of ovariectomy, simvastatin
and sodium alendronate on alveolar bone in rats. Braz Oral Res.
21:247–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kılıç E, Özeç İ, Yeler H, Korkmaz A, Ayas
B and Gümüş C: Effects of simvastatin on mandibular distraction
osteogenesis. J Oral Maxillofac Surg. 66:2233–2238. 2008.PubMed/NCBI
|
|
34
|
Ma B, Clarke SA, Brooks RA and Rushton N:
The effect of simvastatin on bone formation and ceramic resorption
in a peri-implant defect model. Acta Biomater. 4:149–155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang P-C, Lim L, Chong L, et al:
PDGF-simvastatin delivery stimulates osteogenesis in heat-induced
osteonecrosis. J Dent Res. 91:618–624. 2012. View Article : Google Scholar : PubMed/NCBI
|