Introduction
Left ventricular hypertrophy (LVH) is a commonly
encountered complication of hypertension (1). It is also an independent risk factor
for the development of cardiovascular disease, since it may lead to
myocardial ischemia, cardiac arrhythmia, heart failure and even
sudden death (2,3). Tanshinone IIA (TSN), the main active
compound of the Traditional Chinese Medicine Salvia
miltiorrhiza, has been known to exert therapeutic effects in
heart disease via its vasodilative (4), atheroprotective (5), antiarrhythmic (6) and anti-inflammatory properties
(7). In our previous study
(8), the cardioprotective effects
of TSN in preventing the development of LVH were demonstrated in
spontaneously hypertensive rat (SHR) models. A likely mechanism for
this may be that TSN inhibited the renin-angiotensin-aldosterone
system (RAAS) (9). High RAAS
activity in the presence of LVH has previously been associated with
increased an increased level of apoptosis (10). Therefore, the present study
investigated the role of TSN, extracted from Salvia
miltiorrhiza, in the development of LVH and the apoptotic
protein expression in SHRs.
Materials and methods
Animal model and grouping
All procedures were in accordance with the
international, national and institutional rules on animal
experiments. Legal approval from The Review Board of Xiangya Third
Hospital of Central South University (Changsha, China) was granted
for the experiments undertaken. A total of 18 male SHRs (Shanghai
Institute of Hypertension, Shanghai, China) were used in this
study. The rats were fed a standard rat diet and bred in a constant
temperature and humidity for the first 8 weeks before applying any
intervention. The rats were randomly divided into three groups with
six SHRs in each group. Rats in the control group (group S8) were
sacrificed at week 8 of the experiment. Rats in the treatment group
(group D18) were injected with Salvia miltiorrhiza TSN (1
ml/kg body weight/day; S02001300; National Institute for the
Control of Pharmaceutical and Biological Products, Beijing, China)
for 10 weeks, commencing at week 8, and were subsequently
sacrificed week 18. The placebo group (group S18) were treated
similarly to group D18, although distilled water (1ml/kg body
weight/day) was administered instead of TSN.
Reagents
The main reagents used in this study were TSN (China
Pharmaceutical and Biological Products Inspection Center) and
rabbit anti-Bcl-2, Bax and p53 monoclonal antibodies (Santa Cruz
Biotechnology, Inc., Shanghai, China). In addition, a terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
assay kit (Boehringer, Mannheim, Germany) was used.
Measurements
Systolic blood pressure (SBP)
measurement
Following warming the rats at 38ºC for 15 min, SBP
was measured using a tail-cuff sphygmomanometer (MRS2 III,
Shanghai Institute of Hypertension). Five measurements of systolic
pressure were taken for each rat and the SBP was defined as the
mean value of the five measurements. All procedures were performed
with animals in a conscious state.
Left ventricular mass index
(LVMI)
Following sacrifice, the hearts of the rats were
removed. The major vessels, the atria and the right ventricle were
separated from the left ventricle, prior to the left ventricle
being washed with cold phosphate-buffered saline (PBS) and dried.
The wet weight (mg) of the left ventricle was then measured. The
LVMI was defined as the value of the left ventricular weight (mg)
divided by the total body weight (g).
Qualitative and quantitative analysis
of cardiac myocytes and collagen
Tissue samples were obtained for analysis from the
transverse plane of the middle of the left ventricle. Following
fixation, the tissue was embedded and sliced for hematoxylin and
eosin (H&E) and van Gieson (VG) staining. The diameter and area
of cardiac myocytes were measured under H&E staining using
fully automatic morphometry equipment (HPIAS21000, Tongji Medical
Imaging Engineering Co., Wuhan, China). Under VG staining, myocytes
appeared yellow and collagen appeared red. The collagen volume
fraction (CVF) of myocardial tissue and the perivascular
circumferential area (PVCA) were measured under VG staining. The
CVF was defined as the PVCA divided by the total area of the
sample. The PVCA was defined as the collagen area surrounding the
arteriole divided by the arterial lumen area. All values were
calculated as the mean value obtained from five random measurements
for each specimen.
Analysis of cardiac myocyte
apoptosis
Cardiac myocyte apoptosis was analyzed using the
TUNEL method. Following dewaxing, cardiac tissue was hydrated with
alcohol and immersed in protease K (20 μg/ml; Sigma-Aldrich,
Shanghai, China) at 37ºC for 15 min. Peroxidase (POD) activity was
deactivated by the addition of hydrogen peroxide-carbinol (0.3%;
Sigma-Aldrich). The sample was washed with PBS four times prior to
30 μl TUNEL mixture (Boehringer) being added, and the sample then
was incubated in a wet box for 1 h at 37ºC. Subsequently, the
tissue was re-washed with PBS, and re-incubated for 30 min.
Following incubation, the tissue was washed with PBS and
3,3′-diaminobenzidine (DAB) revealed its color. The tissue was then
stained with H&E. The nuclei of normal cells appeared blue
while those of apoptotic cells appeared red.
Western blot assay of Bcl-2, Bax and
p53 genes
TRIzol® (Invitrogen Life Technologies,
Carlsbad, CA, USA) was added to 50 mg myocardial tissue for protein
extraction. Quantitative analysis of the protein was performed
according to Bradford’s method. Protein and buffer were mixed in
the ratio 1:3, boiled for three min prior to vertical
electrophoresis in sodium dodecyl sulfate (SDS) polyacrylamide gel
(separation gel 8% and layering gel 4.5%) and transferred to a
polyvinylidene difluoride (PVDF) membrane. The membrane was blocked
with TBS2T (with 5% skimmed milk powder) for 2 h at room
temperature. Bcl-2, Bax and p53 monoclonal antibodies (Santa Cruz
Biotechnology, Inc.; 1:500 dilution) were added to the samples and
incubated at 4ºC for one night. The following day, membranes were
washed four times with TBS2T, prior to horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG)
antibodies (1:8,000 dilution; Sigma-Aldrich) being added. The
samples were then agitated for 2 h and re-washed with TBS2T four
times. Subsequently, enhanced chemiluminescence (ECL) reagent was
added and the samples were illuminated with X-rays, prior to
scanning with a GDS8000 gel analysis system (UVP, LLC, Upland, CA,
USA) to determine the density of specific light bands. The protein
expression levels of each group were defined as the ratio between
the density of light band in a given group and that of group
S8.
Statistical analysis
Variables are expressed as the mean ± standard
deviation. Differences between groups were analyzed with one way
analysis of variance (ANOVA) using SPSS 16.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of TSN on SBP
In all groups, the SBP began to rise in week 8 in
the treatment and placebo groups. Compared with the baseline, SBP
increased significantly by week 18 (P<0.01). However, a
comparison of SBP between groups D18 and S18 did not reveal a
significant difference (P>0.05). The comparison of SBP between
groups is presented in Table
I.
 | Table IComparison of SBP, LVMI, CD, CA, CVF
and PVCA between the control, placebo and treatment groups. |
Table I
Comparison of SBP, LVMI, CD, CA, CVF
and PVCA between the control, placebo and treatment groups.
| Group | SBP (mmHg) | LVMI (mg/g) | CD (μm) | CA
(μm2) | CVF (%) | PVCA (%) |
|---|
| S8 | 147±9 | 2.86±0.22 | 16.26±2.1 | 218.43±52.44 | 3.77±0.57 | 2.46±0.38 |
| D18 |
171±6b | 3.23±0.24 | 16.35±1.84 | 230.23±69.37 | 4.54±0.80 | 2.94±0.56 |
| S18 |
177±2a |
4.28±0.68a,c |
25.22±4.38a,c |
490.12±118.96a,c |
6.76±0.76a,c |
4.86±0.65a,c |
Effect of TSN on LVH and myocardial
fibrosis
LVMI, cardiac myocyte diameter and area, CVF and
PVCA were significantly higher in group S18 compared with group S8
(as shown in Table I). All these
indices were significantly lower in group D18 than in group
S18.
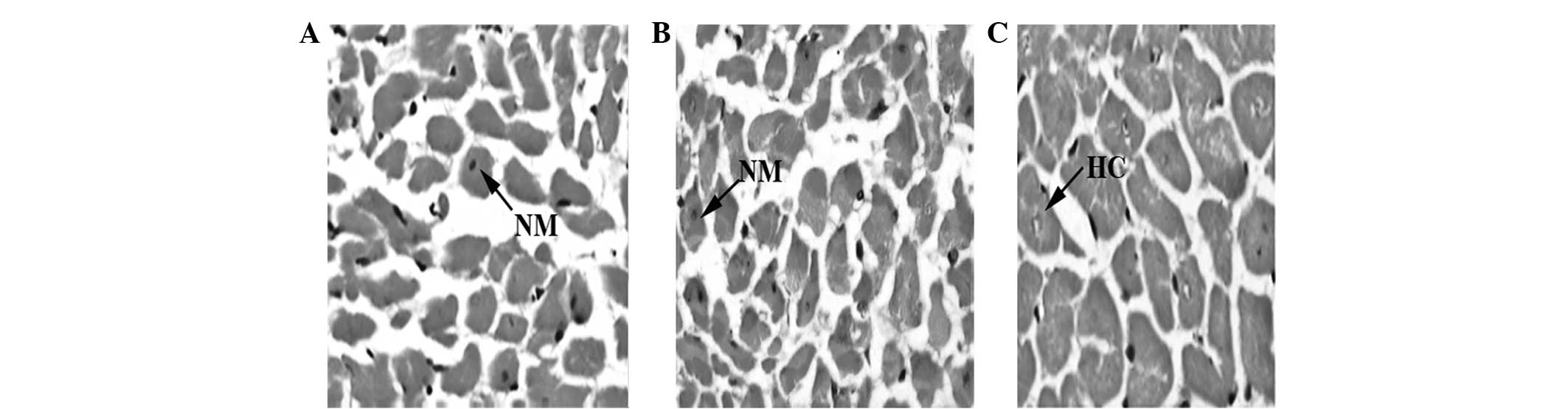
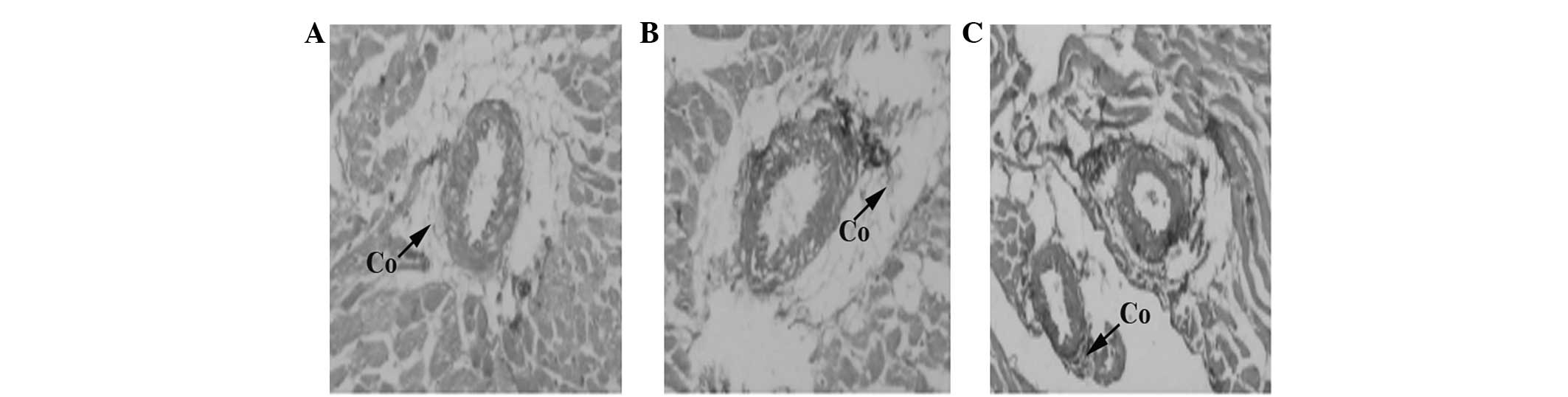
Histopathological study
Under H&E and VG staining, SHRs in group S18
exhibited a greater extent of hypertrophic cardiac myocytes and
collagen hyperplasia compared with those observed in group S8.
Moreover, myocardial fibrosis and left ventricular hypertrophy were
prominent in group S18. In contrast to group S18, SHRs treated with
TSN in group D18 exhibited no significantly hypertrophic cardiac
myocytes and collagen hyperplasia. The histopathological findings
of groups S8, S18 and D18 are shown in Figs. 1 and 2.
Cardiac myocyte apoptosis
Compared with group S8, cardiac apoptotic cell
indices were higher in group S18. Following treatment with TSN,
cardiac apoptotic cell indices decreased significantly compared
with group S18. The comparison between groups is exhibited in
Table II.
 | Table IIComparison of cardiac apoptotic cells
and protein expression levels in the groups of SHRs. |
Table II
Comparison of cardiac apoptotic cells
and protein expression levels in the groups of SHRs.
| Group | Apoptotic cell index
(%) | Bcl-2 | Bax | p53 |
|---|
| S8 | 9.45±1.81 | 1.00±0.17 | 1.00±0.08 | 1.00±0.11 |
| D18 | 7.65±2.36 | 0.95±0.20 | 1.04±0.16 | 1.54±0.14 |
| S18 |
11.59±1.48a,b |
0.81±0.15c,d |
6.22±0.53a,b |
4.03±0.31a,b |
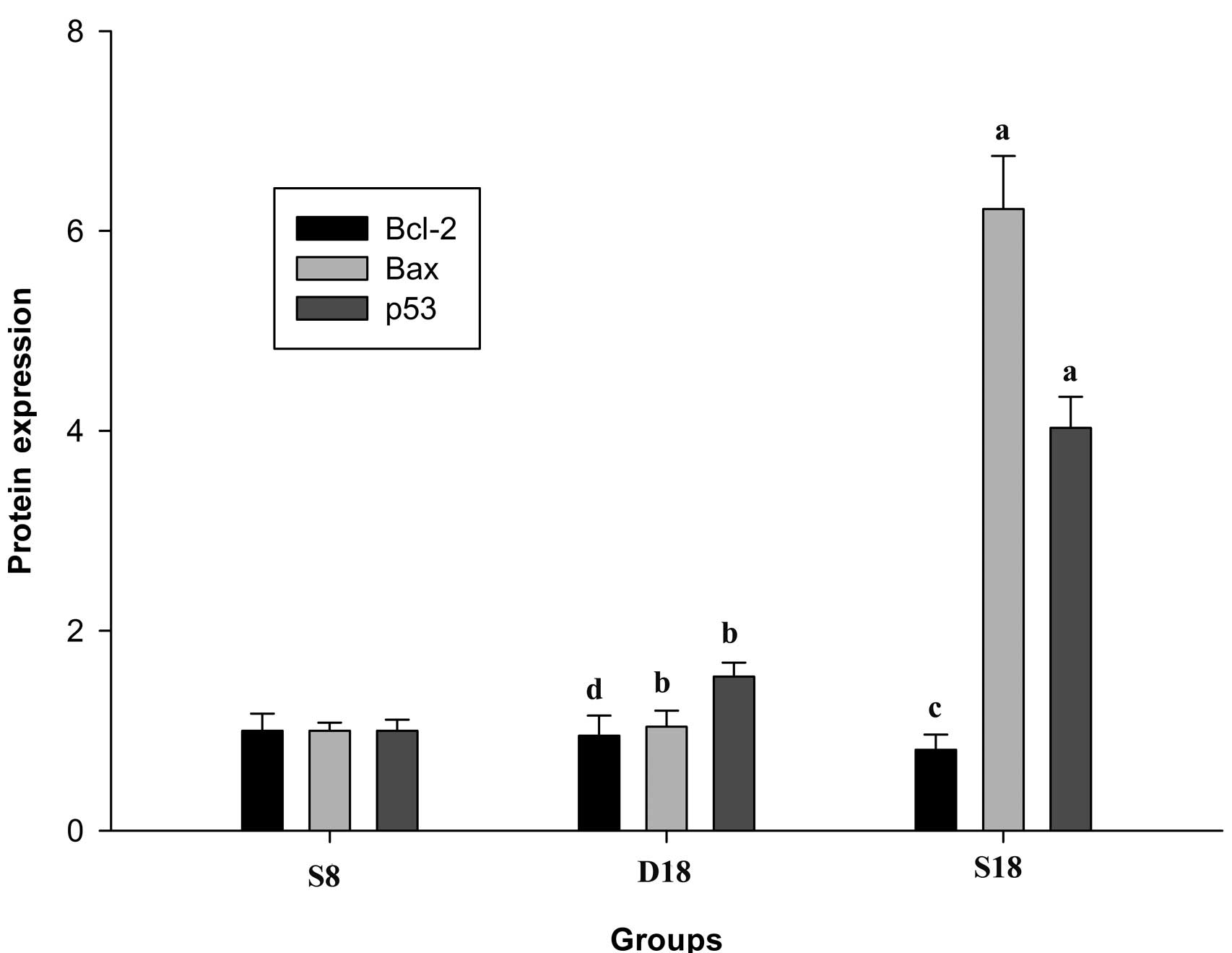
Effect of TSN on myocardial tissue Bc1-2,
Bax and p53 protein expression
Rats in group S18 exhibited lower Bc1-2 protein
expression levels (P<0.05 versus group S8) and higher p53
(P<0.01 versus group S8) and Bax (P<0.01 versus group S8)
protein expression levels. Following treatment with TSN, Bc1-2
protein expression levels increased (P<0.05 versus group S18),
while those of p53 (P<0.01 versus group S18) and Bax (P<0.01
versus group S18) decreased significantly. A comparison of protein
expression levels between groups is shown in Table II and Fig. 3.
Discussion
It has been identified that Salvia
miltiorrhiza is rich in TSN, a member of the tanshinone family.
Sodium tanshinone IIA sulfonate, extracted from Salvia
miltiorrhiza, has been demonstrated to exert therapeutic
effects in heart disease as a result of its vasodilative (4), atheroprotective (5), antiarrhythmic (6) and anti-inflammatory activities
(7). Several studies have reported
an improvement in clinical symptoms, signs and electrocardiography
following the administration of TSN (11–14).
Further research (15–18) has demonstrated that Salvia
miltiorrhiza is able to block L-type calcium ion channels of
ventricular myocardial cells, and therefore exerts calcium channel
blocker-like effects. Moreover, inhibition of NF-κB expression in
myocardial cells has also been reported (7). The present study demonstrated that
Salvia miltiorrhiza attenuated LVH in SHRs, partly through
the inhibition of RAAS activity. In a previous study (18), we focused on left ventricular
norepinephrine (NE), epinephrine (E) and dopamine (DA) levels in
SHRs. It was identified that NE and DA levels increased, while
levels of E decreased in hypertrophic myocardial cells following
administration of TSN. In addition to inhibiting the production of
local cardiac angiotensin II, TSN also inhibited catecholamine
release from sympathetic nerves, lowered the activation of α- and
β-adrenergic receptors and, therefore, prevented SHRs from
developing LVH. Furthermore, TSN downregulated the expression of
cardiac protein kinase C to protect SHRs from LVH.
Apoptotic processes have been associated with LVH
(10). In the present study,
apoptotic processes were investigated in SHRs by observing the
expression of Bcl-2, Bax and p53. It is well known that Bcl-2, also
called the ‘antiapoptotic gene’, inhibits cell apoptosis, while Bax
exerts a proapoptotic effect. Cell apoptosis depends on the
homeostasis between Bax and Bcl-2 proteins. Inhibition of the
apoptotic process has been shown to occur when Bcl-2 protein levels
were higher than Bax protein levels, and vice versa (13). Gene p53 is known to upregulate Bax
protein expression and downregulate Bcl-2 protein expression, and
therefore exerts a proapoptotic effect (12–14).
Results of the present study revealed that group S18 exhibited
hypertrophic myocardial cells to a greater extent than groups S8
and D18. Under a state of LVH, Bax and p53 expression levels were
significantly higher and Bcl-2 expression levels were lower than
groups S8 and D18. The administration of TSN was demonstrated to be
effective in upregulating Bcl-2 expression, while decreasing Bax
and p53 expression. However, no significant difference was
identified between the SBPs of groups S18 and D18. This indicated
that the protective effect of TSN against LVH was not achieved via
a decrease in blood pressure or an improvement in cardiac
afterload. Through this study, we have shown that LVH is closely
associated with apoptotic processes in SHRs, and that TSN is
effective in preventing the development of LVH and downregulating
apoptotic processes in SHRs. The exact mechanism by which apoptosis
leads to LVH remains to be investigated.
In conclusion, the present study has demonstrated
that LVH and apoptosis of cardiac tissues increase with the
increasing age of SHRs. TSN may inhibit the development of LVH and
decrease apoptotic processes in SHRs, possibly via upregulation of
Bcl-2 and downregulation of Bax and p53 expression.
References
|
1
|
Fox E, Taylor H, Andrew M, et al: Body
mass index and blood pressure influences on left ventricular mass
and geometry in African Americans: The Atherosclerotic Risk In
Communities (ARIC) Study. Hypertension. 44:55–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sundström J, Lind L, Arnlöv J, Zethelius
B, Andrén B and Lithell HO: Echocardiographic and
electrocardiographic diagnoses of left ventricular hypertrophy
predict mortality independently of each other in a population of
elderly men. Circulation. 103:2346–2351. 2001.PubMed/NCBI
|
|
3
|
Levy D, Garrison RJ, Savage DD, Kannel WB
and Castelli WP: Prognostic implications of echocardiographically
determined left ventricular mass in the Framingham Heart Study. N
Engl J Med. 322:1561–1566. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan P, Liu IM, Li YX, Yu WJ and Cheng JT:
Antihypertension induced by tanshinone IIA isolated from the roots
of Salvia miltiorrhiza. Evid Based Complement Alternat Med.
2011:3926272011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang FT, Cao Y, Wang TQ, et al: Tanshinone
IIA attenuates atherosclerosis in ApoE(−/−) mice through
down-regulation of scavenger receptor expression. Eur J Pharmacol.
650:275–284. 2011.
|
|
6
|
Shan H, Li X, Pan Z, et al: Tanshinone IIA
protects against sudden cardiac death induced by lethal arrhythmias
via repression of microRNA-1. Br J Pharmacol. 158:1227–1235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jang SI, Kim HJ, Kim YJ, Jeong SI and You
YO: Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW
264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and
JNK pathways. Eur J Pharmacol. 542:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong LY, Zheng Z and Xiong W: The effect
of Salvia miltiorrhiza Bge on preventing left ventricular
hypertrophy in spontaneously hypertensive rats. Chinese Journal of
Hypertension. 11:257–259. 2003.(In Chinese).
|
|
9
|
Yang L, Zou X, Liang Q, et al: Sodium
tanshinone IIA sulfonate depresses angiotensin II-induced
cardiomyocyte hypertrophy through MEK/ERK pathway. Exp Mol Med.
39:65–73. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Velez Rueda JO, Palomeque J and Mattiazzi
A: Early apoptosis in different models of cardiac hypertrophy
induced by high renin-angiotensin system activity involves CaMKII.
J Appl Physiol. 112:2110–2120. 2012.PubMed/NCBI
|
|
11
|
Messerli FH: Hypertension and sudden
cardiac death. Am J Hypertens. 12:181S–188S. 1999. View Article : Google Scholar
|
|
12
|
Kukhta VK, Marozkina NV, Sokolchik IG and
Bogaturova EV: Molecular mechanisms of apoptosis. Ukr Biokhim Zh.
75:5–9. 2003.
|
|
13
|
Dlamini Z, Mbita Z and Zungu M: Genealogy,
expression, and molecular mechanisms in apoptosis. Pharmacol Ther.
101:1–15. 2004. View Article : Google Scholar
|
|
14
|
Goga LM, Vasiliu OM and Ionescu CR:
Molecular mechanisms of apoptosis. Fas and TNF systems. ICE
protease system. Bcl-2 family. Rev Med Chir Soc Med Nat Iasi.
105:23–29. 2001.(In Romanian).
|
|
15
|
Zheng Z, Han HJ and Ren DH: Effects of
Salvia miltiorrhiza Bge on left ventricular hypertrophy and
cardiac aldosterone in spontaneously hypertensive rats. Chin J
Emerg Med. 11:22–24. 2002.(In Chinese).
|
|
16
|
Liu F and Zheng Z: Influence of Radix
Salviac miltiorrhizae(RSM) on cardiac myocyte levels of
transforming growth factor-β1 and preventive effects of RSM on left
ventricular hypertrophy in spontaneously hypertensive rats. Chin J
Crit Care Med. 23:71–73. 2003.(In Chinese).
|
|
17
|
Sun LP and Zheng Z: The effect of
Salvia miltiorrhiza Bge on left ventricular hypertrophy and
the expression of tumor necrosis factor-alpha in spontaneously
hypertensive rats. Chin J Hypertens. 12:238–241. 2004.(In
Chinese).
|
|
18
|
Jiang FL, Feng J and Zheng Z: Effects of
tanshinone II on the mRNA expression of c-fos, c-myc and c-jun in
angiotensin II-induced hypertrophy of cardiomyocytes. Chin
Pharmacol Bull. 23:55–59. 2007.(In Chinese).
|