Introduction
Marine algae, also known as marine vegetables, are
naturally rich in polysaccharides, minerals, polyunsaturated fatty
acids, vitamins and bioactive molecules. Their nutritional value is
markedly higher than that of terrestrial vegetables. The bioactive
compounds in marine algae have been reported to possess strong
antihypertensive (1–3), antitumor (4,5),
anti-inflammatory (6–8), antidiabetic (9,10)
and anticoagulant (11,12) properties. In addition, the
prebiotic health potential of polysaccharides from seaweeds has
been increasingly studied in recent years.
Fatigue, a phenomenon of decreased efficiency
following continuous study or work, may be divided into mental and
physical fatigue. It mainly manifests as a physical decrease in
muscle tone and exercise tolerance due to an accumulation of lactic
acid (LC) and other metabolites (13). Fatigue also leads to yawning, as a
result of a build-up of carbon dioxide that stimulates the
respiratory center. At present, work and life stresses are
escalating with the increasing pace of life. Thus, fatigue is
common and may significantly affect daily routines. However, a
systematic and authoritative hypothesis to explain the induction of
fatigue has yet to be suggested. As technology has progressed, a
number of scientists have begun to investigate fatigue-associated
genes and proteins in order to reveal the mechanisms underlying
fatigue and improve therapeutic strategies (14,15).
Gracilaria eucheumoides Linn (Gracilariaceae;
G. eucheumoides), a species of seaweed, is a type of natural
edible green algae that primarily grows on the southeast coast of
China (16). It contains numerous
vitamins and microelements, and is abundant in dietary fiber, which
aids the clearance of excess cholesterol from the blood and
maintains stable blood glucose levels (17). Therefore, G. eucheumoides
may have medicinal value and has the potential to be exploited as a
functional ingredient in human and animal health applications.
Although G. eucheumoides has previously been used in health
products in China, to the best of our knowledge its antifatigue
effects have not yet been reported. In the present study, the water
extract of G. eucheumoides was administered to three groups
of mice, and the exhaustive swimming times and relevant
physiological indices were measured in order to determine the
antifatigue effects of G. eucheumoides. Furthermore, the
expression levels of glucose transport protein 4 (GLUT4) and
AMP-activated protein kinase (AMPK) were analyzed using
quantitative polymerase chain reaction (qPCR) and western blotting.
The results demonstrated that G. eucheumoides extract may
improve the ability to fight fatigue in mice.
Materials and methods
Preparation of G. eucheumoides
extract
The water extract of G. eucheumoides was
prepared following a method described previously (18).
Grouping and treatment of the mice
Healthy male Kunming mice, weighing 10–22 g, were
purchased from the Institute of Laboratory Animal Sciences of the
Chinese Academy of Medical Sciences (Beijing, China). A total of 40
mice were randomly divided into four groups of ten: Low- (20
mg/kg), medium- (40 mg/kg) and high-dose (80 mg/kg) groups and a
normal control group (normal saline). Each mouse was weighed and
fed with 0.02 ml/g G. eucheumoides extract by gavage daily
for 30 days. The G. eucheumoides extract was diluted with
purified water to the designated concentration. The health status
of the mice was observed each day and all the animals were weighed
every three days. The quantity of feed was determined in line with
the weight of the animals for the 30 days. All mice were freely fed
and watered during the experiment. All animal procedures were
reviewed and approved by the Shanghai University of Sport Science
Research Ethics Committee (chiCTR-TRC-120050028; Shanghai,
China).
Loaded swimming test
Thirty minutes subsequent to the final
administration of G. eucheumoides, a lead sheath, weighing
5% of the body weight of the mouse, was tied to the root of the
tail, prior to the mouse being placed in a water container. The
swimming time (time between being placed in the water and sinking
underwater for >10 sec) was recorded. The water depth was ≥30 cm
and the temperature was 25±1°C.
Determination of hepatic and muscle
glycogen
Thirty minutes subsequent to the final feed,
non-loaded mice were placed in a water container and left to swim
for 90 min at a temperature of 25±1°C. The mice were then
sacrificed by cervical vertebra dislocation, cleaned using normal
saline and dried with filter paper. Subsequently, 100 mg liver
tissue and 500 mg quadriceps femoris tissue were weighed and
diluted to 10% homogenate with normal saline. Following
centrifugation at 800–1,800 × g for 10 min, the supernatant was
used to determine the quantity of glycogen using the Anthrone
colorimetric method.
Measurement of LC, lactate dehydrogenase
(LDH) and blood glucose concentrations
Thirty minutes subsequent to the final feed,
non-loaded mice were left to swim for 90 min at a temperature of
25±1°C. Subsequently, venous blood was collected from the tail and
the LC, LDH and blood glucose concentrations were determined using
the LC kit (Cat. No. A019-1), LDH kit (Cat. No.A020) and glucose
kit (Cat. No.F006) from Nanjing Jiancheng BioEngineering Institute
(Nanjing, China), in accordance with the manufacturer’s
instructions.
qPCR
Thirty minutes subsequent to the final feed,
non-loaded mice were left to swim for 90 min at a temperature of
25±1°C. Subsequently, the mice were sacrificed by cervical vertebra
dislocation and cleaned using normal saline. Following drying with
filter paper, 50–100 mg quadriceps femoris tissue was weighed and
RNA was isolated using TRIzol reagent® (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. The specific primer pairs designed for the
amplification of GLUT4 and AMPK, and the reference gene of 18s RNA,
are exhibited in Table I. All qPCR
reactions were performed using a CFX-96 Real-Time PCR detection
System (Bio-Rad, Hercules, CA, USA). Each 25-μl reaction consisted
of 12.5 μl SYBR Green I, 5 μl cDNA, 2.5 μl forward and reverse
primer, and 2.5 μl sterile water. The conditions for PCR were 40
cycles of: 95°C, 3 sec; 60°C, 5 sec; and 72°C, 30 sec. Following
PCR, a melting curve analysis was performed in order to demonstrate
the specificity of the PCR products. Each reaction was run in
triplicate and the results were analyzed using SPSS 15.0
statistical software (SPSS, Inc., Chicago, IL, USA).
 | Table IPrimer sequences of target and
reference genes used for quantitative polymerase chain reaction
analyses. |
Table I
Primer sequences of target and
reference genes used for quantitative polymerase chain reaction
analyses.
| Primer | Direction | Sequence (5′-3′) |
|---|
| GLUT4 | Forward |
5′-TCGTGGCCATATTTGGCTTTGTGG-3′ |
| Reverse |
5′-TAAGGACCCATAGCATCCGCAACA-3′ |
| AMPK | Forward |
5′-TGACCGGACATAAAGTGGCTGTGA-3′ |
| Reverse |
5′-TGATGATGTGAGGGTGCCTGAACA-3′ |
| 18sRNA | Forward |
5′-CCTGGATACCGCAGCTAGGA-3′ |
| Reverse |
5′-GCGGCGCAATACGAATGCCCC-3′ |
Protein extraction and western blot
analysis
Thirty minutes subsequent to the final feed,
non-loaded mice were left to swim for 90 min at a temperature of
25±1°C. Subsequently, the mice were sacrificed by cervical vertebra
dislocation and cleaned using physiological saline. Following
drying with filter paper, 40 mg quadriceps femoris tissue was
weighed and lysed in a lysis buffer consisting of 50 mM Tris-HCl
(pH 7.4–8.0), 150 mM NaCl, 5 mM EDTA, 1% Triton™ X-100, and 1 mM
PMSF. Protein concentrations were determined using a bicinchoninic
acid protein assay (Pierce Chemical Co., Rockford, IL, USA) using
bovine serum albumin as a standard. Protein samples of a total of
20 μg were separated using sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) technology and were transferred to
polyvinylidene difluoride (PVDF) membranes. The plates were blocked
with 5% (w/v) nonfat milk powder for 2 h at room temperature and
immunoblotted with primary antibodies. Following one night at 4°C,
the secondary antibodies were added and the antigen-antibody
complex was detected using an enhanced chemiluminescence (ECL) kit
(Amersham Pharmacia Biotech, Piscataway, NJ, USA) in accordance
with the manufacturer’s instructions. β-actin was treated as a
control. The primary antibodies and secondary antibodies used were
as follows: goat anti-GLUT4 (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA; sc-1608), goat anti-AMPK (Santa Cruz Biotechnology,
Inc.; sc-19126) and donkey anti-goat IgG-HRP (Santa Cruz
Biotechnology, Inc.; sc-2020).
Statistical analysis
All statistical procedures were performed using SPSS
version 15.0 (SPSS, Inc.). Data are expressed as the mean ±
standard deviation (SD). Differences between groups were analyzed
using one-way analysis of covariance. P<0.05 was considered to
indicate a statistically significant difference and P<0.01 was
considered highly significant.
Results
Effect of G. eucheumoides extract on the
loaded swimming time of mice
The mice in the three G. eucheumoides extract
treatment groups were subjected to the loaded swimming test. The
results of the test are exhibited in Table II.
 | Table IIEffect of G. eucheumoides
extract on the loaded swimming time of mice. |
Table II
Effect of G. eucheumoides
extract on the loaded swimming time of mice.
| Group | Dose (mg/kg) | Number of mice | Loaded swimming time
(sec) | Increment rate of
loaded swimming time (%) |
|---|
| Control | 0 | 10 | 31.3±0.2 | - |
| Low-dose | 20 | 10 | 38.7±0.4 | 19.1a |
| Medium-dose | 40 | 10 | 41.3±0.5 | 32.0b |
| High-dose | 80 | 10 | 36.4±0.2 | 16.3a |
The loaded swimming times of the low-, medium- and
high-dose groups were greater than those of the control group.
Among the three treatment groups, the loaded swimming time of the
medium-dose group was the longest (41.3±0.5 min). The increment
rate of the loaded swimming time for the medium-dose group was 32%,
which was significantly different from the control group.
Furthermore, the increment rates of the low- and high-dose groups
showed significant increases of 19.1 and 16.3%, respectively,
compared with the control group. These results indicated that G.
eucheumoides extract may extend the loaded swimming time of
mice.
Effect of G. eucheumoides extract on
liver and muscle glycogen, LC, LDH and blood glucose concentrations
of mice
In order to investigate the mechanism underlying the
antifatigue effect of G. eucheumoides extract, the liver and
muscle glycogen, LC, LDH and blood glucose concentrations of mice
were measured following the non-loaded swimming test (Table III).
 | Table IIIAnalysis of liver and muscle glycogen,
LC, LDH and blood glucose concentration in mice. |
Table III
Analysis of liver and muscle glycogen,
LC, LDH and blood glucose concentration in mice.
| Group | Liver glycogen (g/100
g) | Muscle glycogen
(g/100 g) | LDH (U/l) | LC concentration
(mM) | Blood glucose
concentration (g/l) |
|---|
| Control | 1.25±0.02 | 0.55±0.00 | 55.3±0.30 | 18.6±0.10 | 0.83±0.10 |
| Low-dose | 1.43±0.04a | 0.78±0.01a | 76.9±0.40a | 15.4±0.06a | 1.10±0.06a |
| Medium-dose | 1.86±0.00b | 1.12±0.11b | 90.3±0.50b | 13.8±0.10b | 1.38±0.10b |
| High-dose | 1.64±0.03a | 0.82±0.09a | 94.2±0.20b | 11.3±0.10b | 1.13±0.10b |
It was revealed that the relevant physiological
indices of the treatment groups were significantly increased
compared with those of the control group (P<0.05), with the
exception of the LC concentration. In the treatment groups, liver
glycogen and muscle glycogen and blood glucose concentrations
initially increased and then decreased as the extract dose was
increased. LDH concentration increased with an increasing dose,
while LC concentration decreased. These results indicated that
G. eucheumoides extract significantly alters the liver and
muscle glycogen, LDH, LC and blood glucose concentrations of
mice.
Effect of G. eucheumoides extract on the
gene expression levels of GLUT4 and AMPK
To investigate the molecular role of G.
eucheumoides extract in mice, the expression levels of GLUT4
and AMPK were determined using qPCR (Fig. 1). Compared with the control group,
the expression levels of GLUT4 and AMPK were significantly
increased in the treatment groups. The expression levels of the two
genes were increased to the greatest extent in the medium-dose
group and were 3.0- and 1.8-fold higher than those in the control
group, for GLUT4 and AMPK respectively. These results demonstrated
that G. eucheumoides extract may regulate the expression
levels of GLUT4 and AMPK in the muscle of mice.
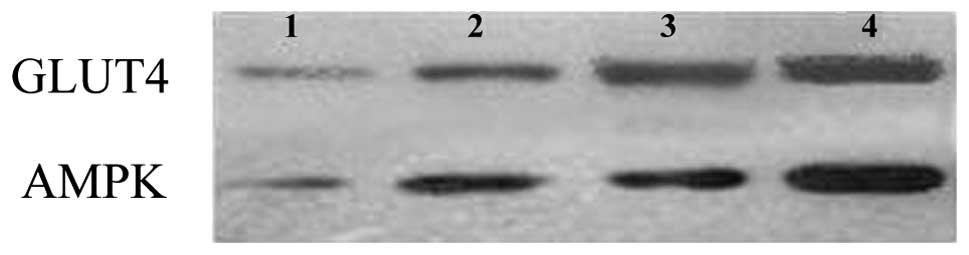
Effect of G. eucheumoides extract on
protein expression levels of GLUT4 and AMPK
The protein expression levels of GLUT4 and AMPK were
further characterized by western blotting (Fig. 2). The results showed that protein
expression levels of GLUT4 and AMPK increased gradually with an
increasing extract dose. Although the expression levels of these
proteins reached their peaks in the high-dose group, the increasing
trends were different between the proteins. GLUT4 was significantly
increased from the low-dose group to the medium-dose group, while
AMPK was significantly increased from the medium-dose group to the
high-dose group. In combination, these results suggested that G.
eucheumoides extract enhanced the protein expression of GLUT4
and AMPK in mice.
Discussion
Fatigue is a normal physiological or psychological
phenomenon, usually associated with physical and/or mental
weakness, varying from a general state of lethargy to a specific
work-induced burning sensation within the muscles. Prolonged and
high intensity exercise leads to an inadequate oxygen supply at the
physiological level. In order to maintain physical functioning, the
body primarily performs glucose metabolism, which is dependent on
the glycolytic pathway and results in significant consumption of
glycogen and accumulation of fatigue-inducing metabolites,
including LC (13).
The bioactive compounds derived from seaweed may be
classified into two types: Indigestible intercellular viscous
polysaccharides, including alginic acid, fucoidan, rhodophyte agar
and carrageenan (19,20), and digestible compounds with low
molecular weights, including halide, terpenoid, bromophenol
compound, algae tannin and laminin (5,21,22).
The latter type are capable of being absorbed by the human body and
regulate metabolism directly and indirectly. G. eucheumoides
contains several types of vitamins and microelements, and is
abundant in dietary fiber. The human consumption of dietary fiber
has been correlated with a number of health-promoting effects,
including growth promotion, the protection of beneficial intestinal
flora and the reduction of the overall glycemic response. Thus, at
present G. eucheumoides is is a topic of interest.
In the present study, the loaded swimming times of
mice fed with G. eucheumoides extract were significantly
increased compared with those of the control group, indicating that
the extract may enhance tolerance to fatigue in mice. Analysis of
physical indices demonstrated that the liver and muscle glycogen,
LDH and blood glucose concentrations of mice in the treatment
groups were significantly increased compared with those in the
control group. Furthermore, the LC concentrations of the treatment
groups were significantly decreased compared with those of the
control group. Glycogen serves as a form of energy storage in
animals and is stored primarily in the cells of the liver and the
muscles (23). Energy is supplied
via hepatic glycogen degradation when blood glucose is depleted.
The results of this study suggested that G. eucheumoides may
improve the energy efficiency of mice.
During high intensity exercises, blood glucose is
metabolized and oxidized to pyruvate, and LC is produced from the
pyruvate at a greater speed than tissues are capable of removing
it. Therefore, the LC concentration begins to rise (24,25).
The LC concentration of mammalian blood is 1–2 mM when sitting
still and may increase to 20 mM during strenuous exercise. During
the present experiments, the LC concentration decreased to 11.3 mM
in the high-dose group and the concentration of LDH increased
following exercise. LDH catalyzes the oxidation of LC, forming
pyruvate. Therefore, mice fed with G. eucheumoides extract
experienced a reduction in blood LC concentration as a result of
increased LDH concentration.
In order to investigate the molecular mechanisms
underlying the antifatigue effect of G. eucheumoides, the
mRNA and protein expression of AMPK and GLUT4 were examined using
qPCR and western blotting, respectively. AMPK has a central role in
the control of metabolism in cells, and operates an alternative
pathway of energy synthesis when cellular energy is low (26,27).
GLUT4 transports glucose in adipose tissue and muscle, and is
important in energy metabolism and the antifatigue process
(28). In this study, mRNA and
protein expression levels of AMPK and GLUT4 were significantly
increased in the treatment groups compared with those of the
control group. However, the expression levels were increased in
different manners. The mRNA and protein levels of GLUT4 increased
to their highest levels in the high-dose group. The mRNA expression
of AMPK also increased to its highest level in the medium-dose
group; however, the protein expression level of AMPK increased to
its highest level in the high-dose group. It is possible that G.
eucheumoides may not only promote the levels of gene and
protein expression of AMPK and GLUT4, but also initiate other
methods of energy synthesis in order to meet energy requirements
and avoid severe hypoglycemia during vigorous exercise.
Furthermore, the enhanced expression of GLUT4 protein may improve
glucose transport rates, thus increasing its utilization.
In conclusion, the antifatigue effect of G.
eucheumoides was analyzed and the physiological and molecular
mechanisms underlying this effect were investigated. The results
demonstrated that G. eucheumoides exerts marked antifatigue
effects, which may be achieved by regulating antifatigue-associated
genes and increasing LDH concentration. Further studies are
required to investigate the mechanims underlying these properties
of G. eucheumoides.
References
|
1
|
Bocanegra A, Bastida S, Benedí J, Nus M,
Sánchez-Montero JM and Sánchez-Muniz FJ: Effect of seaweed and
cholesterol-enriched diets on postprandial lipoproteinaemia in
rats. Br J Nutr. 102:1728–1739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wada K, Nakamura K, Tamai Y, et al:
Seaweed intake and blood pressure levels in healthy pre-school
Japanese children. Nutr J. 10:832011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blokhuis TJ and Arts JJ: Bioactive and
osteoinductive bone graft substitutes: definitions, facts and
myths. Injury. 42(Suppl 2): S26–S29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed HH, Hegazi MM, Abd-Alla HI, Eskander
EF and Ellithey MS: Antitumour and antioxidant activity of some Red
Sea seaweeds in Ehrlich ascites carcinoma in vivo. Z Naturforsch C.
66:367–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wijesinghe WA and Jeon YJ: Exploiting
biological activities of brown seaweed Ecklonia cava for
potential industrial applications: a review. Int J Food Sci Nutr.
63:225–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coura CO, de Araújo IW, Vanderlei ES, et
al: Antinociceptive and anti-inflammatory activities of sulphated
polysaccharides from the red seaweed Gracilaria cornea.
Basic Clin Pharmacol Toxicol. 110:335–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siqueira RC, da Silva MS, de Alencar DB,
et al: In vivo anti-inflammatory effect of a sulfated
polysaccharide isolated from the marine brown algae Lobophora
variegata. Pharm Biol. 49:167–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuvaraj N, Kanmani P, Satishkumar R, Paari
A, Pattukumar V and Arul V: Seagrass as a potential source of
natural antioxidant and anti-inflammatory agents. Pharm Biol.
50:458–467. 2012.PubMed/NCBI
|
|
9
|
Xu HL, Kitajima C, Ito H, et al:
Antidiabetic effect of polyphenols from brown alga Ecklonia
kurome in genetically diabetic KK-A(y) mice. Pharm Biol.
50:393–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YS, Shin KH, Kim BK and Lee S:
Anti-diabetic activities of fucosterol from Pelvetia
siliquosa. Arch Pharm Res. 27:1120–1122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Albuquerque IR, Queiroz KC, Alves LG,
Santos EA, Leite EL and Rocha HA: Heterofucans from Dictyota
menstrualis have anticoagulant activity. Braz J Med Biol Res.
37:167–171. 2004.
|
|
12
|
Silva TM, Alves LG, de Queiroz KC, et al:
Partial characterization and anticoagulant activity of a
heterofucan from the brown seaweed Padina gymnospora. Braz J
Med Biol Res. 38:523–533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bogdanis GC: Effects of physical activity
and inactivity on muscle fatigu. Front Physiol. 3:1422012.
View Article : Google Scholar
|
|
14
|
Saiki T, Kawai T, Morita K, et al:
Identification of marker genes for differential diagnosis of
chronic fatigue syndrome. Mol Med. 14:599–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finsterer J: Biomarkers of peripheral
muscle fatigue during exercise. BMC Musculoskelet Disord.
13:2182012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takano R, Hayashi K and Hara S: Highly
methylated agars with a high gel-melting point from the red
seaweed, Gracilaria eucheumoides. Phytochemistry.
40:487–490. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krishnaiah D, Sarbatly R, Prasad DMR and
Bono A: Mineral content of some seaweeds from Sabah’s south China
sea. Asian J Sci Res. 1:166–170. 2008.
|
|
18
|
Zhang X, Li H, Wu G, Ban S and Park H:
Extraction of Eupatorium odoratum and its inhibition on
toxic cyanobacteria. J Hainan Norm Uni (Nat Sci). 23:427–432.
2010.(In Chinese).
|
|
19
|
Jiménez-Escrig A, Gómez-Ordóñez E and
Rupérez P: Seaweed as a source of novel nutraceuticals: sulfated
polysaccharides and peptides. Adv Food Nutr Res. 64:325–337.
2011.PubMed/NCBI
|
|
20
|
Vera J, Castro J, Gonzalez A and Moenne A:
Seaweed polysaccharides and derived oligosaccharides stimulate
defense responses and protection against pathogens in plants. Mar
Drugs. 9:2514–2525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewis AS: Organic versus inorganic arsenic
in herbal kelp supplements. Environ Health Perspect. 115:A5752007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun XW, Weng HX and Qin YC: Release of
bioactive active iodine in kelp. J Environ Sci (China). 17:241–244.
2005.PubMed/NCBI
|
|
23
|
Greenberg CC, Jurczak MJ, Danos AM and
Brady MJ: Glycogen branches out: new perspectives on the role of
glycogen metabolism in the integration of metabolic pathways. Am J
Physiol Endocrinol Metab. 291:E1–E8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brook GA, Dubouchard H, Brown M, Sicurello
JP and Butz CE: Role of mitochondrial lactate dehydrogenase and
lactate oxidation in the intracellular lactate shuttle. Proc Natl
Acad Sci USA. 96:1129–1134. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scott CB: Contribution of anaerobic energy
expenditure to whole body thermogenesis. Nutr Metab (Lond).
2:142005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heidrich F, Schotola H, Popov AF, et al:
AMPK - activated protein kinase and its role in energy metabolism
of the heart. Curr Cardiol Rev. 6:337–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oakhill JS, Steel R, Chen ZP, et al: AMPK
is a direct adenylate charge-regulated protein kinase. Science.
332:1433–1435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song H, Guang Y, Zhang L, Li K and Dong C:
SPARC interacts with AMPK and regulates GLUT4 expression. Biochem
Biophys Res Commun. 396:961–966. 2010. View Article : Google Scholar : PubMed/NCBI
|