Introduction
Breast cancer accounts for 30% of primary malignant
tumors in women (1). Radiotherapy
is an important method for the clinical treatment of breast cancer,
but its curative effect is often affected by damage to the
surrounding normal tissues and tumor radiation tolerance, so
radiotherapy alone has certain limitations (2). Gene-radiotherapy, as a new therapy
that combines gene therapy and radiation therapy, has attracted
much interest and has broad application prospects (3,4). The
basic principle of gene-radiotherapy is the use of the
radiation-induced characteristics of early growth response-1
(Egr-1) to increase the expression of a target gene following
radiation and thereby enhance the treatment effect. Egr-1,
containing the six serum response elements of CArG [CC (A + T-rich)
6GG], is a key component of radiation-activated expression.
Numerous studies have observed that if the Egr-1 promoter gene is
placed upstream of TNF-α, IFN-γ, endostatin and TRAIL genes, it
promotes the expression of these genes by radiation induction
(5–7). In the present study, the application
of the radiotherapy-induced Egr-1 promoter gene is considered.
The target gene of tumor gene-radiotherapy may be a
pro-apoptotic, cytokine or suicide gene (7–9).
Ionizing radiation is able to induce the apoptosis and cell cycle
arrest of tumor cells, and the failure to repair DNA damage
following cell cycle arrest causes cell apoptosis (10). Therefore, second
mitochondria-derived activator of caspase (Smac) was used as the
target gene in the current study. Smac is localized in the
mitochondria and released into the cytoplasm, triggering a cascade
reaction of the caspase family through a variety of pathways, and
promoting apoptosis. Smac is expressed in a variety of tumors, and
is closely associated with the occurrence and development of
various tumors (11). The
overexpression of the Smac gene may promote the apoptosis of tumor
cells and enhance the sensitivity of the cells to chemotherapy and
radiotherapy. A previous study has shown that overexpression of the
Smac gene may cause cancer cells to become more sensitive to
apoptotic stimuli. In particular, a short amino acid sequence,
which is separated from the N-terminus of the Smac protein, also
reacts with XIAP and may kill tumor cells overexpressing IAPs
(12,13). The purpose of the current study was
to investigate the dual effects of apoptosis induced by ionizing
radiation and the Smac gene.
Egr-1 may be activated by radiation to deliver gene
therapy, but often the hypoxic microenvironment in solid tumors
markedly reduces the effect of the Egr-1 promoter. Overcoming solid
tumor hypoxia (leading to radiation tolerance) is a key challenge
in the treatment of tumors. The core sequence of hypoxia response
elements (HREs), 5′-(A/G)CGT(G/C)(G/C)-3′, has clear
hypoxia-inducible characteristics (14–16).
In addition, the use of specific replication with the conditionally
replicative adenovirus (CRAd) in tumor cells is able to greatly
increase the copy number and cause the high level expression of
therapeutic genes (17). The
conditionally replicative adenovirus mediated by HREs may achieve
increased gene expression under hypoxic conditions and overcome the
low efficiency of radiotherapy caused by the hypoxic
environment.
Therefore, in the present study, HRE and Egr-1 were
used to construct a CRAd vector to mediate the expression of the
Smac gene when induced by the dual stimuli of hypoxia and
radiation. The effects of the vector on the proliferation, cell
cycle and apoptosis of MDA-MB-231 human breast cancer cells were
then observed. This exploration of the gene-radiotherapy effect was
conducted in order to provide new insight for the clinical
radiotherapy of breast cancer.
Materials and methods
Cell lines and culture
MDA-MB-231 human breast cancer cells were purchased
from the Shanghai Institute of Cell Biology, Chinese Academy of
Science (Shanghai, China). The cells were cultured at 37°C with 5%
CO2, using L15 medium containing 10% fetal bovine serum,
100 U/ml penicillin and streptomycin (Gibco BRL, Carlsbad, CA,
USA). HEK293 human embryonic kidney cells were maintained by the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Science (Shanghai, China). The cells were cultured under the same
conditions as were used for the MDA-MB-231 cells, with a high sugar
DMEM medium containing 10% fetal bovine serum, 100 U/ml penicillin
and 100 U/ml streptomycin (Sigma, St. Louis, MO, USA).
CRAd
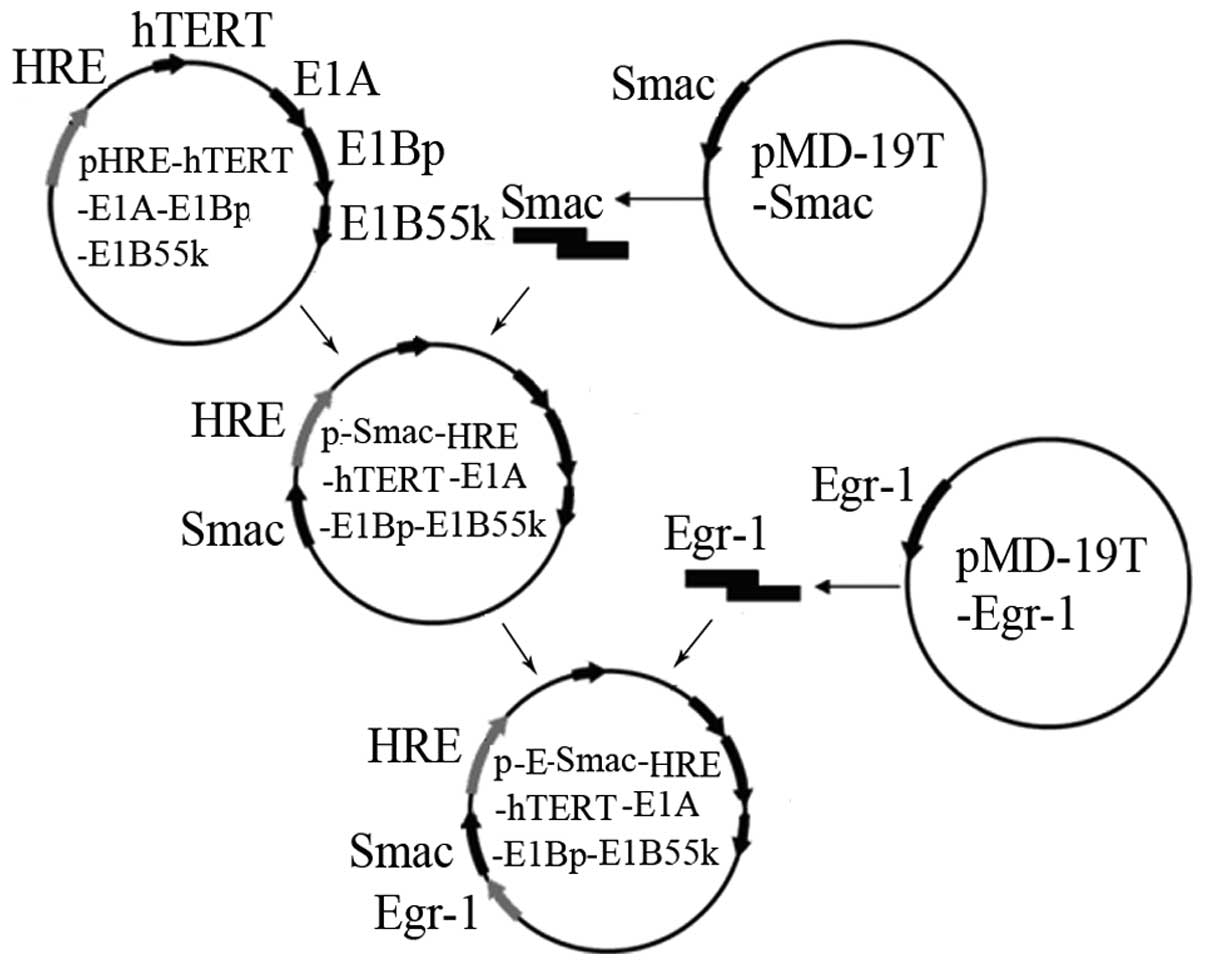
A shuttle vector,
pShuttle-Egr-1-Smac-HRE-hTERT-E1A-E1Bp-E1B55K was constructed. It
was activated by hypoxia and radiation, resulting in the
overexpression of Smac. The shuttle vector was transferred into
BJ5183 (AdEasy-1 +) by electrotransformation, where it underwent
homologous recombination with pAdEasy-1 to form the recombinant
adenovirus plasmid. The plasmid was then transfected into HEK293
cells using the Lipofectamine 2000 regeants reagents (Invitrogen,
Carlsbad, CA, USA). After packing, the CRAd was named
CRAd.pEgr-1-Smac. The empty virus CRAd.p served as the control.
After 3–5 generations of amplification, the virus was collected,
the virus titer was confirmed by determining the 50% tissue culture
infection dose (TCID50) and the virus was stored below
−70°C. The establishment process of
pShuttle-Egr-1-Smac-HRE-hTERT-E1A-E1Bp-E1B55K is shown in Fig. 1.
Cell transfection, hypoxia and X-ray
irradiation
The MDA-MB-231 cells were divided in six groups:
normal control, CRAd.pEgr-1-Smac, hypoxia (H), empty virus
(CRAd.p), CRAd.pEgr-1-Smac + H and CRAd.p + H. Briefly, the cells
were seeded in 6-, 24- or 96-well culture plates. As the cells
reached 80–90% confluence, they were infected with the adenovirus
at a MOI [multiplicity of infection (virus/cell)] of five for 24 h
according to the literature method (18). CoCl2 (Sigma) was added
at a final concentration of 150 μmol/l for 24 h to simulate hypoxia
(19). The X-ray irradiation was
performed using an X-ray machine (X.S.S.250FZ; Guiyang Medical
Instrument, Guiyang, China) under the following conditions: 200 kV,
10 mA, 0.5 mm Cu filter and 1.0 mm Al filter, a target skin
distance of 50 cm, and 0 or 4 Gy of radiation at a dose rate of
0.287 Gy/min. The dose and dose rate options were in accordance
with studies by the United Nations Scientific Committee on Atomic
Radiation (UNSCAR) in 1986 and our previous work (7,20).
Detection of the Smac protein
The MDA-MB-231 cells were seeded at a density of
1×106 cells in each well of 6-well culture plates,
followed by the treatment described in the preceding section. The
cells were collected 24 h after irradiation and lysed using a lysis
buffer [10 mmol/l Tris-HCl, pH 7.4; 1 mmol/l EDTA, pH 8.0; 0.1
mol/l NaCl; 1 μg/ml aprotinin; 100 μg/ml phenylmethanesulfonyl
fluoride (PMSF)]. The total protein was extracted, quantified using
a Coomassie blue protein quantification kit (Nanjing Jiancheng
Biological Institute, Nanjing, China) and separated by 12% SDS-PAGE
(loading: 50 μg). The protein was electroblotted onto a
nitrocellulose membrane. Then, the membrane was incubated
consecutively in 5% nonfat milk for blocking for 1 h and with
anti-GAPDH or anti-Smac antibodies (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) overnight at 4°C. The membrane was then washed
in TBST buffer and incubated with horseradish peroxidase-conjugated
secondary antibody at 37°C for 1 h (Thermo Fisher Scientific Inc.,
Lake Barrington, IL, USA). The western blotting luminal reagent
(Santa Cruz Biotechnology, Inc.) was used to develop the blots,
followed by the photographic capturing of images for analysis.
Detection of cell proliferation
The 3-(4,5-dimethylthiazol-2-yl)
-2,5-diphenyltetrazolium bromide (MTT) method was used to detect
cell proliferation. Briefly, the MDA-MB-231 cells were seeded at a
density of 2×104 cells per well in a 96-well culture
plate, with six replicates for each group, and treated as described
previously. At 12, 24 and 48 h after irradiation, 10 μl MTT (5
mg/ml; Sigma) was added for 4 h. The supernatants were discarded
and 100 μl dimethylsulfoxide (DMSO; Sigma) was added to dissolve
the crystals. The optical density (OD) value at 570 nm was measured
using a microplate reader (Bio-Rad, Hercules, CA, USA) (7). The experiment was repeated three
times.
Flow cytometric analysis of the cell
cycle and apoptosis
The cell cycle and apoptosis were analyzed by flow
cytometry (FCM; Becton-Dickinson, Franklin Lakes, NJ, USA) with PI
single dye (Sigma) or PI + Annexin V-FITC double staining (Nanjing
KeyGEN Biotech Co., Ltd., Nanjing, China), respectively. Briefly,
the MDA-MB-231 cells were seeded at a density of 3×105
cells per well in 24-well culture plates, and treated as described
previously. Cells were collected in an Eppendorf tube 24 h after
irradiation and washed twice with PBS by centrifugation. The
supernatants were discarded. To analyze the cell cycle, 50 μl RNase
A and 200 μl PI were added to each tube, and the tube contents were
mixed in the dark at room temperature for 20 min, followed by FCM
testing. To detect apoptosis, 500 μl PBS, 5 μl Annexin V-FITC and 5
μl PI were added to each tube, and the contents of the tube were
mixed in the dark at room temperature for 15 min, followed by FCM
testing. Cell Quest software (Becton-Dickinson) was used to acquire
and analyze the data, and the data are expressed as cell
percentages.
Statistical processing
The experimental data are expressed as mean ±
standard deviation (SD). A one-way ANOVA test was used for
statistical analysis using the statistical software SPSS 12.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant result.
Results
Expression of Smac protein in MDA-MB-231
cells
At 6, 12 and 24 h after sham irradiation (0 Gy), the
difference in Smac protein expression between groups was small,
which indicated that the Egr-1 promoter had no function in the
absence of irradiation and was unable to express the characteristic
of regulating the downstream gene (Fig. 2). At 6 h after 4-Gy irradiation,
there was no significant increase in the level of Smac expression;
while after 12 and 24 h, the Smac expression level was increased in
the CRAd.pEgr-1-Smac and CRAd.pEgr-1-Smac + H groups, particularly
in the latter.
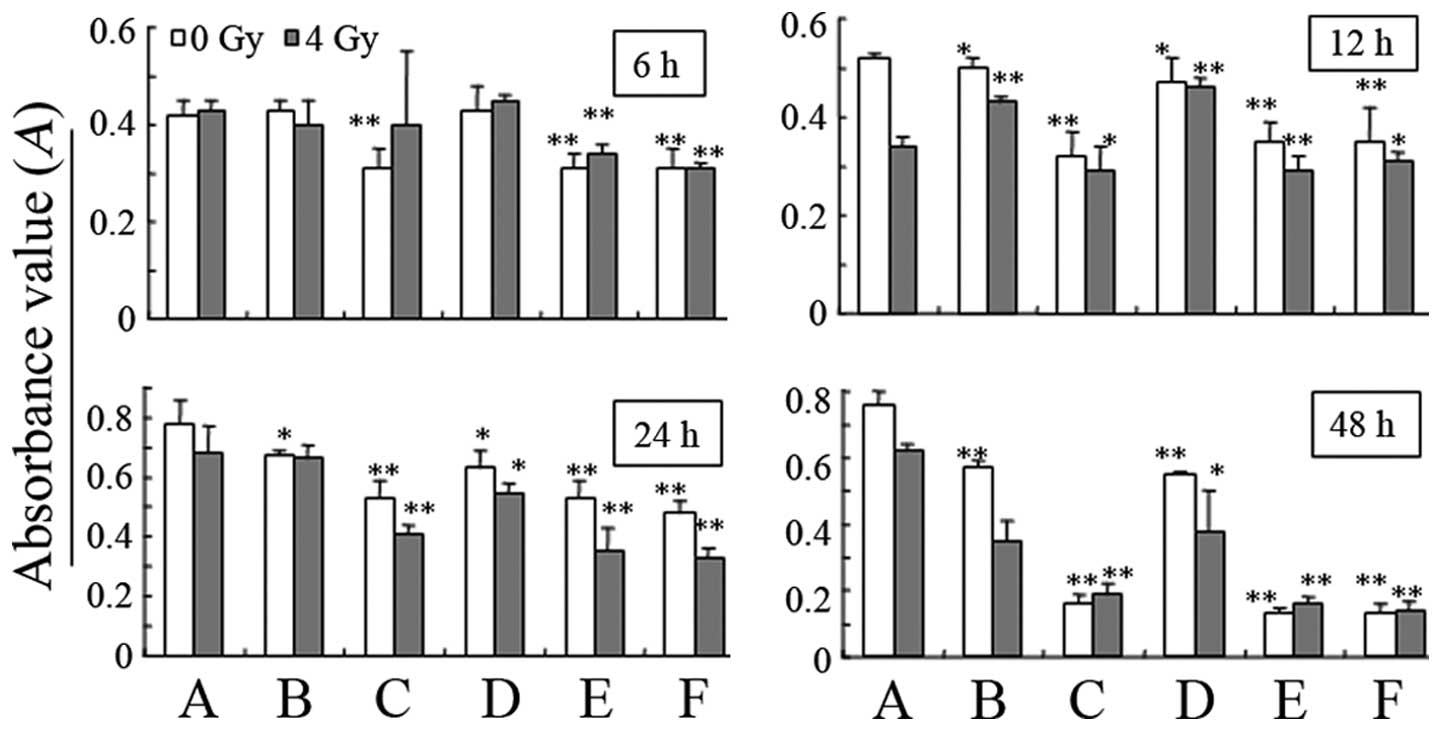
Changes in cell proliferation
As shown in Fig. 3,
at 6 h after 0-Gy irradiation, hypoxia inhibited the growth of
MDA-MB-231 cells; there was a statistically significant difference
in cell proliferation between the hypoxia and normal control groups
(P<0.01). At 12 h, the transfected virus also inhibited
MDA-MB-231 cell growth, and the inhibition was time-dependent. At 6
h after 4-Gy irradiation, there was no significant reduction in the
proliferation of the cells transfected with the CRAd.pEgr-1-Smac
virus, while the proliferation was reduced significantly following
hypoxia (P<0.01). At 12 h, the cell proliferation of each group
decreased significantly (P<0.01), and at 48 h, it reached
minimum values.
Changes in MDA-MB-231 cell cycle
progression
Apoptosis occurs in various cell cycle phases, and
cell cycle progression and apoptosis are closely associated. As
shown in Fig. 4, the transfection
with CRAd.pEgr-1-Smac or CRAd.p in combination with hypoxia may
lead to an increase in the percentage of MDA-MB-231 cells in the S
phase. The CRAd.pEgr-1-Smac + H and CRAd.p + H groups had a
significantly increased percentage of cells in the S phase compared
with the control group (P<0.01), and a significantly increased
percentage of cells in the G2/M phase compared with the
normal control group (P<0.05). The 4-Gy irradiation and hypoxia
led to increases in the percentages of cells in the S and
G2/M phases, while transfection with CRAd.pEgr-1-Smac
did not change the cell cycle markedly. The 4-Gy irradiation of
normal control cells increased the percentage of cells in the S and
G2/M phases significantly compared with 0-Gy irradiation
(P<0.05), while no significant changes occurred in the other
groups.
Changes in apoptosis of MDA-MB-231
cells
In Figs. 5 and
6, transfection with
CRAd.pEgr-1-Smac and hypoxia following 0-Gy irradiation is shown to
induce MDA-MB-231 cell apoptosis significantly when compared with
the normal control (P<0.05); transfection with CRAd.p had no
such effects, but when combined with hypoxia was able to induce
cell apoptosis significantly (P<0.01). When treated with 4 Gy of
radiation, the cell apoptosis events in each group were the same as
those when 0 Gy of radiation was used. The CRAd.pEgr-1-Smac + H
group presented the greatest increase in cell apoptosis. In
addition, in this group, the percentage of cell apoptosis when 4 Gy
of radiation was administered was significantly higher compared
with that when 0 Gy of radiation was used (P<0.01).
Discussion
The efficient expression of radiation-inducible
therapeutic genes in tumor cells is crucial in gene-radiotherapy.
Only by the overexpression of the gene with its corresponding
functions may the combined effects of gene therapy and radiotherapy
be achieved. CRAd, also called oncolytic adenovirus, is an ideal
expression vector, which is able to replicate in tumor cells and
produce a cascade effect (21,22).
Relevant studies (5–7,23)
have confirmed that ionizing radiation is able to induce the Egr-1
promoter to activate the expression of the downstream gene, which
generates oxygen-free radicals acting on the six serum response
element CArG. Hypoxia in solid tumors leads to the reduction of the
free radicals induced by radiation, affecting the efficiency of the
Egr-1 promoter.
The present study used the hypoxia-inducible
characteristic of HRE to enhance the replication ability of CRAd
under hypoxic conditions, and also strengthened the mediated
efficiency of radiation on the Egr-1 promoter, increasing the
expression of Smac, and thus realized dual activation by radiation
and hypoxia.
At 6, 12 and 24 h after sham irradiation (0 Gy), the
difference in Smac protein expression between the groups was small,
indicating that the Egr-1 promoter had no function in the absence
of irradiation, and was not able to regulate the downstream gene.
The presence of Smac expression observed in each group may be due
to endogenous expression in the cell, not the exogenous Smac
expression. At 6 h after treatment with 4 Gy of radiation, there
was no significant increase in Smac expression, while after 12 and
24 h, Smac expression was increased in the CRAd.pEgr-1-Smac group,
and markedly increased in the CRAd.pEgr-1-Smac + H group. These
results indicated that radiation activated the Egr-1 promoter.
Hypoxia induced an increase in the replication of CRAd, suggesting
that this experiment achieved the targeted overexpression of the
therapeutic Smac gene with radiation and hypoxia.
At 12 h after 0-Gy irradiation, the transfected
virus inhibited the MDA-MB-231 cell growth, and the inhibitory
effect was time-dependent. This was likely due the CRAd itself
being able to inhibit the tumor cell proliferation (24). At 6 h after 4-Gy irradiation, there
was no significant reduction in the proliferation of the cells
transfected by the CRAd.pEgr-1-Smac virus, while the proliferation
reduced significantly following hypoxia (P<0.01). At 12 h, the
cell proliferation of each group decreased significantly
(P<0.01), and at 48 h, the proliferation reached minimum values.
These results indicate that the expression of Smac protein was at a
low level 6 h after irradiation and had no function. With the
progression of time, the expression level increased and Smac was
able to fully inhibit cell proliferation.
After exposure to ionizing radiation, most cells are
in G2 arrest, ensuring the DNA damage is repaired and conducive to
cell survival (25). Furthermore,
G2 arrest is also associated with cell radiosensitivity and may
increase cell radiosensitivity, which is useful in the radiation
therapy of tumor cells (26). The
changes in MDA-MB-231 cell cycle progression demonstrate that the
conditions in the present study maintained the MDA-MB-231 cells in
G2/M phase arrest, which may increase the cellular
radiosensitivity and aid tumor radiotherapy.
The aim of tumor gene-radiotherapy includes not only
the inhibition of cell proliferation, but also the induction of
cell death. Apoptosis is one type of cell death. Ionizing radiation
is the basis of clinical radiotherapy, which is able to induce
tumor cell apoptosis directly and block the cell cycle checkpoint.
Initiating apoptosis by using certain radiotherapy strategies may
inhibit the growth of tumor cells effectively, being quite
important to tumor radiotherapy. Smac is one type of
apoptosis-inducing factor. An anti-tumor study conducted with Smac
in recent years indicated that the overexpression of Smac may cause
tumor cell apoptosis, and increase the sensitivity of tumor cells
to chemotherapy (27). The
increase in apoptosis of MDA-MB-231 cells with Smac overexpression
suggests that when the cells transfected with CRAd.pEgr-1-Smac are
in a hypoxic condition, treatment with ionizing radiation is able
to induce the highest level of cell apoptosis. Therefore, the
present study indicates that the maximum efficacy may be achieved
by using the gene-radiotherapy strategy.
In conclusion, this study achieves the
overexpression of Smac with hypoxia and radiation; its ability to
inhibit cell proliferation and induce the apoptosis of MDA-MB-231
breast cancer cells is clearly apparent. The overexpression of Smac
may also result in cell cycle arrest by enabling the
G2/M phase, i.e. the radiation-sensitive cell cycle
phase, which is also likely to facilitate the subsequent
radiotherapy. This study explored a gene-radiotherapy strategy for
treating breast cancer, and this strategy is expected to solve gene
selection and targeting problems. First, the hypoxic
microenvironment of solid tumors was used to overcome the
ineffectiveness of gene-radiotherapy caused by hypoxia. Secondly,
the CRAd vector was used to deliver a therapeutic gene to tumor
cells subjected to increased radiation levels and a hypoxic status.
Radiotherapy may be fully integrated with the favorable and
unfavorable conditions of genes to maximize the benefits of
radiotherapy. This study provides a new method for the clinical
treatment of breast cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation No. 30870747 and No. 30970681.
References
|
1
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar
|
|
2
|
Kwong DL, Sham JS, Leung LH, Cheng AC, Ng
WM, Kwong PW, Lui WM, Yau CC, Wu PM, Wei W and Au G: Preliminary
results of radiation dose escalation for locally advanced
nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 64:374–381.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng AQ, Song XR, Yu JM, Wei L and Wang
XW: Liposome transfected to plasmid-encoding endostatin gene
combined with radiotherapy inhibits liver cancer growth in nude
mice. World J Gastroenterol. 11:4439–4442. 2005.PubMed/NCBI
|
|
4
|
Harari PM and Huang SM: Head and neck
cancer as a clinical model for molecular targeting of therapy:
combining EGFR blockade with radiation. Int J Radiat Oncol Biol
Phys. 49:427–433. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu LL, Smith MJ, Sun BS, Wang GJ, Redmond
HP and Wang JH: Combined IFN-gamma-endostatin gene therapy and
radiotherapy attenuates primary breast tumor growth and lung
metastases via enhanced CTL and NK cell activation and attenuated
tumor angiogenesis in a murine model. Ann Surg Oncol. 16:1403–1411.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang W and Li XY: Anti-tumor effect of
pEgr-interferon-γ-endostatin gene-radiotherapy in mice bearing
Lewis lung carcinoma and its mechanism. Chin Med J (Engl).
118:296–301. 2005.PubMed/NCBI
|
|
7
|
Li Y, Guo C, Wang Z, Gong P, Sun Z and
Gong S: Enhanced effects of TRAIL-endostatin-based
double-gene-radiotherapy on suppressing growth, promoting apoptosis
and inducing cell cycle arrest in vascular endothelial cells. J
Huazhong Univ Sci Technolog Med Sci. 32:167–172. 2012. View Article : Google Scholar
|
|
8
|
Wu C, Li X and Tian M: Effect of
pEgr-TNFalpha gene radiotherapy on mice melanoma. Melanoma Res.
15:185–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Yu XH, Zha X and Kong W:
Adenovirus-mediated HSV-TK gene therapy using hTERT promoter in CNE
cells in vitro. Chem Res Chinese Universities. 25:60–63. 2009.
|
|
10
|
Wang WD, Chen ZT, Li DZ, Duan YZ, Wang ZX
and Cao ZH: HSV-TK gene therapy of lung adenocarcinoma xenografts
using a hypoxia/radiation dual-sensitive promoter. Ai Zheng.
23:788–793. 2004.(In Chinese).
|
|
11
|
Mizutani Y, Nakanishi H, Yamamoto K, Li
YN, Matsubara H, Mikami K, Okihara K, Kawauchi A, Bonavida B and
Miki T: Downregulation of Smac/DIABLO expression in renal cell
carcinoma and its prognostic significance. J Clin Oncol.
23:448–454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pluta P, Cebula-Obrzut B, Ehemann V, Pluta
A, Wierzbowska A, Piekarski J, Bilski A, Nejc D, Kordek R, Robak T,
Smolewski P and Jeziorski A: Correlation of Smac/DIABLO protein
expression with the clinico-pathological features of breast cancer
patients. Neoplasma. 58:430–435. 2011. View Article : Google Scholar
|
|
13
|
Fulda S, Wick W, Weller M and Debatin KM:
Smac agonists sensitize for Apo2L/TRAIL or anticancer drug-induced
apoptosis and induce regression of malignant glioma in vivo. Nat
Med. 8:808–815. 2002.PubMed/NCBI
|
|
14
|
Lok CN and Ponka P: Identification of a
hypoxia response element in the transferin receptor gene. J Biol
Chem. 274:24147–24152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okino ST, Chichester CH and Whitlock JP
Jr: Hypoxia-inducible mammalian gene expression analyzed in vivo at
a TATA-driven promoter and at an initiator-driven promoter. J Biol
Chem. 273:23837–23843. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwon OJ, Kim PH, Huyn S, Wu L, Kim M and
Yun CO: A hypoxia- and α-fetoprotein-dependent oncolytic adenovirus
exhibits specific killing of hepatocellular carcinomas. Clin Cancer
Res. 16:6071–6082. 2010.
|
|
17
|
Barnes MN, Coolidge CJ, Hemminki A,
Alvarez RD and Curiel DT: Conditionally replicative adenoviruses
for ovarian cancer therapy. Mol Cancer Ther. 1:435–439.
2002.PubMed/NCBI
|
|
18
|
Wu JH, Wang HF, Wang ZC, Xu K, Qi YL, Li
JH, Gong SL and Liu Y and Liu Y: Conditionally replicating
adenovirus combined with gene-targeted radiotherapy induces
apoptosis via TRAIL death receptors in MDA-MB-231 cells. Mol Med
Rep. 8:299–305. 2013.PubMed/NCBI
|
|
19
|
Dai M, Cui P, Yu M, Han J, Li H and Xiu R:
Melatonin modulates the expression of VEGF and HIF-1 alpha induced
by CoCl2 in cultured cancer cells. J Pineal Res.
44:121–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu G, Gong P, Zhao H, Wang Z, Gong S and
Cai L: Effect of low-level radiation on the death of male germ
cells. Radiat Res. 165:379–389. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang SW, Chanda D, Coody JJ, Rivera AA,
Waehler R, Siegal GP, Douglas JT and Ponnazhagan S: Conditionally
replicating adenovirus expressing TIMP2 in creases survival in a
mouse model of disseminated ovarian cancer. PLoS One. 6:e251312011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng FQ, Xu Y, Yang RJ, Wu B, Tan XH, Qin
YD and Zhang QW: Combination effect of oncolytic adenovirus therapy
and herpes simplex virus thymidine kinase/ganciclovir in hepatic
carcinoma animal models. Acta Pharmacol Sin. 30:617–627. 2009.
View Article : Google Scholar
|
|
23
|
Senzer N, Mani S, Rosemurgy A, Nemunaitis
J, Cunningham C, Guha C, Bayol N, Gillen M, Chu K, Rasmussen C,
Rasmussen H, Kufe D, Weichselbaum R and Hanna N: TNFerade biologic,
an adenovector with a radiation-inducible promoter, carrying the
human tumor necrosis factor alpha gene: a phase I study in patients
with solid tumors. J Clin Oncol. 22:592–601. 2004. View Article : Google Scholar
|
|
24
|
Kasuya H, Takeda S, Nomoto S and Nakao A:
The potential of oncolytic virus therapy for pancreatic cancer.
Cancer Gene Ther. 12:725–736. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teyssier F, Bay JO, Dionet C and Verrelle
P: Cell cycle regulation after exposure to ionizing radiation. Bull
Cancer. 86:345–357. 1999.(In French).
|
|
26
|
Deplanque G, Céraline J, Mah-Becherel MC,
Cazenave JP, Bergerat JP and Klein-Soyer C: Caffeine and the G2/M
block override: a concept resulting from a misleading cell kinetic
delay, independent of functional p53. Int J Cancer. 94:363–369.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo C, Li Y, Zhang H, Wang Z, Jin M, Zhang
L, An L, Hu G, Liu X, Liu Y, Du H and Sun Z: Enhancement of
antiproliferative and proapoptotic effects of cadmium chloride
combined with hSmac in hepatocellular carcinoma cells.
Chemotherapy. 57:27–34. 2011. View Article : Google Scholar : PubMed/NCBI
|