Introduction
The hallmark of severe acute pancreatitis (SAP) is
extreme rapidity of disease progression with a high risk of
mortality, ranging from 30 to 50% (1). The aetiology of SAP is heterogeneous
and involves a complex cascade of events, however, the exact
pathophysiological mechanisms remain a subject of debate.
It is believed that the coagulation dysregulation
and thromboembolism that occur during disease progression are
critical in the pathogenesis of SAP, which is associated with its
severity (2,3). The hemostatic system is activated in
the course of SAP with the production of microthrombi in the
microvessels, and pathological changes ranging from scattered
intravascular thrombosis to disseminated intravascular coagulation
are integral to coagulative disorders (4). Severe complications, such as multiple
organ dysfunction syndrome, which are due to microcirculatory
disturbances and microvascular thrombosis, originate from vascular
endothelial cell injuries and hypercoagulation (5). Adding to the complexity, there are
interactions between acute inflammatory mediators, which are
released from the inflammation cascade during SAP and the
coagulation system, suggesting that the two events are closely
linked processes (1–3,6). In
turn, the inflammation-mediated initiation of coagulation (1,2,6),
fibrin deposition and formation of thrombin contribute to the local
inflammatory response (7).
Treatment with systemic anti-coagulants may improve the
microcirculatory perfusion and are capable of exerting an
anti-inflammatory effect (7,8).
However, the precise mechanisms remain to be determined.
Fibrinogen-like protein 2 (fgl2), also known as fgl2
prothrombinase, is a novel member of the fibrinogen-associated
protein superfamily, which includes tenascin and angiopoietin
(9). Fgl2 is expressed and
differentially regulated in various cell types (10). Fgl2 has been suggested as crucial
in microthrombosis and its biological characteristics, in line with
coagulation factor Xa, may cleave prothrombinase into activated
thrombinase, thereby initiating microthrombosis (11,12).
Fgl2 is involved in viral hepatitis (11,13),
acute vascular xenograft rejection (14,15)
and cytokine-induced fetal loss syndrome (16,17)
by mediating ‘immune coagulation’ (11), which facilitates microthrombosis,
resulting in microvascular disturbance. However, whether human fgl2
(hfgl2) is involved in patients with SAP remains to be
elucidated.
In the present study, the expression and
histological localization of hfgl2 were detected in peripheral
blood mononuclear cells (PMBCs) and pancreatic tissues and its
correlation with disease progression was assessed in patients with
SAP, with the expectation of providing a novel point of view on the
pathogenesis of SAP and a novel biomarker for predicting the
occurrence of SAP.
Materials and methods
Patients and collection of specimens
The definition of SAP is based on the typical
clinical features, laboratory evidence, typical manifestation
during ultrasonography and contrast-enhanced computed tomography
(CT), and intraoperative findings. All participants signed consent
forms for this study and the study protocol was reviewed and
approved by the Institutional Review Board of the First Affiliated
Hospital of Wenzhou Medical College (Wenzhou, China). Samples from
25 patients diagnosed with SAP (11 male and 14 female; mean age
62.6 years, range 21–83 years) and 37 patients diagnosed with mild
acute pancreatitis (MAP) (17 male and 20 female; mean age 59.0
years, range 19–85 years), who were admitted to the hospital within
24–48 h of the onset of disease, were collected from June 2011 to
September 2012. The etiology of the cases of MAP and SAP are
presented in Table I. In addition,
20 healthy volunteers (9 male and 11 female; mean age 58.95 years,
range 23–84 years) were recruited as healthy controls. No
significant differences in age and gender among the three groups
were detected (Table II).
 | Table IEtiology of MAP and SAP in the
patients. |
Table I
Etiology of MAP and SAP in the
patients.
| Etiology | No. (%) of mild
cases | No. (%) of severe
cases |
|---|
|
Gallstone-associated | 19 (51.4) | 16 (64) |
|
Alcohol-associated | 10 (27) | 5 (20) |
| Miscellaneousa | 5 (13.5) | 2 (8) |
| Unknown | 3 (8.1) | 2 (8) |
| Total | 37 | 25 |
 | Table IIAge and gender of the three
groups. |
Table II
Age and gender of the three
groups.
| Variables | Mild (n=37) | Severe (n=25) | Healthy control
(n=20) | P-value |
|---|
| Age (years) | 59.0 | 62.6 | 58.95 | 0.192 |
| Male/female
ratio | 17/20 | 11/14 | 9/11 | 0.989 |
All clinical definitions complied with the Atlanta
classification system for acute pancreatitis (18). Evaluations of scores of Ranson and
APACHE II were performed within 24 h after admission. PBMCs were
freshly isolated from the 82 participants and stored at −80ºC for
subsequent use. Paraffin sections were also reviewed and obtained
for pancreatic tissues from 10 postoperative patients (7 male and 3
female; mean age 53.2 years, range 34–77 years) with SAP and eight
(3 male and 5 female; mean age 50.8 years, range 35–71 years) with
MAP between 2003 and 2011, respectively. Of the eight MAP cases,
three were patients with pancreatic cancers, as the normal tissues
other than the tumor tissues were collected, and the other five
cases were patients with gallstones.
Real-time quantitative PCR (qPCR)
Total RNA was isolated from the PBMCs using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The first-strand cDNA was subsequently
synthesized (MBI Fermentas, Burlington, Canada). The PCR primer
sequences (Generay Biotech Co., Ltd., Shanghai, China) used were as
follows: 5-CCTGGAGATTGTGGTTTCGT-3 and 5-TACCATGCCTTTCTCCAAGG-3 for
hfgl2; and 5-TGTCACCAACTGGGACGATA-3 and 5-GGGGTGTTGAAGGTCTCAAA-3
for β-actin, which was used as an internal control. The qPCR was
performed with the ABI 7500 Sequence Detection system (Applied
Biosystems, Carlsbad, CA, USA) according to the manufacturer’s
instructions. The cDNA was amplified over 40 cycles, denatured at
95ºC for 15 sec, annealed at 60ºC for 45 sec and extended at 72ºC
for 60 sec. The samples were run in triplicate and the relative
expression was detected by normalizing to the β-actin levels. The
expression levels of the targeted genes were calculated by using
the 2−ΔΔCT method.
Immunohistochemical staining of
hfgl2
Immunohistochemical staining was performed to
evaluate the expression of hfgl2 in the pancreatic tissues and
PBMCs. Paraffin-embedded pancreatic tissues (4 μm) were sectioned.
Microwave antigen retrieval was conducted for 20 min in citrate
buffer (pH 6.0) to activate antigens. Blocking of endogenous
peroxidase was achieved using 0.3% H2O2 at
room temperature for 10 min. The pancreatic tissue slices or PBMCs
were incubated with mouse anti-fgl2 monoclonal antibody (Abnova
Corp., Taipei, Taiwan) at 4ºC overnight. Subsequently, the slices
were incubated with a secondary antibody (Zhongshan Goldenbridge,
Beijing, China) at 37ºC for 30 min, followed by developing with
diaminobenzidine and counterstaining with hematoxylin. PBS was used
instead of the primary antibody as the negative control.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Statistical Program for the Social Sciences Software,
version 15.0 (SPSS, Inc., Chicago, IL, USA) was used to conduct the
analysis. Statistical analysis was performed by one-way analysis of
variance. The Spearman grade correlation analysis was used to
evaluate the associations among the parameters. P≤0.05 was
considered to indicate a statistically significant difference.
Results
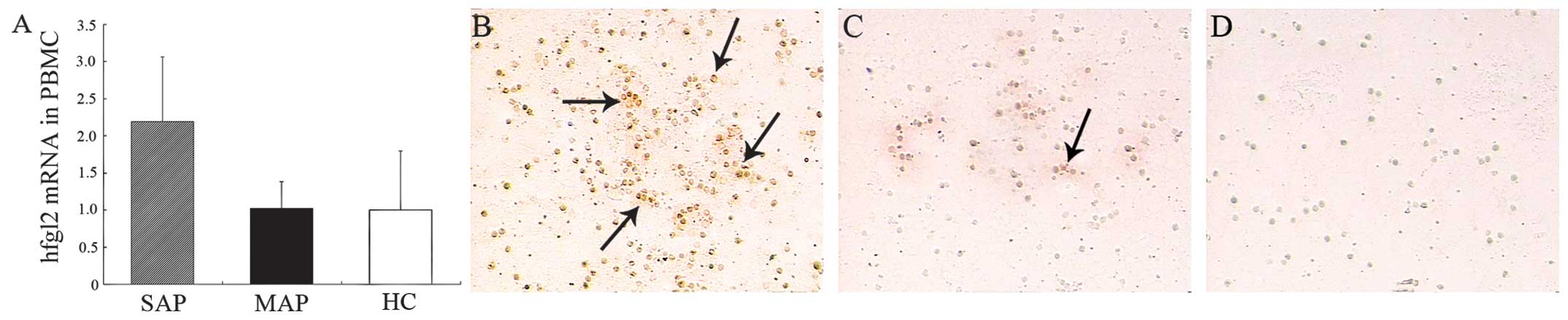
Upregulated hfgl2 expression in PBMCs
from patients with SAP
Hfgl2 expression in the PBMCs was assessed by qPCR
analysis and immunohistochemistry. The levels of hfgl2 mRNA were
significantly (P<0.01) upregulated in the patients with SAP
compared with those in the patients with MAP and the healthy
controls, whereas there was no significant difference (P>0.05)
between those of the MAP group and the healthy controls (Fig. 1A). Hfgl2 protein was highly
detectable in the PBMCs from the patients with SAP, in those from
the MAP group or the healthy controls (Fig. 1B–D).
Hfgl2 expression in pancreatic
tissues
Paraffin sections were reviewed and collected from
postoperative patients with SAP or MAP to evaluate hfgl2 expression
in pancreatic tissues. Results of immunohistochemistry demonstrated
that hfgl2 was primarily localized in infiltrating interstitial and
endothelial cells of the microvasculature, whereas little fgl2 was
identified in the pancreatic tissues of the patients with MAP
(Fig. 2).
Hfgl2 expression correlates with the
severity of SAP
The Ranson and APACHE II scores were significantly
elevated in the SAP group compared with the MAP group (Table III). Spearman grade correlation
analysis was conduced to determine the strength of the association
between fgl2 expression and the severity of SAP, as indicated by
the scoring systems. The data demonstrated that there was a strong
correlation between fgl2 expression and the scores of Ranson
(r=0.937, P<0.01) and APACHE II (r=0.976, P<0.01) (Fig. 3).
 | Table IIIMean severity scores of patients with
MAP and SAP. |
Table III
Mean severity scores of patients with
MAP and SAP.
| Type of score | Mild (n=37) | Severe (n=25) | P-value |
|---|
| Ranson | 1.54±0.61 (2) | 3.64±1.19 (3) | <0.001 |
| 24-h APACHE II | 6.86±1.60 (7) | 10.88±2.28 (11) | <0.001 |
Discussion
SAP is characterized as an inflammatory disease, in
which dysregulated cytokines such as TNF-α may ‘drive’ the
progression of this disorder (2,3).
During this process, the inflammation and coagulation cascade are
associated (1,3–6).
Furthermore, microthrombosis due to fibrin deposition has been
demonstrated to be pivotal in the pathogenesis of SAP and occurs
early on the onset (2,3,19).
In the present study, an important role for hfgl2 was defined in
the generation of fibrin and subsequently the formation of
microthrombi in patients with SAP.
Procoagulants other than tissue factor, which are
induced specifically by immune mediators, may be critical in
promoting localized fibrin deposition and result in disturbance of
microcirculation (20).
Fgl2/fibroleukin, a novel procoagulant, is a 70-kDa type-2
transmembrane protein containing a C-terminal fibrinogen-related
extracellular domain which has been found to directly cleave
prothrombin to thrombin in an alternative way and be crucial in
microthrombosis (9,11,12).
Moreover, fgl2 was demonstrated to be primarily expressed on
activated macrophages, T cells and endothelial cells (21). In the present study, marked hfgl2
expression as a source of procoagulant activity was identified in
PBMCs (containing lymphocytes, monocytes, dendritic cells and a few
other types of cell) from patients with SAP by qPCR analysis and
compared with patients with MAP and healthy controls. Concomitant
to the qPCR, results of immunohistochemistry demonstrated that
hfgl2 was localized on the infiltrating interstitial cells and
endothelial cells of the microvasculature in the areas of
inflammation in pancreatic tissues, therefore hfgl2 upregulation
may be pivotal in the morbid state of SAP and lead to pathological
injuries of the pancreas.
The pathway of fgl2-initiating coagulation is via
‘immunity blood coagulation’, which means fgl2 is expressed on
microvascular endothelial cells, macrophages and certain other
immunocytes and its expression is regulated by the mediation of a
series of proinflammatory cytokines as stimuli (17,22,23).
Fgl2 functions as a bridge molecule between immune and coagulation
reactions. Studies have demonstrated that fgl2 was strongly
expressed in endothelial cells with the induction of TNF-α
(17,23), however, IFN-γ was essential for the
induction of fgl2 on macrophages (23). Clark et al suggested that
elevating fgl2 prothrombinase in trophoblasts and in the deciduas
induces abortion in CBAxDBA/2 mice by stimulation of TNF-α
(24–26). Based on previous studies, it may be
considered that hfgl2, as an important mediator of prothrombinase
activity in fibrin formation, is a key effector molecule in the
pathogenesis of SAP with the induction of proinflammatory cytokines
such as TNF-α as accelerators.
To determine the relevance of hfgl2 expression in
PMBCs and the severity of SAP, as indicated by the Ranson scores
and APACHE II within 24 h following admission, Spearman grade
correlation analysis was performed. The three methods are well
known for the evaluation of SAP. The results demonstrated strong
correlations between hfgl2 expression in PMBCs and the assessments
by the three different methods. The potential of hfgl2 measurement
in PBMCs is convincing and it may be of prognostic value for
predicting the severity of SAP in the early stage.
The usefulness of injection of a neutralizing
antibody or gene therapy against fgl2 has been demonstrated in
diseases including MHV-3 hepatitis and graft rejection, which may
attenuate fibrin deposition as well as the pathological injury and
prevent mice from mortality (27–30).
Thus, in-depth investigation should be conducted to identify
whether the inhibition of fgl2 or the application of antibodies
against fgl2 are able to delay or ameliorate the course of SAP.
In conclusion, hfgl2, serving as a novel
prothrombinase, and the potent function it encodes contribute to
the pathogenesis of SAP. The detection of hfgl2 in PBMCs may serve
as a useful marker in predicting the severity of SAP and as a
promising target for therapeutic intervention.
References
|
1
|
Lisman T and Porte RJ: Activation and
regulation of hemostasis in acute liver failure and acute
pancreatitis. Semin Thromb Hemost. 36:437–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hagiwara S, Iwasaka H, Shingu C, Matsumoto
S, Uchida T and Noguchi T: Antithrombin III prevents
cerulein-induced acute pancreatitis in rats. Pancreas. 38:746–751.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kakafika A, Papadopoulos V, Mimidis K and
Mikhailidis DP: Coagulation, platelets, and acute pancreatitis.
Pancreas. 34:15–20. 2007. View Article : Google Scholar
|
|
4
|
Agarwal N and Pitchumoni CS: Acute
pancreatitis: a multisystem disease. Gastroenterologist. 1:115–128.
1993.PubMed/NCBI
|
|
5
|
Yasuda T, Ueda T, Kamei K, et al: Plasma
tissue factor pathway inhibitor levels in patients with acute
pancreatitis. J Gastroenterol. 44:1071–1079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warzecha Z, Dembiński A, Ceranowicz P, et
al: Influence of ischemic preconditioning on blood coagulation,
fibrinolytic activity and pancreatic repair in the course of
caerulein-induced acute pancreatitis in rats. J Physiol Pharmacol.
58:303–319. 2007.
|
|
7
|
Andersson E, Axelsson J, Pedersen LC, Elm
T and Andersson R: Treatment with anti-factor VIIa in acute
pancreatitis in rats: blocking both coagulation and inflammation?
Scand J Gastroenterol. 42:765–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dobosz M, Mionskowska L, Hac S,
Dobrowolski S, Dymecki D and Wajda Z: Heparin improves organ
microcirculatory disturbances in caerulein-induced acute
pancreatitis in rats. World J Gastroenterol. 10:2553–2556.
2004.PubMed/NCBI
|
|
9
|
Doolittle RF: The structure and evolution
of vertebrate fibrinogen. Ann N Y Acad Sci. 408:13–27. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu M, Leibowitz JL, Clark DA, et al: Gene
transcription of fgl2 in endothelial cells is controlled by Ets-1
and Oct-1 and requires the presence of both Sp1 and Sp3. Eur J
Biochem. 270:2274–2286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levy GA, Liu M, Ding J, et al: Molecular
and functional analysis of the human prothrombinase gene (HFGL2)
and its role in viral hepatitis. Am J Pathol. 156:1217–1225. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan CW, Chan MW, Liu M, et al: Kinetic
analysis of a unique direct prothrombinase, fgl2, and
identification of a serine residue critical for the prothrombinase
activity. J Immunol. 168:5170–5177. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marsden PA, Ning Q, Fung LS, et al: The
Fgl2/fibroleukin prothrombinase contributes to immunologically
mediated thrombosis in experimental and human viral hepatitis. J
Clin Invest. 112:58–66. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mendicino M, Liu M, Ghanekar A, et al:
Targeted deletion of Fgl-2/fibroleukin in the donor modulates
immunologic response and acute vascular rejection in cardiac
xenografts. Circulation. 112:248–256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghanekar A, Mendicino M, Liu H, et al:
Endothelial induction of fgl2 contributes to thrombosis during
acute vascular xenograft rejection. J Immunol. 172:5693–5701. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knackstedt MK, Zenclussen AC, Hertwig K,
et al: Th1 cytokines and the prothrombinase fgl2 in
stress-triggered and inflammatory abortion. Am J Reprod Immunol.
49:210–220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clark DA, Foerster K, Fung L, et al: The
fgl2 prothrombinase/fibroleukin gene is required for
lipopolysaccharide-triggered abortions and for normal mouse
reproduction. Mol Hum Reprod. 10:99–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bradley EL 3rd: A clinically based
classification system for acute pancreatitis. Summary of the
International Symposium on Acute Pancreatitis, Atlanta, Ga,
September 11 through 13, 1992. Arch Surg. 128:586–590. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang XP, Zhang J, Ma ML, et al:
Pathological changes at early stage of multiple organ injury in a
rat model of severe acute pancreatitis. Hepatobiliary Pancreat Dis
Int. 9:83–87. 2010.PubMed/NCBI
|
|
20
|
Levitt S: Proprioceptive neuromuscular
facilitation techniques in cerebral palsy. Physiotherapy. 52:46–51.
1966.PubMed/NCBI
|
|
21
|
Schwartz BS, Levy GA, Fair DS and
Edgington TS: Murine lymphoid procoagulant activity induced by
bacterial lipopolysaccharide and immune complexes is a monocyte
prothrombinase. J Exp Med. 155:1464–1479. 1982. View Article : Google Scholar
|
|
22
|
Su K, Chen F, Yan WM, et al:
Fibrinogen-like protein 2/fibroleukin prothrombinase contributes to
tumor hypercoagulability via IL-2 and IFN-gamma. World J
Gastroenterol. 14:5980–5989. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu M, Mendicino M, Ning Q, et al:
Cytokine-induced hepatic apoptosis is dependent on
FGL2/fibroleukin: the role of Sp1/Sp3 and STAT1/PU.1 composite cis
elements. J Immunol. 176:7028–7038. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clark DA, Ding JW, Chaouat G, Coulam CB,
August C and Levy GA: The emerging role of immunoregulation of
fibrinogen-related procoagulant Fgl2 in the success or spontaneous
abortion of early pregnancy in mice and humans. Am J Reprod
Immunol. 42:37–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark DA, Chaouat G, Arck PC, Mittruecker
HW and Levy GA: Cytokine-dependent abortion in CBA × DBA/2 mice is
mediated by the procoagulant fgl2 prothrombinase [correction of
prothombinase]. J Immunol. 160:545–549. 1998.
|
|
26
|
Clark DA, Ding JW, Yu G, Levy GA and
Gorczynski RM: Fgl2 prothrombinase expression in mouse trophoblast
and decidua triggers abortion but may be countered by OX-2. Mol Hum
Reprod. 7:185–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ning Q, Sun Y, Han M, et al: Role of
fibrinogen-like protein 2 prothrombinase/fibroleukin in
experimental and human allograft rejection. J Immunol.
174:7403–7411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Fung LS, Chung S, et al: Monoclonal
antiprothrombinase (3D4.3) prevents mortality from murine hepatitis
virus (MHV-3) infection. J Exp Med. 176:689–697. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao S, Wang M, Ye H, et al: Dual
interference with novel genes mfgl2 and mTNFR1 ameliorates murine
hepatitis virus type 3-induced fulminant hepatitis in BALB/cJ mice.
Hum Gene Ther. 21:969–977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu C, Sun Y, Luo X, Yan W, Xi D and Ning
Q: Novel mfgl2 antisense plasmid inhibits murine fgl2 expression
and ameliorates murine hepatitis virus type 3-induced fulminant
hepatitis in BALB/cJ mice. Hum Gene Ther. 17:589–600. 2006.
View Article : Google Scholar : PubMed/NCBI
|