Introduction
Since its discovery, apoptosis has been shown to be
important in the pathogenesis of many diseases (1,2).
Myocardial apoptosis is the main means of myocardial cell death
during myocardial ischemia, anoxia and ischemia-reperfusion
(3), and is an important
cytological factor leading to a number of heart diseases. The
reduction of non-physiological myocardial apoptosis is of great
significance in the protection of cardiac structure and
function.
Alpinetin is a natural flavonoid predominantly found
in the ginger family, such as turmeric, cardamom and radix
curcumae. In recent years, with the increased study of flavonoids,
it has been demonstrated that flavonoids exhibit a number of
functions, including exerting antibacterial, antioxidative,
anticancer, antithrombotic, antihypertensive, antidiabetic,
antiemetic and analgesic effects (4–6), in
addition to restraining the growth of tumor cells (7). However, to date, there remains little
insight into the function of alpinetin in the growth of normal
human myocardial cells. Furthermore, the detailed mechanism of
action of alpinetin remains unclear.
Opioid receptors are G-protein-coupled receptors and
are extensively distributed throughout the human body. A number of
studies have shown that δ receptor activation may promote the
proliferation of rat myocardial cells (8,9) and
imitate ischemia preconditioning to protect the heart and brain
(10,11). As studies of δ receptor functions
have continued, it has been shown that the δ receptor is critical
in the regulation of the onset and development of cardiac diseases.
Aitchison et al(12)
observed in an in vitro rat heart model that preconditioning
with (D-Ala2, D-Leu5)enkephalin (DADLE), a δ
receptor agonist, was able to significantly reduce the area of
myocardial infarction. Furthermore, the δ receptor blocker
naltrindole has been shown to reverse the cardioprotective effects
of ischemic preconditioning (IPC) by abolishing the reduction in
the myocardial infarction area (13). The results of these studies have
indicated that the δ receptor is important in cell growth and
proliferation.
Protein kinase C (PKC) is a member of the
serine/threonine kinase family, which is widely distributed in the
body. Under quiescent conditions, intracellular PKC exists in the
cytoplasm in a passivation form. However, when the cell is
stimulated, PKC is induced to translocate from the cytoplasm to the
cytomembrane to be activated. PKC exhibits extensive biological
activity. It has been demonstrated that PKC participates in
protection against myocardial ischemia, and that cell proliferation
and apoptosis and the regulation of myocardial contraction may
induce myocardial hypertrophy, myocardial fibrosis and cardiac
failure. The PKC signaling pathway is an important pathway for the
proliferation, differentiation and survival of numerous types of
cells (14). In recent years, the
opioid receptor has been revealed to exhibit the common sequence
for PKC phosphorylation. It was observed in an isolated heart model
that the protective effect of morphine in reducing the myocardial
infarction area was blocked by chelerythrine, a PKC blocker
(15). This indicated that PKC
induced the protective effects of morphine preconditioning.
As shown in previous studies, the δ receptor,
similar to other G-protein-coupled receptors, is able to activate
extracellular signal-regulated kinase (ERK), a member of the
mitogen-activated protein kinase (MAPK) family (16–20).
ERK may participate in the proliferation process of hepatoma cells
(21); therefore, it has been
inferred that the δ receptor may cause the proliferation of
myocardial cells via the ERK signal transduction pathway. A
previous study has demonstrated that the activated δ receptor
protected PC12 cells via the MEK (MAP kinase kinase)-ERK pathway
(22). Furthermore, it has been
shown that the activation of the δ receptor in SH-SY5Y cells
resulted in the activation of Ca2+/calmodulin-dependent
protein kinase II (CaMKII) and PKC, which, in turn, activated ERK
(23). This indicated that the ERK
pathway was also crucial to the proliferation of myocardial
cells.
In the current study, the myocardial cells of newly
born rats were cultured in vitro and myocardial apoptosis
was induced by serum deprivation. This was used as a model to
investigate the impacts of alpinetin and the δ receptor on
myocardial apoptosis and its molecular mechanism.
Materials and methods
Animals and experimental reagents
Healthy male Sprague-Dawley (SD) rats, born within
the previous seven days, were provided by the Laboratory Animal
Center of Jilin University (Changchun, China). All surgical
procedures were performed in accordance with the Guide for the Care
and Use of Laboratory Animals (1993) and followed the ethical
standards. Alpinetin, naltrindole, GF109203X, U0126 and Dulbecco’s
modified Eagle’s medium (DMEM) were obtained from Sigma (St. Louis,
MO, USA) and fetal calf serum was purchased from Gibco-BRL (Grand
Island, NY, USA). The Annexin V-fluorescein isothiocyanate (FITC)
kit used in the study was obtained from Bio-Rad (Hercules, CA,
USA), while δ, κ and μ opioid receptor antibodies and anti-actin,
PKC, ERK, Bcl-2 and Bcl-2-associated X protein (Bax) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Cytochrome c (Cyt c), caspase-3 and caspase-9
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The present study was approved by the Ethics
Committee of The Basic Medical College of Jilin University.
Separation and culture of rat myocardial
cells
The hearts of the healthy male SD rats (born within
the previous seven days) were extracted via a thoracotomy, washed
three times with phosphate-buffered saline (PBS) solution and cut
into tissue fragments measuring 1 mm3. Following this,
trypsin, at a concentration of 0.08%, was added to digest the
cells. The digested cells were subsequently suspended in DMEM
culture medium containing 15% fetal calf serum and 1%
double-antibody, prior to being suspended in culture medium with
uniform distribution. Following this, the cells were placed into a
CO2 incubator containing 5% CO2 and 95%
oxygen for culture. Rat myocardial cells were cultured for 48 h and
the medium of each group, with the exception of the control group,
was replaced with serum-free DMEM and cultured further. The cells
were collected at different time points (0, 24, 48, and 72 h) for
analysis. During serum deprivation, the intervention groups were
treated with different concentrations of Alpinetin (0, 40, 80 and
120 mg/ml) for 48 h.
Assessment of myocardial cell
viability
The myocardial cells of the various groups were
cultured in vitro and treatment factors were administered.
The cell density was then adjusted to 1×105 cells/ml,
prior to inoculation into 96-well culture plates, with eight
complex wells for each group. A total of 20 μl of 5 mg/ml MTT
solution was added to each well 4 h prior to testing and then the
cells were cultured for a further 4 h in the CO2
incubator. Following this, the culture liquid was removed and 0.15
ml dimethylsulfoxide (DMSO) was added to each well, prior to 15 min
oscillation being performed. The optical density (OD) value of each
well was subsequently measured at a wavelength of 570 nm and the
measured light absorption value was converted into the number of
cells, in order to determine the cell viability. Cell viability was
calculated using the following formula: Cell viability = light
absorption value of the experimental group/light absorption value
of the control group × 100.
Analysis of apoptosis using the Annexin
V-FITC/propidium iodide (PI) double-labeling method
Trypsin (0.25%) digestion was used to collect the
cells of all the experimental groups and the cell density was
adjusted to 1×106 cells/ml. A total of 10 μl Annexin
V-FITC and 5 ml PI were added, respectively, for 30 min staining at
4°C, prior to analysis being performed using flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA).
Analysis of mitochondrial membrane
potential
The myocardial cells of all the experimental groups
were collected and the mitochondria were extracted using the method
of Tang et al(24). The
mitochondrial density in the cell count under a microscope plate
was adjusted for the test. Following this, 10 μg/ml JC-1 solution
(dissolved in DMSO) was added, fully mixed and incubated in a 5%
CO2 dark incubator for 30 min at 37°C. The cells were
subsequently analyzed using a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) with an emission wavelength of 488 nm.
Each sample consisted of 1×104 cells. JC-1 monomers and
aggregates were respectively visualized using FL1 and FL2
detectors. FL1-H and FL2-H represented the fluorescence intensity
of red and green. The quantitative analysis was conducted using
CellQuest™ analysis software (BD Biosciences).
Cell cycle analysis
The myocardial cells in each of the groups were
digested with 0.25% trypsin and fixed overnight with 95% cold
ethanol at 4°C. Following this, the cells were washed twice with
PBS and the cell density was adjusted to 1×106 cells/ml.
The final volume was 100 μl. A total of 500 μl DNAStain synthetic
dye liquor (with final concentrations of 50 mg/l RNase, 100 mg/l PI
and 1 ml/l Triton X-100) was subsequently added and stored for 30
min at room temperature in a dark place. Analysis was conducted
using flow cytometry.
Extraction of total RNA and the
reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA of the myocardial cells was extracted in
accordance with the instructions of the RNAiso™ Plus kit (Takara
Bio, Inc., Shiga, Japan). Subsequent to calculating the RNA
concentration, an RT-PCR kit (Takara Bio, Inc.) was used to perform
the RT-PCR, in accordance with the manufacturer’s instructions.
Primers for the δ, μ and κ opioid receptors (DOR, MOR and KOR,
respectively) and β-actin were synthesized by Invitrogen Life
Technologies (Carlsbad, CA, USA) and were as follows: DOR, forward
primer 5′-GTTCGGAGAGCTGCTGTGC-3′ and reverse primer
5′-ATTGATGTCCACCAGCGTCC-3′; MOR, forward primer
5′-GCCCTCTACTCTATCGTGTGT-3′ and reverse primer
5′-GGAAACAGTCTGGAAAGTGGT-3′; KOR, forward primer
5′-CGTCTGCTACACCCTGATGATC-3′ and reverse primer
5′-CTCTCGGGAGCCAGAAAGG-3′; β-actin, forward primer
5′-CTGGGACGACATGGAGAAAA-3′ and reverse primer
5′-AAGGAAGGCTGGAAGAGTGC-3′. The reaction conditions for the 50-μl
PCR reaction system were as follows: 94°C for 2 min, 94°C
degeneration for 30 sec, 60°C annealing for 30 sec and 72°C
extension for 30 sec (total, 30 cycles). The PCR products were
analyzed using 1.0% agarose gel electrophoresis (AGE) and were
scanned and analyzed with a gel imaging system (G:BOX Chemi XR5;
Syngene, Cambridge, UK).
Western blot analysis
The myocardial cells of all the groups were
collected and washed twice with PBS, prior to 2 ml lysis solution
(Sigma) being added for cell lysis. Once the protein concentration
had been determined using the bicinchoninic acid (BCA) method, the
protein was stained with bromophenol blue. Identical amounts of
protein were added to each well, prior to the proteins being
separated with 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The proteins were subsequently
transferred to a polyvinylidene difluoride (PVDF) membrane using
the semi-dry method and sealed with 5% skimmed mild powder
overnight. On the following day, the membrane was washed with
Tris-buffered saline and Tween 20 (TBST) and the primary antibody
[PKC antibody (1:300), ERK antibody (1:500), Bcl-2 and Bax antibody
(1:700), Cyt C antibody (1:200), δ, μ, κ opioid receptor antibody
(1:400)] was added. This was incubated for 2 h, prior to bleaching
with TBST. The secondary antibody (goat anti-rabbit and goat
anti-mouse) was then added and incubated for 2 h. A
chemiluminescent reagent was added prior to the capturing of images
by exposure of an X-ray film and strip scanning. A gray scale
analysis was conducted and β-actin was used for
standardization.
Statistical analysis
SPSS 16.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis. The values are
shown as the mean ± standard deviation. The statistical analysis
was performed using the Student’s t-test, and P<0.05 was
considered to indicate a statistically significant difference.
Results
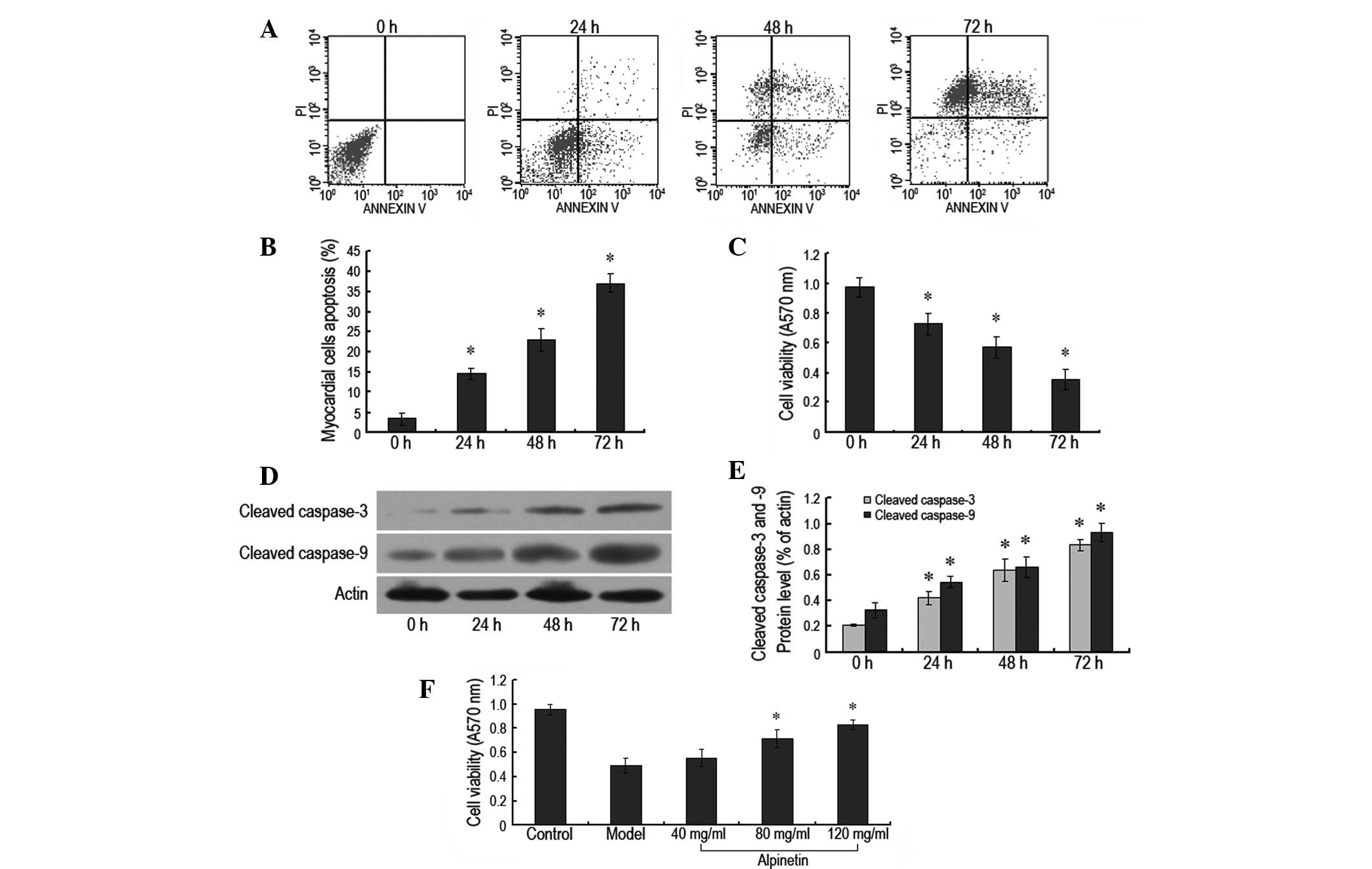
Rat myocardial cell apoptosis induced by
serum deprivation is attenuated by alpinetin in a
concentration-dependent manner
In this experiment, the myocardial cell culture
medium was serum-deprived and flow cytometry and the MTT method
were used to analyze apoptosis and cell viability, respectively.
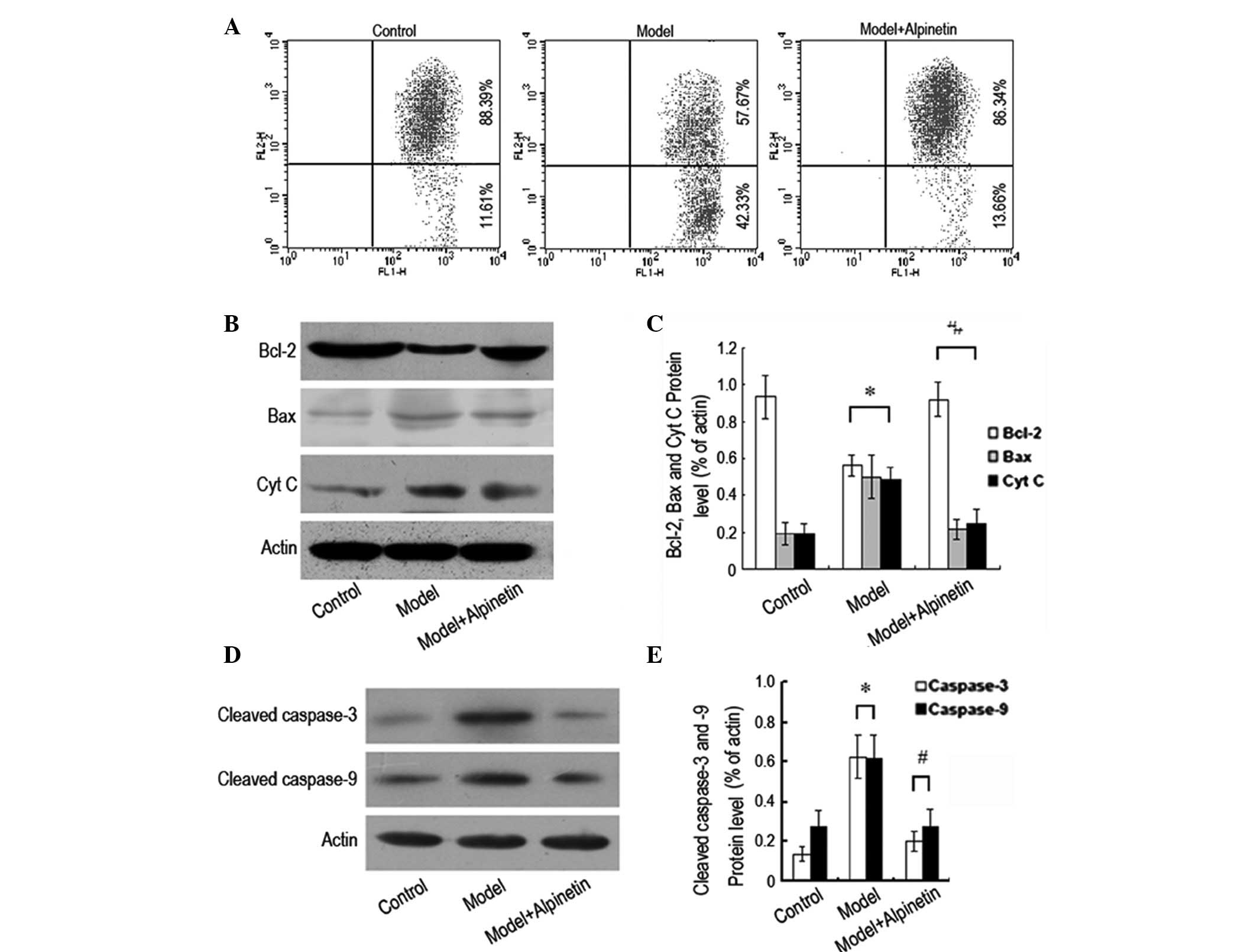
The cell apoptosis rate of the myocardial cells was demonstrated to
be 14.54, 23.18 and 37.11% following 24, 48 and 72 h of serum
deprivation, respectively, which was significantly higher than that
of the control group (P<0.05; Fig.
1A and B). The A570 nm values of the serum-deprived cells were
0.73±0.17, 0.57±0.17 and 0.35±0.16, respectively, which were also
significantly lower than those of the control group (P<0.05;
Fig. 1C). In addition, it was
observed that the levels of cleaved caspase-3 and cleaved caspase-9
proteins increased as the duration of serum deprivation progressed,
which was consistent with the results mentioned previously
(Fig. 1D and E). This indicated
that serum deprivation was able to simulate in vivo ischemia
and anoxia to result in rat myocardial apoptosis. Alpinetin, at
various concentrations, was administered to the rat myocardial
cells at the same time as the serum deprivation. The A570 nm values
of the myocardial cells treated with alpinetin were observed to be
increased compared with those for the cells undergoing serum
deprivation only. As the concentration of alpinetin increased from
40 to 120 mg/ml, the A570 nm values of the rat myocardial cells
increased in a concentration-dependent manner. This indicated that
alpinetin conferred protection against the rat myocardial apoptosis
induced by serum deprivation in a concentration-dependent manner,
with the most notable effects apparent when the concentration was
120 mg/ml (Fig. 1F).
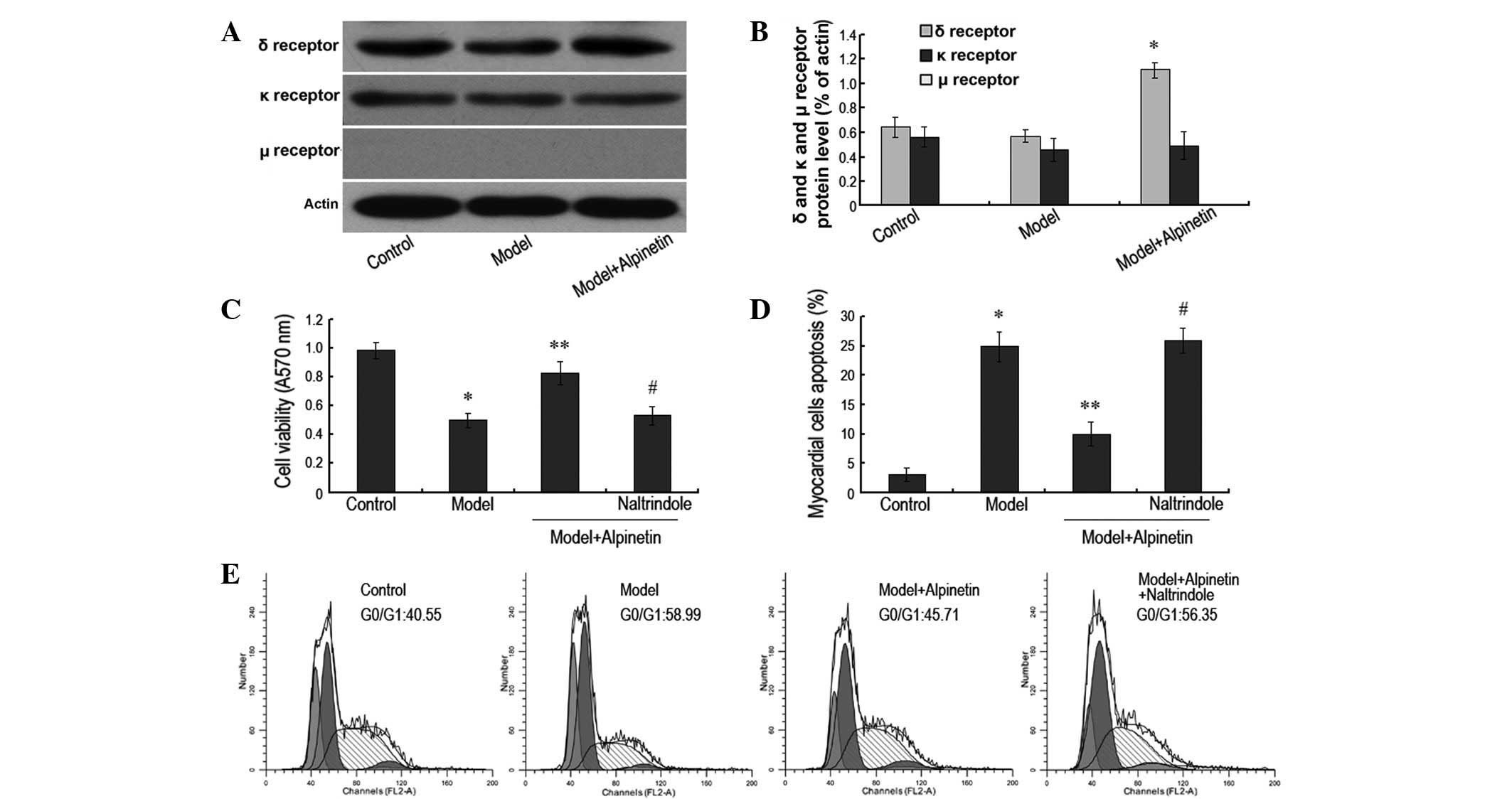
Alpinetin protects myocardial cells via
activation of the δ receptor
As indicated by previous studies, the activation of
the δ receptor may promote the proliferation of myocardial cells
and protect them to a certain extent (8,9). In
this experiment, following the administration of a therapeutic
concentration of alpinetin, western blotting revealed the
expression level of the δ receptor to have increased significantly
in the rat myocardial cells. However, there were no apparent
changes in the expression levels of the κ and μ receptors (Fig. 2A and B). When the δ receptor
antagonist naltrindole was also administered, it was observed that
the myocardial apoptosis rate increased significantly (P<0.05)
and the A570 value of the myocardial cell decreased markedly
(P<0.05), in comparison with those of the cells treated with
alpinetin only (Fig. 2C and D). In
addition, cell cycle analysis by flow cytometry demonstrated that
the percentage of the myocardial cells in the G0/G1 phase increased
significantly following serum deprivation (P<0.05), while the
percentage decreased significantly following the administration of
120 mg/ml alpinetin (P<0.05). However, when the δ receptor
antagonist was also administered, the percentage of the myocardial
cells in G0/G1 phase increased again (P<0.05; Fig. 2E). These results indicate that the
protective effect of alpinetin on the myocardial cells was
dependent on the activation of the δ receptor, and that this was
closely associated with the inhibition of apoptosis and the
promotion of cell proliferation and the cell cycle.
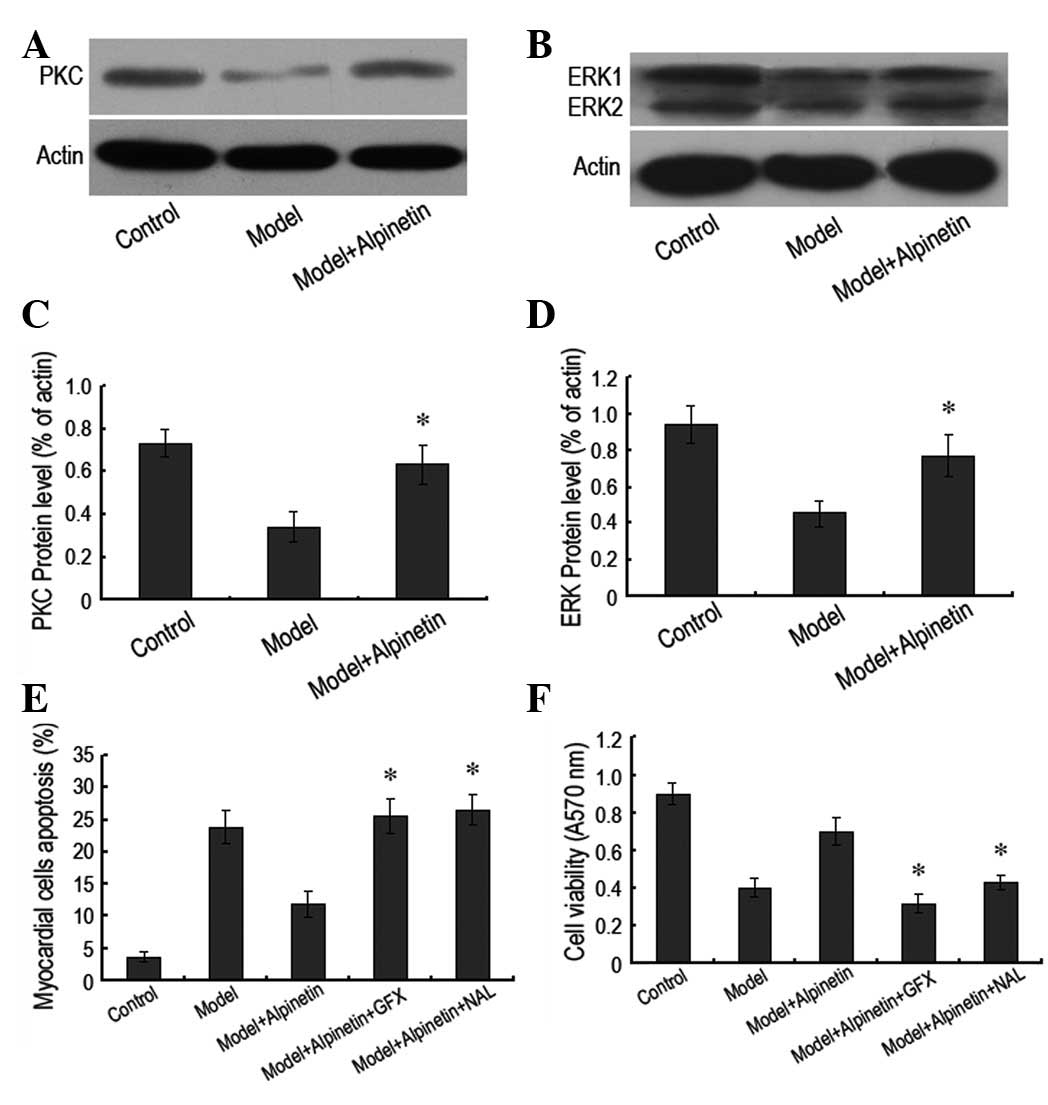
Protection of rat myocardial cells by
alpinetin is mediated by the PKC/ERK signaling pathway
In order to verify the molecular mechanism by which
alpinetin protects rat myocardial cells, the signaling pathways of
PKC and ERK were studied. Notably, it was observed that following
alpinetin administration, the intracellular PKC and ERK protein
expression levels increased significantly compared with those in
the serum deprivation model (Fig.
3A–D). However, when the PKC inhibitor GF109203X or the ERK
inhibitor U0126 was administered to block the corresponding
signaling pathways, it was observed that level of myocardial cell
apoptosis increased, irrespective of alpinetin administration.
Furthermore, the A570 value of the myocardial cells decreased
(Fig. 3E and F). These results
indicated that the protective effects of alpinetin on the rat
myocardial cells were closely associated with the PKC/ERK signaling
pathway.
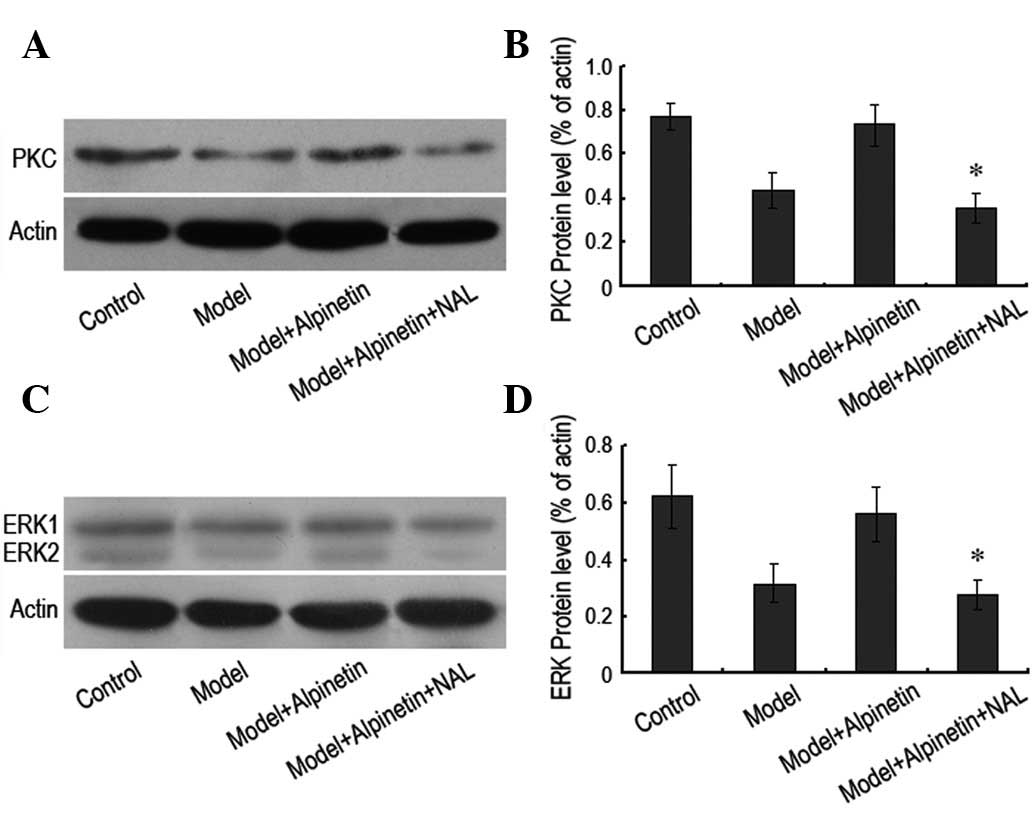
Downregulating the δ receptor may inhibit
the PKC/ERK signaling pathway
In order to further demonstrate the interrelation
between the δ receptor and the PKC/ERK pathway in the protection of
rat myocardial cells, the δ receptor antagonist naltrindole (10 μM)
was administered and the expression levels of PKC and ERK protein
were assessed. It was observed that the inhibition of the δ
receptor resulted in a significant reduction in the intracellular
expression levels of PKC and ERK proteins (Fig. 4). This indicated that the δ
receptor and the PKC/ERK pathway were important for the protection
of rat myocardial cells by alpinetin. Of note was the fact that the
function of the δ receptor was upstream of the PKC/ERK pathway.
Alpinetin inhibits rat myocardial
apoptosis via the mitochondrial pathway
In order to further study the molecular mechanism of
the rat myocardial apoptosis caused by serum deprivation, flow
cytometry and western blotting were used to analyze the changes in
the mitochondrial membrane potential and the changes in the
expression levels of Bcl-2, Bax and Cyt c, respectively.
Following 48 h of serum deprivation, the mitochondrial membrane
potential of the rat myocardial cells was observed to have
decreased, as was the expression level of Bcl-2 protein. However,
the expression levels of Bax and Cyt c proteins were
observed to have increased markedly. Following the administration
of alpinetin, the mitochondrial membrane potential of the rat
myocardial cells was restored to its original level, the expression
level of Bcl-2 protein increased and the expression levels of Bax
and Cyt c proteins decreased (Fig. 5A–C). In addition, western blotting
was used for the analysis of the changes in the expression levels
of cleaved caspase-3 and cleaved caspase-9 protein. Following 48 h
of serum deprivation, it was observed that the intracellular
expression levels of cleaved caspase-3 and cleaved caspase-9
proteins increased significantly. With the administration of
alpinetin, the expression levels of the two proteins decreased
(Fig. 5D and E). These results
indicate that the inhibition of the serum deprivation-induced rat
myocardial apoptosis by alpinetin was associated with the
mitochondrial pathway.
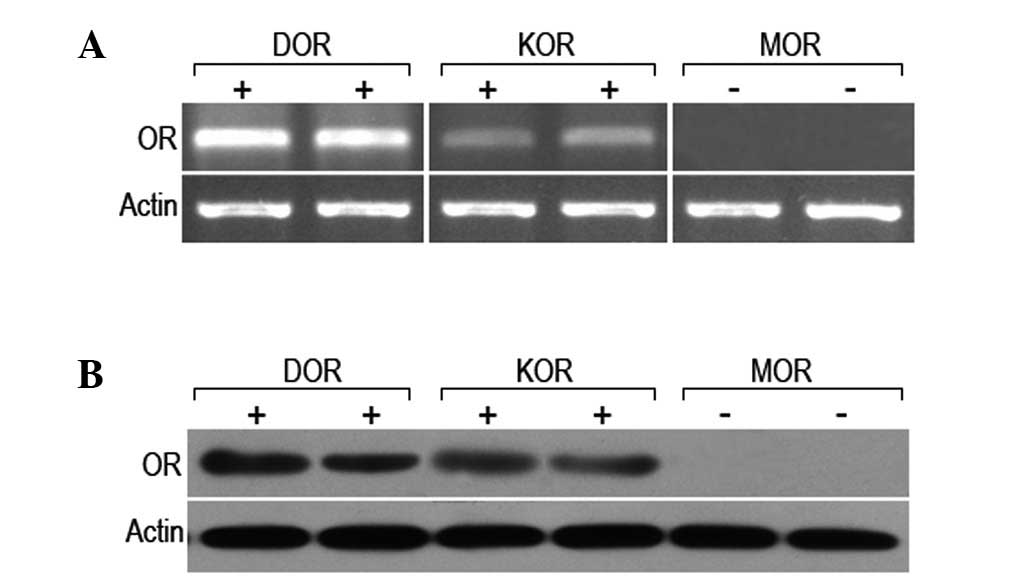
Expression of the δ, κ and μ receptors in
rat myocardial cells
In order to determine the expression levels of the
δ, κ and μ receptors in rat myocardial cells, the mRNA and protein
levels of the three receptors were analyzed. It was observed that
the mRNA and protein of the δ and κ receptors was expressed in the
rat myocardial cells; however, no mRNA or protein expression of the
μ receptor was detected (Fig. 6).
This, in combination with the results from previous studies,
indicate that the δ receptor is important in the protective effect
of alpinetin in rat myocardial cells.
Discussion
It has been shown in previous studies that
flavonoids are able to protect cells against fatal injury in
ischemia-reperfusion, which promotes the proliferation of various
cells. This may be used in the treatment of a number of diseases
(25–27). As a flavonoid with numerous
biological activities, alpinetin has been the subject of much focus
globally in recent years, due to its wide distribution and low
toxicity.
Apoptosis is important in the regulation of normal
organismal development and in the maintenance of the stability of
the internal environment. With regard to the cardiovascular system,
apoptosis participates in the formation of the heart and blood
vessel structure in the early stage of morphogenesis and, at a
later stage, regulates the growth and development of the
cardiovascular system. Thus, apoptosis is an indispensible process
in the development of the cardiovascular system (28). Cell apoptosis and proliferation are
coordinated and complement each other to maintain the stability of
the internal environment and the normal growth of the body. When
the internal environment changes, such a balance may be lost,
leading to the onset of disease. The development of studies into
cardiovascular diseases has revealed that myocardial apoptosis
prevails in the physiological and pathological changes of the
cardiovascular system, and is a critical cytological factor in the
development of numerous cardiovascular diseases. At present,
myocardial apoptosis is considered to be an important factor
causing cardiovascular diseases and one of the major reasons
leading to a decline in cardiac function (29). Therefore, exploring the mechanism
and development of myocardial apoptosis and reducing its occurrence
is of great significance in the safeguarding of cardiac structure
and function.
It has been demonstrated that apoptosis may be
stimulated by a number of techniques, with hydrogen peroxide and
ischemia/reaeration used as the common methods to induce myocardial
apoptosis. However, the use of strong chemical provocative methods
to induce apoptosis may ultimately result in irreversible injury to
the cells. In the current study, serum deprivation was used to
induce cell apoptosis, which was milder than that induced by the
previously mentioned methods and closer to the cell injury caused
by ischemia and anoxia in vivo. This involved removing the
serum from the cell culture medium to maintain the state of serum
deprivation, thereby forming the model of the apoptosis and, in
addition, alleviating the impact of the varying nature of the serum
on the experimental results (30).
In this experiment, following 48 h of serum deprivation, the cell
proliferation of the cultured myocardial cells was inhibited and
apoptosis occurred, which indicated that the growth of the
myocardial cells was inhibited.
δ receptors are widely distributed around the body,
with numerous receptors existing on the myocardial membrane and
blood vessel walls, in addition to the central nervous system
(31). In the opioid receptor
superfamily, the δ receptor is closely associated with the
viability and proliferation of cells (32,33).
As demonstrated in previous studies, the activation of the δ
receptor promotes the proliferation of rat ventricular muscle cells
(34,35). In the current study, alpinetin was
administered to rat myocardial cells, leading to an increase in the
expression level of the δ receptor, a significant reduction in the
myocardial apoptosis rate and an increase in cell proliferation.
This indicated that the δ receptor was important in the protection
of rat myocardial cells by alpinetin. However, when a specific
antagonist to downregulate the δ receptor expression was
administered, it was observed that the protective effect of
alpinetin in rat myocardial cells disappeared. This indicated that
the protective effect of alpinetin was closely correlated with the
function and state of the δ receptor.
As demonstrated by previous studies, the δ receptor
functions via the G-protein and KATP channel signal transduction
pathways (36,37) and is closely associated with PKC
(38). PKC is a member of the
serine/threonine kinase family and exhibits extensive biological
activities, including regulating the proliferation and
differentiation of numerous cell types (39–41).
It has been suggested that the activation of PKC may induce various
cells to proliferate (42), and
PKC has been shown to participate in the proliferation and
differentiation of myocardial cells. In the present study, it was
revealed that alpinetin increased intracellular PKC expression
following the serum deprivation of rat myocardial cells. However,
when PKC was inhibited, the protective effects of alpinetin on the
myocardial cells disappeared. Therefore, it was inferred that the
PKC pathway was involved in the protection of the the rat
myocardial cells. It has been demonstrated that the participation
of PKC in cell proliferation differs according to the various
isoforms of the enzyme (43). In a
number of experimental models, the different subtypes of PKC have
been shown to possess a variety of functions. The complicated
nature of the intracellular apoptosis signaling pathways makes it
crucial to understand the relationship between the different PKC
subtypes.
It was demonstrated in a previous study that the δ
receptor acted via the ERK signaling pathway to promote the
survival and proliferation of cells (35). In this study, we proposed that
alpinetin exerted its protective effects on the rat myocardial
cells by exciting the δ receptor to activate PKC, leading to the
further activation of ERK. As shown in our results, while the
protective effects of alpinetin on the myocardial cells were
reversed by a PKC-specific inhibitor, they were also reversed by an
ERK-specific inhibitor, U0126. This result supported our previous
assumption and was consistent with previous studies (22,23).
However, further investigations into the specific mechanism behind
the PKC-mediated regulation of ERK are required.
Apoptosis occurs via two pathways, the mitochondrial
and death receptor pathways. As demonstrated in a previous study, a
δ receptor agonist protected against apoptosis via the
mitochondrial pathway (44). In
the present study, whether the mitochondrial pathway was relevant
to the protective effects of alpinetin on myocardial cells was
investigated. It was observed that following alpinetin
administration, the mitochondrial membrane potential was restored,
the level of Bcl-2 protein in the cytoplasm was increased and the
expression levels of Bax and Cyt c were decreased. The
intracellular expression levels of cleaved caspase-3 and cleaved
caspase-9 proteins were also shown to have decreased markedly. It
was concluded that the protective effects of alpinetin on rat
myocardial cells were elicited via the mitochondrial pathway.
In conclusion, alpinetin protects against rat
myocardial cell apoptosis induced by serum deprivation.
Furthermore, alpinetin activates the δ receptor to induce the
endogenous protection of myocardial cells via the PKC/ERK signaling
pathway.
Acknowledgements
The authors would like to thank Professor Luo from
the First Hospital of Jilin University and Professor Li from the
College of Medicine of Jilin University for their guidance in this
study.
References
|
1
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gottlieb RA, Burleson KO, Kloner RA,
Babior BM and Engler RL: Reperfusion injury induces apoptosis in
rabbit cardiomyocytes. J Clin Invest. 94:1621–1628. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang ZT, Lau CW, Chan FL, Yao X, Chen ZY,
He ZD and Huang Y: Vasorelaxant effects of cardamonin and alpinetin
from Alpinia henryi K. Schum. J Cardiovasc Pharmacol.
37:596–606. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He W, Li Y, Xue C, Hu Z, Chen X and Sheng
F: Effect of Chinese medicine alpinetin on the structure of human
serum albumin. Bioorg Med Chem. 13:1837–1845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He W, Li Y, Tang J, Luan F, Jin J and Hu
Z: Comparison of the characterization on binding of alpinetin and
cardamonin to lysozyme by spectroscopic methods. Int J Biol
Macromol. 39:165–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang J, Li N, Dai H and Wang K: Chemical
constituents from seeds of Alpinia katsumadai, inhibition on
NF-kappaB activation and anti-tumor effect. Zhongguo Zhong Yao Za
Zhi. 35:1710–1714. 2010.(In Chinese).
|
|
8
|
Feng Y, He X, Yang Y, Chao D, Lazarus LH
and Xia Y: Current research on opioid receptor function. Curr Drug
Targets. 13:230–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang D, Wang H, Wu G, Yang Y, Yang J, Liu
C and Wong TM: Protein kinase C mediates the effects of
delta-opioid receptor stimulation on survival and apoptosis in
neonatal cardiomyocytes cultured in serum-deprived condition.
Pharmazie. 64:466–471. 2009.
|
|
10
|
Maslov LN, Barzakh EI, Krylatov AV,
Chernysheva GA, Krieg T, Solenkova NV, Lishmanov AY, Cybulnikov SY
and Zhang Y: Opioid peptide deltorphin II simulates the
cardioprotective effect of ischemic preconditioning: role of
δ2-opioid receptors, protein kinase C, and K(ATP)
channels. Bull Exp Biol Med. 149:591–593. 2010.PubMed/NCBI
|
|
11
|
Wang S, Duan Y, Su D, Li W, Tan J, Yang D,
Wang W, Zhao Z and Wang X: Delta opioid peptide (D-Ala2, D-Leu5)
enkephalin (DADLE) triggers postconditioning against transient
forebrain ischemia. Eur J Pharmacol. 658:140–144. 2011. View Article : Google Scholar
|
|
12
|
Aitchison KA, Baxter GF, Awan MM, Smith
RM, Yellon DM and Opie LH: Opposing effects on infarction of delta
and kappa opioid receptor activation in the isolated rat heart:
implications for ischemic preconditioning. Basic Res Cardiol.
95:1–11. 2000. View Article : Google Scholar
|
|
13
|
Schultz JJ, Hsu AK and Gross GJ: Ischemic
preconditioning and morphine-induced cardioprotection involve the
delta (delta)-opioid receptor in the intact rat heart. J Mol Cell
Cardiol. 29:2187–2195. 1997. View Article : Google Scholar
|
|
14
|
Genade S, Moolman JA and Lochner A: Opioid
receptor stimulation acts as mediator of protection in ischaemic
preconditioning. Cardiovasc J S Afr. 12:8–16. 2001.PubMed/NCBI
|
|
15
|
Miki T, Cohen MV and Downey JM: Opioid
receptor contributes to ischemic preconditioning through protein
kinase C activation in rabbits. Mol Cell Biochem. 186:3–12. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopez-Ilasaca M, Crespo P, Pellici PG,
Gutkind JS and Wetzker R: Linkage of G protein-coupled receptors to
the MAPK signaling pathway through PI 3-kinase gamma. Science.
275:394–397. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Polakiewicz RD, Schieferl SM, Gingras AC,
Sonenberg N and Comb MJ: mu-Opioid receptor activates signaling
pathways implicated in cell survival and translational control. J
Biol Chem. 273:23534–23541. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Xin SM, Wu GX, Zhang WB, Ma L and
Pei G: Endogenous delta-opioid and ORL1 receptors couple to
phosphorylation and activation of p38 MAPK in NG108-15 cells and
this is regulated by protein kinase A and protein kinase C. J
Neurochem. 73:1502–1509. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilson MA, Burt AR, Milligan G and
Anderson NG: Mitogenic signalling by delta opioid receptors
expressed in rat-1 fibroblasts involves activation of the
p70s6k/p85s6k S6 kinase. Biochem J. 325:217–222. 1997.PubMed/NCBI
|
|
20
|
Fukuda K, Kato S, Morikawa H, Shoda T and
Mori K: Functional coupling of the delta-, mu-, and kappa-opioid
receptors to mitogen-activated protein kinase and arachidonate
release in Chinese hamster ovary cells. J Neurochem. 67:1309–1316.
1996. View Article : Google Scholar
|
|
21
|
Wang S, Huang X, Li Y, Lao H, Zhang Y,
Dong H, Xu W, Li JL and Li M: RN181 suppresses hepatocellular
carcinoma growth by inhibition of the ERK/MAPK pathway. Hepatology.
53:1932–1942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayashi T, Tsao LI and Su TP:
Antiapoptotic and cytotoxic properties of delta opioid peptide
[D-Ala2, D-Leu5]enkephalin in PC12 cells.
Synapse. 43:86–94. 2002.PubMed/NCBI
|
|
23
|
Bilecki W, Zapart G, Ligeza A,
Wawrzczak-Bargiela A, Urbański MJ and Przewłocki R: Regulation of
the extracellular signal-regulated kinases following acute and
chronic opioid treatment. Cell Mol Life Sci. 62:2369–2375. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang B, Zhao L, Liang R, Zhang Y and Wang
L: Magnetic nanoparticles: an improved method for mitochondrial
isolation. Mol Med Rep. 5:1271–1276. 2012.PubMed/NCBI
|
|
25
|
Akhlaghi M and Bandy B: Preconditioning
and acute effects of flavonoids in protecting cardiomyocytes from
oxidative cell death. Oxid Med Cell Longev.
2012:7823212012.PubMed/NCBI
|
|
26
|
Sato Y, Itagaki S, Oikawa S, Ogura J,
Kobayashi M, Hirano T, Sugawara M and Iseki K: Protective effect of
soy isoflavone genistein on ischemia-reperfusion in the rat small
intestine. Biol Pharm Bull. 34:1448–1454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan E, Liu XX, Guo DJ, Kwan YW, Leung GP,
Lee SM and Chan SW: Extract of Scutellaria baicalensis
Georgi root exerts protection against myocardial
ischemia-reperfusion injury in rats. Am J Chin Med. 39:693–704.
2011.
|
|
28
|
Fisher SA, Langille BL and Srivastava D:
Apoptosis during cardiovascular development. Circ Res. 87:856–864.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka M, Ito H, Adachi S, Akimoto H,
Nishikawa T, Kasajima T, Marumo F and Hiroe M: Hypoxia induces
apoptosis with enhanced expression of Fas antigen messenger RNA in
cultured neonatal rat cardiomyocytes. Circ Res. 75:426–433. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goyeneche AA, Harmon JM and Telleria CM:
Cell death induced by serum deprivation in luteal cells involves
the intrinsic pathway of apoptosis. Reproduction. 131:103–111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zimlichman R, Gefel D, Eliahou H, Matas Z,
Rosen B, Gass S, Ela C, Eilam Y, Vogel Z and Barg J: Expression of
opioid receptors during heart ontogeny in normotensive and
hypertensive rats. Circulation. 93:1020–1025. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su TP: Delta opioid peptide
[D-Ala2,D-Leu5]enkephalin promotes cell
survival. J Biomed Sci. 7:195–199. 2000.
|
|
33
|
Kim H, Lee SW, Park JS, Min JH and Kim HK:
Genomic analysis of [d-Ala2, d-Leu5]
enkephalin preconditioning in cortical neuron and glial cell injury
after oxygen deprivation. Brain Res. 1447:91–105. 2012.
|
|
34
|
Xin W, Yang X, Rich TC, Krieg T,
Barrington R, Cohen MV and Downey JM: All preconditioning-related G
protein-coupled receptors can be demonstrated in the rabbit
cardiomyocyte. J Cardiovasc Pharmacol Ther. 17:190–198. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao M, Wang HX, Yang J, Su YH, Su RJ and
Wong TM: delta-Opioid receptor stimulation enhances the growth of
neonatal rat ventricular myocytes via the extracellular
signal-regulated kinase pathway. Clin Exp Pharmacol Physiol.
35:97–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olianas MC, Dedoni S, Olianas A and Onali
P: δ-Opioid receptors stimulate the metabolic sensor AMP-activated
protein kinase through coincident signaling with G(q/11)-coupled
receptors. Mol Pharmacol. 81:154–165. 2012.
|
|
37
|
Pateliya BB, Singh N and Jaggi AS:
Possible role of opioids and KATP channels in neuroprotective
effect of postconditioning in mice. Biol Pharm Bull. 31:1755–1760.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang B, Zhang Y, Liang R, Yuan P, Du J,
Wang H and Wang L: Activation of the δ-opioid receptor inhibits
serum deprivation-induced apoptosis of human liver cells via the
activation of PKC and the mitochondrial pathway. Int J Mol Med.
28:1077–1085. 2011.
|
|
39
|
Saberi B, Shinohara M, Ybanez MD, Hanawa
N, Gaarde WA, Kaplowitz N and Han D: Regulation of
H2O2-induced necrosis by PKC and
AMP-activated kinase signaling in primary cultured hepatocytes. Am
J Physiol Cell Physiol. 295:C50–C63. 2008.
|
|
40
|
Molè D, Gentilin E, Gagliano T, Tagliati
F, Bondanelli M, Pelizzo MR, Rossi M, Filieri C, Pansini G, degli
Uberti EC and Zatelli MC: Protein kinase C: a putative new target
for the control of human medullary thyroid carcinoma cell
proliferation in vitro. Endocrinology. 153:2088–2098.
2012.PubMed/NCBI
|
|
41
|
Wickley PJ, Ding X, Murray PA and Damron
DS: Propofol-induced activation of protein kinase C isoforms in
adult rat ventricular myocytes. Anesthesiology. 104:970–977. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ali AS, Ali S, El-Rayes BF, Philip PA and
Sarkar FH: Exploitation of protein kinase C: a useful target for
cancer therapy. Cancer Treat Rev. 35:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kao HH, Wu CJ, Won SJ, Shin JW, Liu HS and
Su CL: Kinase gene expression and subcellular protein expression
pattern of protein kinase C isoforms in curcumin-treated human
hepatocellular carcinoma Hep 3B cells. Plant Foods Hum Nutr.
66:136–142. 2011. View Article : Google Scholar
|
|
44
|
Tsao LI and Su TP: Hibernation-induction
peptide and cell death:
(D-Ala2,D-Leu5)enkephalin blocks Bax-related
apoptotic processes. Eur J Pharmacol. 428:149–151. 2001. View Article : Google Scholar : PubMed/NCBI
|