Introduction
Gestational diabetes mellitus (GDM) is defined as a
glucose intolerance of varying severity with an onset or first
diagnosis during pregnancy (1).
GDM occurs in 4–10% of pregnancies and is associated with maternal
and fetal complications, as well as long-term consequences,
including metabolic syndrome, type 2 diabetes mellitus (T2DM) and
cardiovascular disease (2,3). Although the pathogenesis of GDM has
not been completely elucidated, the innate immune system has been
reported to be involved (4). In
addition, several types of cytokines have been shown to have the
ability to interfere with insulin signaling, as well as the
development of insulin resistance (IR), in patients with GDM
(5).
Toll-like receptors (TLRs) recognize preserved
patterns and are important in the regulation of innate immune
responses and inflammation (6).
TLRs are expressed in numerous types of cells, including adipose
cells and monocytes. These cells are the predominant cells of the
innate immune system and are pivotal in diabetes (7). Among the TLRs, TLR4 is a particularly
important receptor. TLR4 is the receptor for lipopolysaccharide
(LPS) from Gram-negative bacteria, and affects the innate immune
response, the prevalence of T2DM and the metabolic system (8). TLR4 expression has been identified in
numerous cells and tissues, primarily in monocytes (9). TLR4 is important for the regulation
of the immune response and inflammatory reaction (10). In addition, TLR4 induces the
production of proinflammatory cytokines, leading to an impairment
of tissue-specific effects (11,12).
However, whether TLR4 is expressed in maternal monocytes of
patients with GDM has not been evaluated.
The current study was conducted to investigate
whether TLR4 is expressed in maternal monocytes of patients with
GDM and to elucidate the roles of TLR4 in the pathogenesis of GDM.
Novel insights into the involvement of TLR4 in the pathogenesis of
GDM may provide an opportunity to trace the underlying pathogenesis
of GDM and, if proven, may be conducive to improving the treatment
of the disease.
Materials and methods
Subjects
A total of 62 females (21–39 years old) at ≥37 weeks
of gestation were involved in this study, including 32 females with
GDM and 30 healthy pregnant females. Females with GDM were selected
by a screening and diagnostic program according to the criteria of
the Fourth Workshop Conference of Gestational Diabetes (13). Females with multiple pregnancies,
fetal anomalies, preexisting hypertension or DM, or chronic disease
were excluded. All females with GDM were treated with insulin and
IR was estimated using the homoeostasis model assessment (HOMA)-IR.
The protocol was approved by the local Ethics Committee of College
of life and Technology, Jinan University (Guangzhou, China) and
written informed consent was obtained from all females.
Isolation of monocytes
Maternal peripheral blood was collected using tubes
treated with EDTA and the peripheral blood monocytes were isolated
by density gradient centrifugation using Ficoll-Paque PLUS
(Amersham, Piscataway, NJ, USA). The negative isolation of human
monocytes was performed using a Monocyte Isolation kit II (Miltenyi
Biotech, Bergisch Gladbach, Germany), in accordance with the
manufacturer’s instructions. Following centrifugation at 400 × g
for 30 min, the cells were washed with phosphate-buffered saline at
4°C. The viabilities of monocytes at >90% confluence were
calculated using 0.4% trypan blue. The monocytes were stored at
−80°C until use.
RNA isolation and quantitative polymerase
chain reaction (qPCR)
Total RNA from the monocytes was isolated using a
commercial kit (Omega Bio-Tek, Norcross, GA, USA), in accordance
with the manufacturer’s instructions. The RNA concentration and
purity were determined using 1.0% agarose gel electrophoresis with
an optical density 260/280 absorption ratio of >1.8. cDNA was
synthesized in a 20-μl reaction mixture containing 2 μg total RNA,
using the Omniscript reverse transcription kit (Takara Bio, Inc.,
Otsu, Japan) and oligo(dT) primers, following the manufacturer’s
instructions. TLR4 mRNA expression was measured with the ABI PRISM
7300 Sequence Detection System using the SYBR® Green PCR
Master mix (Applied Biosystems, Foster City, CA, USA). The
following primers were used for analysis: TLR4 mRNA forward,
5′-AGTGTGTGTGTCCGCATGAT-3′ and reverse, 5′-CCACTTGGGGTCTAAGAACG-3′;
18S rRNA forward, 5′-TTCGGAACTGAGGCCATGAT-3′ and reverse,
5′-CGAACCTCCGACTTTCGTTT-3′. qPCR was performed with a 20-μl
reaction mixture, using the SYBR Premix Ex Taq™ II kit
(Takara Bio, Inc.), according to the manufacturer’s instructions.
The PCR profile was obtained as follows: Initial activation step at
95°C for 5 min, followed by 40 cycles of denaturation, annealing
and amplification (95°C for 15 sec, 60°C for 30 sec and 72°C for 15
sec, respectively). With regard to the internal control, the
expression of the house-keeping gene, 18S rRNA, was examined under
the same reaction conditions. The experiment was repeated in
triplicate. Following amplification, melting curve analysis was
conducted for the product formed.
Western blotting
Western blotting was performed using the same
monocytes as qPCR. Total protein was extracted from the monocytes
using a cell lysis buffer and protease inhibitor cocktail.
Following centrifugation at 10,000 × g and 4°C for 15 min, the
protein concentration was assessed using the Bradford protein assay
kit (Bio-Rad Laboratories, Hercules, CA, USA). The protein samples
(20 μg) were loaded onto a 12% SDS-PAGE gel and transferred onto
polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences,
Piscataway, NJ, USA). The membranes were blocked using 5% non-fat
dry milk in a Tris-buffered sodium chloride-Tween-20 (TBST)
solution (20 mmol/l Tris, pH 7.6, 137 mmol/l sodium chloride and
0.1% Tween-20) at room temperature for 1 h. The PVDF membranes were
subsequently incubated with monoclonal antibodies against human
TLR4 (1:1,000; Abcam, Cambridge, MA, USA) overnight at 4°C.
Following this, the membranes were incubated with secondary
antibody goat anti-rabbit horseradish peroxidase conjugate
(1:2,000; Abcam) at room temperature for 2 h. Following three
10-min washes in TBST, the immunoreactive bands were detected using
western blotting chemiluminescence luminol reagents (Santa Cruz
Biotechnology, Inc., Santa Cruz CA, USA). The band intensities were
quantified using scanning densitometry (Bio-Rad Quantity One
software; Bio-Rad).
Serum tumor necrosis factor (TNF)-α level
analysis
The maternal peripheral blood samples were collected
and transferred to centrifuge tubes. The blood samples were
centrifuged at 3,000 × g for 15 min to separate the plasma and
maintained at −80°C until analysis. Serum TNF-α levels were
measured using a commercial TNF-α ELISA kit (BioSource
International, Inc., Camarillo, CA, USA), in accordance with the
manufacturer’s instructions.
Statistical analysis
All experimental data are expressed as the mean ±
standard deviation. Statistical analyses were performed with SPSS
17.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Inter-group differences were compared using the Student’s t-test
and the correlation analyses were conducted using Pearson’s linear
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The characteristics of the studied patients are
listed in Table I. There were no
significant differences in age, body mass index, blood pressure,
gestational age or birth weight between the normal control and GDM
groups (P>0.05). However, the blood glucose levels and HOMA-IR
score were significantly higher in the GDM group than in the normal
control group (P<0.05).
 | Table IDemographic data in the GDM and normal
control groups. |
Table I
Demographic data in the GDM and normal
control groups.
| Characteristics | Normal control
(n=32) | GDM (n=30) | P-value |
|---|
| Age, years | 29.6±3.3 | 30.9±4.7 | NS |
| Gestational age,
days | 272.6±4.5 | 273.9±5.6 | NS |
| Fetal weight, g | 3,230.0±304.7 | 3,373.0±418.4 | NS |
| Systolic blood
pressure, mmHg | 114.5±14.7 | 108.6±8.3 | NS |
| Diastolic blood
pressure, mmHg | 72.9±8.7 | 72.5±6.8 | NS |
| BMI,
kg/m2 | 22.1±1.9 | 23.3±2.8 | NS |
| Fasting glucose,
mmol/l | 4.1±0.5 | 5.8±0.8a | <0.05 |
| Serum fasting
insulin, μU/ml | 8.6±4.2 | 14.5±10.2a | <0.05 |
| HOMA-IR | 1.58±0.7 | 4.6.0±2.2a | <0.05 |
qPCR and western blotting
The TLR4 mRNA expression results are shown in
Fig. 1. TLR4 mRNA expression
levels were significantly higher in the GDM group than in the
normal control group (P<0.05).
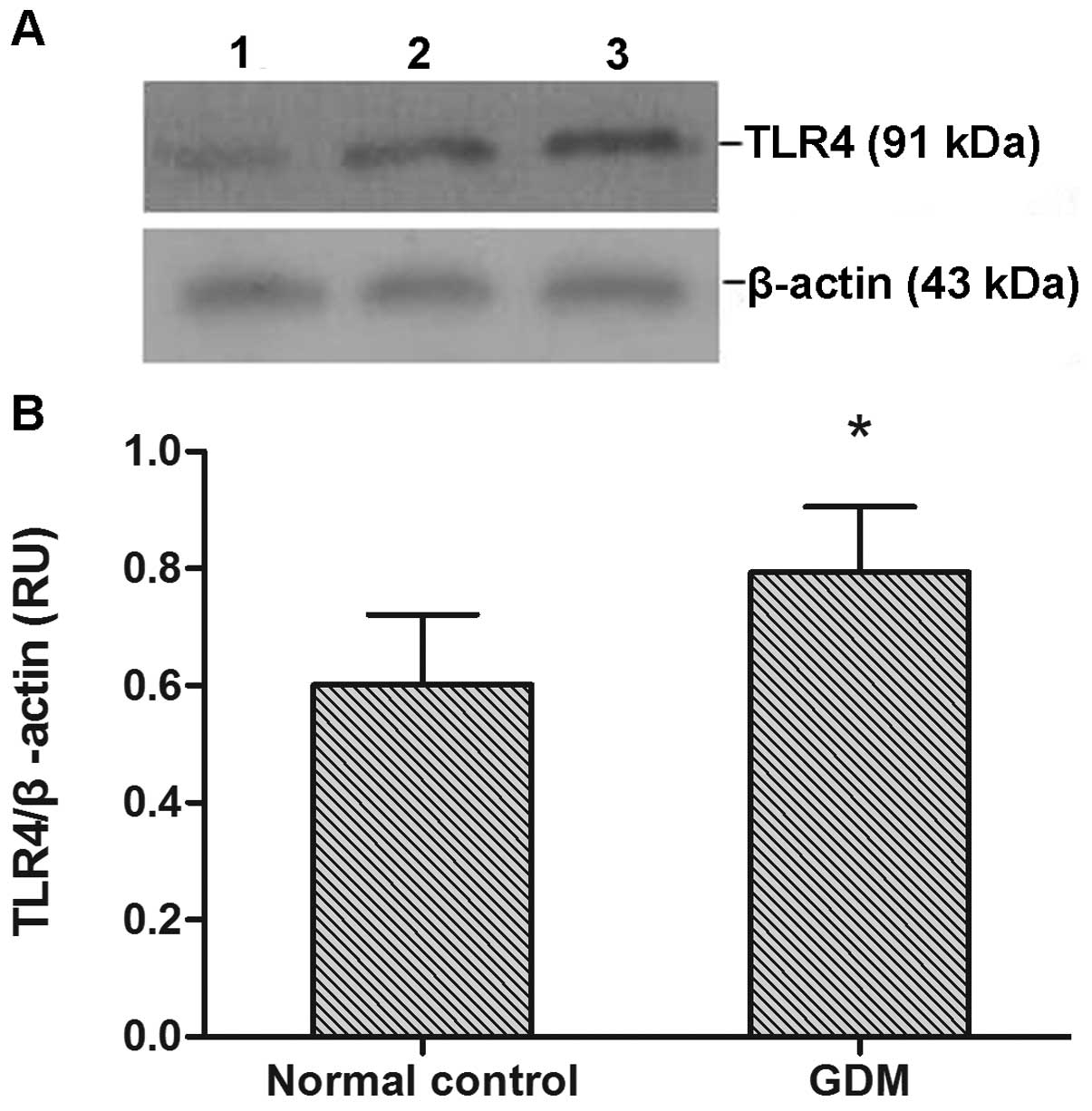
TLR4 protein expression was observed in all monocyte
samples (Fig. 2A). TLR4 protein
expression levels were significantly increased in the GDM group
compared with the normal control group (P<0.05; Fig. 2B).
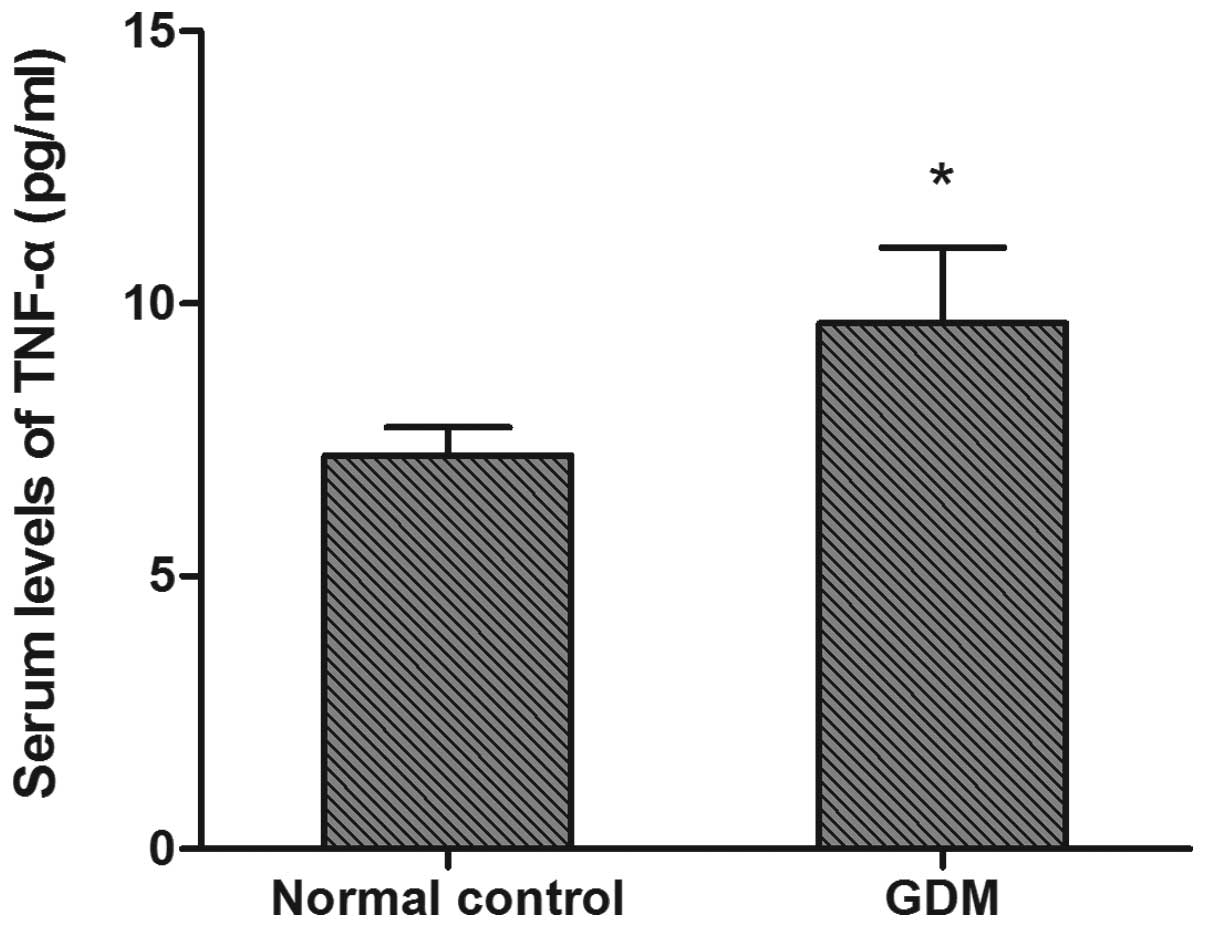
Serum TNF-α levels
Significantly elevated serum TNF-α levels were
observed in the GDM group (9.50±1.73 pg/ml) compared with the
control group (7.27±0.45 pg/ml) (P<0.05, Fig. 3).
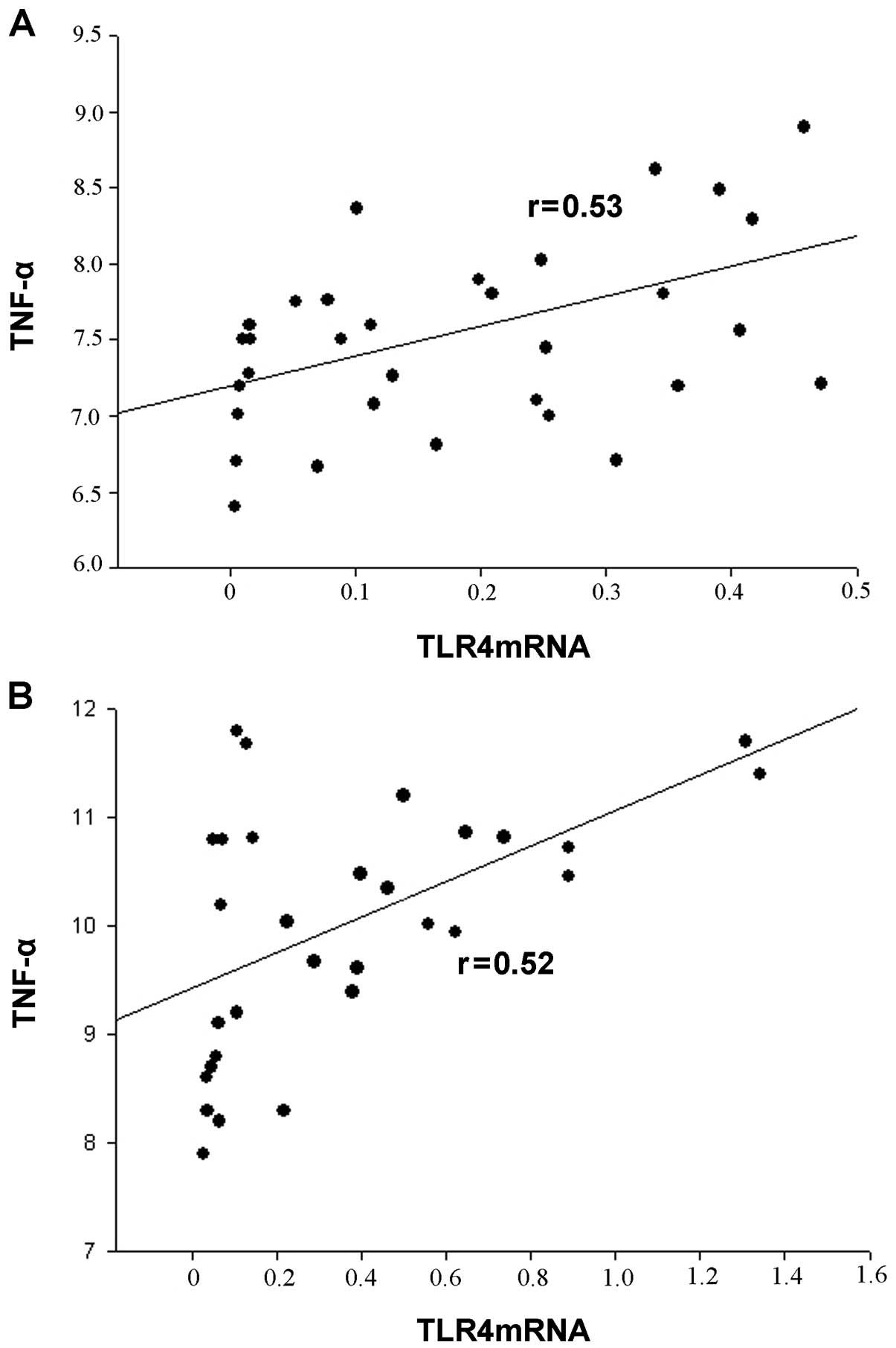
Correlation between TLR4 expression and
serum TNF-α levels
TLR4 mRNA levels correlated with serum TNF-α levels
in all participants. There was a positive correlation between the
serum TNF-α levels and TLR4 mRNA expression in monocytes (Fig. 4).
Discussion
To the best of our knowledge, the present is the
first to compare the levels of TLR4 gene expression between females
with GDM and healthy pregnant females. The results showed that TLR4
gene expression levels were significantly increased in patients
with GDM compared with healthy pregnant females. Serum TNF-α levels
were higher in the GDM group than in the normal control group. In
addition, a positive correlation was also observed between TLR4
mRNA expression and serum TNF-α levels in this study.
Inflammation is characterized by increased levels of
circulating biomarkers of inflammation and monocyte activity. The
relationships between inflammation, hyperglycemia and IR have been
widely studied in patients with diabetes and have been hypothesized
to be mediated via the activation of the innate immune system. TLR4
is an important mediator in the innate and cytokine-activated
immune systems. In addition, TLR4 is required for the adaptive
immune response, and its activation mediates a signaling pathway
involved in the transcriptional expression of proinflammatory
cytokines and chemokines (14).
TLR4 has also been identified as the primary receptor for LPS and
is expressed in numerous types of cells. TLR4 gene expression and
activation have been demonstrated to be increased in the monocytes
of patients with hyperglycemia (15). In addition, upregulated TLR4
expression is likely to lead to disease-resistant effects, and TLR4
has a proinflammatory role in the occurrence of diabetes and its
complications (11,16).
It has been suggested that GDM is a state of chronic
inflammation (17). In DM, the
aggravated inflammation may be mediated by TLRs via the activation
of the innate immune pathway (18). TLR4 activation and downstream
cytokine production may lead to the development of diabetes
(19). However, the underlying
mechanisms remain unknown. Increased TLR4 expression has been
observed to correlate with enhanced nuclear factor (NF)-κB
activation in response to the TLR4 ligand, resulting in elevated
levels of proinflammatory cytokines (19). NF-κB is a well-recognized
transcription factor that regulates the production of
proinflammatory cytokines, including TNF-α and interleukin 1 (IL-1)
(20). TLR4 also activates the
mitogen-activated protein kinase (MAPK) signaling pathway, leading
to the increased transcription of genes involved in inflammation
(21). In addition, TLR4
activation affects chemokine (C-X-C motif) ligand 10, inducing
apoptosis of islet β cells (22).
These observations suggest that TLR4 may lead to the activation of
various downstream pathways and cause diverse pathophysiological
events. These events may induce the development of GDM.
GDM is characterized by decreased maternal insulin
sensitivity or increased IR. In a previous study, the majority of
females with GDM appeared to exhibit β-cell dysfunction under a
background of chronic IR (23). IR
was revealed to be associated with the innate immune response
(24). A recent study suggested
that TLR4 and downstream pathways (MAPK and NF-κB) are important in
the pathogenesis of IR (25). In
addition, abnormal TLR4 expression may induce an inflammatory
response in insulin-resistant subjects (26). These results indicate that TLR4
expression in females with GDM may result in IR. In patients with
GDM, IR may lead to hyperglycemia, fetal macrosomia, a higher
likelihood of developing obstetric complications and a higher risk
of stillbirth (27).
The function of the TLR4 gene has been widely
studied in immune system cells, including monocytes and
macrophages. Monocytes effectively regulate specific and
non-specific immunological responses, which avoid damage to the
organisms by pathogens. Thus, phagocytosis by monocytes is
important in the innate immune response. Monocytes phagocytose a
portion of debris left from the digestion of a pathogen and present
it as an antigen to the adaptive immune system (28). The upregulation of TLR4 expression
has been demonstrated to promote the phagocytic capacity of
monocytes (29). Hence, TLR4 is
expressed in monocytes and TLR4 signaling is necessary for
phagocytosis by monocytes.
TNF-α is a proinflammatory cytokine secreted
predominantly by monocytes and macrophages. The results of the
present study are consistent with a previous observation that serum
TNF-α levels were increased in females with GDM compared with
healthy pregnant females (30).
Therefore, hyperglycemia-induced TNF-α release in patients with GDM
may contribute to the underlying pathogenesis of GDM. It has been
suggested that the production of TNF-α is induced by the activation
of TLR4. The activation of TLR4 has been shown to lead to the
activation of NF-kB, which results in the production of TNF-α
(31). In addition, the activation
of monocytes, induced by the TLR4-mediated c-Jun N-terminal kinase
signaling, may also cause the secretion of TNF-α (32). These results suggest that the
activation of TLR4 is associated with the upregulation of TNF-α.
Therefore, the elevated expression of TLR4 is able increase serum
TNF-α level. An increase in serum TNF-α levels from early to late
pregnancy was correlated with a decrease in insulin sensitivity
(33), which suggested that TNF-α
is associated with the development of IR. TNF-α is a significant
predictor of IR in patients with GDM through its ability to
decrease the tyrosine kinase activity of the insulin receptor
(27).
In conclusion, the novel observations of the present
study indicate that TLR4 expression increases in monocytes in GDM
and a positive correlation exists between TLR4 mRNA expression in
monocytes and serum TNF-α level in females with GDM. These results
suggest that a selective interference with TLR4 may present an
opportunity for the treatment of IR and GDM.
References
|
1
|
Metzger BE, Buchanan TA, Coustan DR, et
al: Summary and recommendations of the Fifth International
Workshop-Conference on Gestational Diabetes Mellitus. Diabetes
Care. 30:S251–S260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiong X, Saunders L, Wang F and Demianczuk
N: Gestational diabetes mellitus: prevalence, risk factors,
maternal and infant outcomes. Int J Gynaecol Obstet. 75:221–228.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Landon MB, Spong CY, Thom E, et al; Eunice
Kennedy Shriver National Institute of Child Health and Human
Development Maternal-Fetal Medicine Units Network. A multicenter,
randomized trial of treatment for mild gestational diabetes. N Engl
J Med. 361:1339–1348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fernández-Real JM and Pickup JC: Innate
immunity, insulin resistance and type 2 diabetes. Trends Endocrinol
Metab. 19:10–16. 2008.PubMed/NCBI
|
|
5
|
Greenberg A and McDaniel M: Identifying
the links between obesity, insulin resistance and β-cell function:
potential role of adipocyte-derived cytokines in the pathogenesis
of type 2 diabetes. Eur J Clin Invest. 32(Suppl 3): 24–34.
2002.
|
|
6
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hill HR, Hogan NA, Rallison ML, et al:
Functional and metabolic abnormalities of diabetic monocytes. Adv
Exp Med Biol. 141:621–628. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolek MJ, Carlquist JF, Muhlestein JB, et
al: Toll-like receptor 4 gene Asp299Gly polymorphism is associated
with reductions in vascular inflammation, angiographic coronary
artery disease, and clinical diabetes. Am Heart J. 148:1034–1040.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nitsche JF, Jiang SW and Brost BC:
Toll-like receptor-2 and toll-like receptor-4 expression on
maternal neutrophils during pregnancy. Am J Reprod Immunol.
64:427–434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kajikawa O, Frevert CW, Lin SM, et al:
Gene expression of Toll-like receptor-2, Toll-like receptor-4, and
MD2 is differentially regulated in rabbits with Escherichia
coli pneumonia. Gene. 344:193–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi H, Kokoeva MV, Inouye K, Tzameli I,
Yin H and Flier JS: TLR4 links innate immunity and fatty
acid-induced insulin resistance. J Clin Invest. 116:3015–3025.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nguyen MA, Favelyukis S, Nguyen AK, et al:
A subpopulation of macrophages infiltrates hypertrophic adipose
tissue and is activated by free fatty acids via Toll-like receptors
2 and 4 and JNK-dependent pathways. J Biol Chem. 282:35279–35292.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
American Diabetes Association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 29(Suppl
1): S43–S48. 2006.
|
|
14
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dasu MR, Devaraj S, Zhao L, Hwang DH and
Jialal I: High glucose induces toll-like receptor expression in
human monocytes: mechanism of activation. Diabetes. 57:3090–3098.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhande R, Dauphinee SM, Thomas JA,
Yamamoto M, Akira S and Karsan A: FADD negatively regulates
lipopolysaccharide signaling by impairing interleukin-1
receptor-associated kinase 1-MyD88 interaction. Mol Cell Biol.
27:7394–7404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Benedetto A, Russo G, Corrado F, et al:
Inflammatory markers in women with a recent history of gestational
diabetes mellitus. J Endocrinol Invest. 28:34–38. 2005.PubMed/NCBI
|
|
18
|
Devaraj S, Dasu MR, Rockwood J, Winter W,
Griffen SC and Jialal I: Increased toll-like receptor (TLR) 2 and
TLR4 expression in monocytes from patients with type 1 diabetes:
further evidence of a proinflammatory state. J Clin Endocrinol
Metab. 93:578–583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohammad MK, Morran M, Slotterbeck B, et
al: Dysregulated Toll-like receptor expression and signaling in
bone marrow-derived macrophages at the onset of diabetes in the
non-obese diabetic mouse. Int Immunol. 18:1101–1113. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar
|
|
21
|
Mullick AE, Tobias PS and Curtiss LK:
Toll-like receptors and atherosclerosis: key contributors in
disease and health? Immunol Res. 34:193–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schulthess FT, Paroni F, Sauter NS, et al:
CXCL10 impairs β cell function and viability in diabetes through
TLR4 signaling. Cell Metab. 9:125–139. 2009.
|
|
23
|
Buchanan TA and Xiang AH: Gestational
diabetes mellitus. J Clin Invest. 115:485–491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dandona P, Aljada A and Bandyopadhyay A:
Inflammation: the link between insulin resistance, obesity and
diabetes. Trends Immunol. 25:4–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hussey SE, Liang H, Costford SR, et al:
TAK-242, a small-molecule inhibitor of Toll-like receptor 4
signalling, unveils similarities and differences in
lipopolysaccharide- and lipid-induced inflammation and insulin
resistance in muscle cells. Biosci Rep. 33:37–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reyna SM, Ghosh S, Tantiwong P, et al:
Elevated toll-like receptor 4 expression and signaling in muscle
from insulin-resistant subjects. Diabetes. 57:2595–2602. 2008.
View Article : Google Scholar
|
|
27
|
Kirwan JP, Hauguel-De Mouzon S, et al:
TNF-α is a predictor of insulin resistance in human pregnancy.
Diabetes. 51:2207–2213. 2002.
|
|
28
|
Qureshi M: Avian macrophage and immune
response: an overview. Poult Sci. 82:691–698. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng S, Wu Q, Yu K, et al: Changes in the
relative inflammatory responses in sheep cells overexpressing of
toll-like receptor 4 when stimulated with LPS. PLoS One.
7:e471182012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hotamisligil G: The role of TNFα and TNF
receptors in obesity and insulin resistance. J Intern Med.
245:621–625. 1999.
|
|
31
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar
|
|
32
|
Paik YH, Schwabe RF, Bataller R, Russo MP,
Jobin C and Brenner DA: Toll-like receptor 4 mediates inflammatory
signaling by bacterial lipopolysaccharide in human hepatic stellate
cells. Hepatology. 37:1043–1055. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Catalano P, Highman T, Huston L and
Friedman J: Relationship between reproductive hormones/TNF alpha
and longitudinal changes in insulin sensitivity during gestation.
Diabetes. 45:175A1996.
|