Introduction
Arteriosclerosis (AS) is a chronic inflammatory
disease. Reactive oxygen species (ROS) are produced during exposure
to oxidative stress, such as hydrogen peroxide
(H2O2), and bind to the nuclear receptor of
vascular endothelial cells and smooth muscle cells (SMCs) as
ligands. ROS directly regulate the gene expression of various types
of signaling molecules, such as interleukin (IL), intercellular
adhesion molecule (ICAM) and vascular cell adhesion molecule
(VCAM), to enhance the adhesion and migration of monocytes to the
tunica intima, which is vital in the early stage of AS (1–3).
Various types of cells and inflammatory mediators participate in
the occurrence of AS (4–7), particularly the vascular smooth
muscle cells (VSMCs) of the tunica media. The VSMCs of the tunica
media form SMC-derived foam cells after proliferation, phenotypic
transition and migration to the intima in the presence of a
stimulating factor. The VSMCs of the tunica media are significant
in the early, middle and advanced stages of AS, as well as in the
pathogenesis of vascular stenosis diseases. VSMCs are categorized
as important pathological features of AS (8–11).
Xin Mai Jia (XMJ) is a Chinese medicinal formulation
that is available in capsule form. XMJ contains 10–35% functional
red kojic rice powder, 1–10% kudzu flavonoid powder, 1–8% soybean
isoflavone powder, 1–8% bamboo leaf flavone powder, 1–8%
resveratrol powder, 1–6% hawthorn powder, 1–6% gastrodia powder,
1–30% Auricularia auricula powder, 0.1–0.2% hippocampus
powder, 0.008–0.04% astaxanthin powder, 0.1–0.3% menthol powder and
20–50% resistant starch.
This health food contains several natural
antioxidants, which enhance immunity and decrease the effects of
ageing (12–15). A previous study has shown that XMJ
is able to alleviate cardiovascular and cerebrovascular diseases,
reduce blood lipids, normalize blood pressure, increase energy
levels and improve sleep after several months of intake (16). Although the effect of XMJ is
satisfactory, the exact mechanism of its antiarteriosclerotic
action has not been confirmed.
Materials and methods
Drugs and chemicals
The crude components of XMJ were purchased from
Beijing Tong Ren Tang Chinese Medicine Co. Ltd. (Beijing, China).
Lovastatin, H2O2,
2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
(XTT) and phenazine methosulfate (PMS) were purchased from Sigma
Chemical Co. (St. Louis, Mo, USA). Zhibituo was obtained from
Chengdu Diao Pharmaceutical Group Co., Ltd. (Chengdu, China). The
RPMI-1640 culture medium and fetal calf serum were acquired from
Gibco (Carlsbad, CA, USA). Wright-Giemsa (R-G) stain was purchased
from Changsha Li Xin Biotechnology Co. (Changsha, China). IL-1,
IL-6, ICAM-1, VCAM-1, matrix metalloproteinase-2 (MMP-2), tissue
inhibitor of metalloproteinase (TIMP-2) and nuclear factor (NF)-κB
were purchased from R&D Systems (Minneapolis, MN, USA). All
other reagents were analytically pure and purchased in China.
Cell experiment protocol
Human aortic smooth muscle cells (HASMCs) were
obtained from HASMC cell lines purchased from American Type Culture
Collection (Manassas, VA, USA). The cells were routinely maintained
in phenol red containing Dulbecco’s modified Eagle’s medium
supplemented with 15% fetal calf serum, 100 U/ml penicillin and 0.1
mg/ml phytomycin in a 37°C incubator containing 5% CO2.
The third generation of HASMCs was used in the study. The cells
were randomly divided into eight groups and incubated with the
corresponding drugs for 24 h. The cells in the first group were
incubated with Kreb’s solution and were assigned to the blank
control group (n=6). The cells in the second group were incubated
with 500 mg/l XMJ and were assigned to the XMJ control group (n=6).
The cells in the third group were incubated with 200 μmol/l
H2O2 and were assigned to the model group
(n=6). The cells in the fourth group were incubated with 1 μmol/l
lovastatin and 200 μmol/l H2O2 and were
assigned to the lovastatin group (n=6). The cells in the fifth
group were incubated with 50 μmol/l zhibituo and 200 μmol/l
H2O2 and were assigned to the zhibituo group
(n=6). The cells in the sixth group were incubated with 25 μmol/l
XMJ and 200 μmol/l H2O2 and were assigned to
the low-dose XMJ group (n=6). The cells in the seventh group were
incubated with 50 μmol/l XMJ and 200 μmol/l
H2O2 and were assigned to the middle-dose XMJ
group (n=6). The cells in the eighth group were incubated with 100
μmol/l XMJ and 200 μmol/l H2O2 and were
assigned to the high-dose XMJ group (n=6). The cultured cells were
collected after each treatment for subsequent tests.
Preparation of the ultra-filtration
membrane extracts for XMJ
Approximately 1,000 g of the crude components of XMJ
were placed in a container containing 6,000 ml water and heated for
1 h in a microwave oven at 1,000 W. The decoction of XMJ was
obtained after filtering the extract through four gauzes.
Thereafter, 6,000 ml water was added to the container and the above
procedure was repeated. After mixing the former decoction of XMJ
with the latter one, the mixture was filtered using sterile
absorbent cotton. XMJ was refined by ultra-filtration technology
with water decoction at a pressure of 0.5 kPa/m3, a
temperature of 25°C, and a flow rate of 100 l/h/m2.
Approximately 5,000 ml of filtrate was then condensed to 1,000 ml,
which was equivalent to 1 g of XMJ/ml of liquid medicine. Finally,
the refined liquid was labeled and stored in a refrigerator at
4°C.
HASMC proliferation
Seed cells were placed into 96-well plates (100
μl/well) and cultured in RPMI-1640. Thereafter, the null medium was
discarded and replaced with 100 μl fresh medium and 25 μl XTT and
PMS mixture. After cell culture for 4 h, the optical density (OD)
was measured at 450 and 630 nm wavelengths using an enzyme-linked
immunosorbent assay (ELISA) plate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). Each group contained six wells. The result was
obtained from the average value of these six wells.
Wright-Giemsa staining
The cells were plated in a 24-well plate at
5×104 cells/ml, treated with XMJ (25, 50 and 100
μmol/l), lovastatin and zhibituo, respectively, for 24 h. The
sterile slides were placed in a 24-well plate in advance for the
adherence of cells. The slides were then taken out and washed twice
with phosphate-buffered saline (PBS). After air-drying at room
temperature, Wright-Giemsa dye was added to rapidly cover the
slide. The same amount of PBS was added a few minutes later. The
Wright-Giemsa dye and the PBS were mixed using a rubber pipette
bulb. The dye was then rinsed with water, dried and sealed, and the
cells were examined under a microscope (Olympus, Fukushima,
Japan).
Detection of biochemical indicators in
HASMC supernatant fluid
Seed cells were placed into 6-well plates in 2 ml
concentrations/well. To detect the biochemical indicator of the
cell supernatant fluid, the culture fluid was collected to measure
the superoxide dismutase (SOD) and malondialdehyde (MDA) activity,
following the manufacturer’s instructions. The reagents and kit
used to measure SOD and MDA were purchased from Nanjing Jiancheng
Biological Engineering Institute (Nanjing, China).
ELISA
Seed cells were placed into 6-well plates (2
ml/well). The supernatant fluid of the various wells in the plates
was collected by an ELISA reagent kit, according to the
manufacturer’s instructions (R&D Systems Inc., Minneapolis, MN,
USA). The OD value of each well was measured at a 450 nm
wavelength. The OD value was assigned as the abscissa, while the
standard liquid concentration of the reagent was assigned as the
ordinate. The relevant curve was drawn and the curve equation was
calculated. The OD values of the samples were substituted into the
equation of the standard curve, and the IL-1, IL-6, ICAM-1, VCAM-1,
MMP-2, TIMP-2 and NF-κB values were calculated.
HASMC transfer ability
Seed cells were placed into 24-well plates (1
ml/well). After 24 h of cell culture, a line of the same width was
drawn in each well with a 200 μl pipette tip, and the plates were
washed three times with sterile PBS. The cells were cultured in
RPMI-1640 medium without serum, and exposed to XMF (25, 50 and 100
μmol/l), lovastatin and zhibituo, respectively, for 24 h. The
entire process was recorded. The scratch width was detected using
scratch image software 3.22 (Olympus). Six scratch belt widths were
collected in each well, and the average value of the widths was
calculated and compared.
Statistical analysis
All data are shown as the mean ± standard error.
Single factor variance and Student-Newman-Keuls multiple comparison
analyses were used to compare data from different groups.
Comparisons were performed using statistical software SPSS 13.0
(SPSS, Inc., Chicago, IL, USA. P<0.05 was considered to indicate
a statistically significant result.
Results
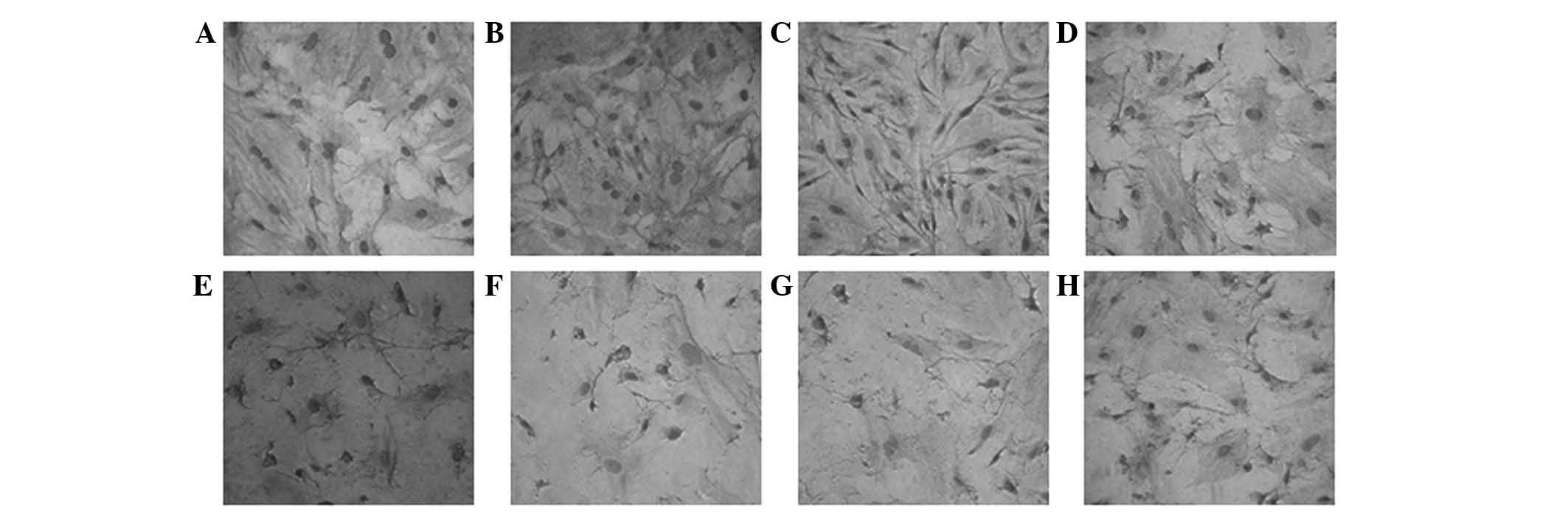
Different concentrations of XMJ and
H2O2 stimulate morphological changes in
HASMCs
The HASMCs in the control group were arranged
closely with abundant cytoplasms and intact cell membranes. The
HASMCs demonstrated typical ‘peak-valley’-like growth. The number
of cells in the normal group was lower than that in the model
group. The normal group had loose cell connections and exhibited
cytoplasm shrinkage, whereas the model group lost its typical
growing appearance but had a fusiform cell presentation. The drugs
inhibited cell proliferation to varying degrees. Certain damaged
cells were restored with plumper cytoplasms and clearer profiles.
However, these effects were much clearer in the high-dose XMJ
group. Certain cells in the high-dose XMJ group reverted to a
near-normal state. However, no significant effects were observed in
the zhibituo and lovastatin groups. The cell shrinkage in the
lovastatin group was particularly evident (Fig. 1).
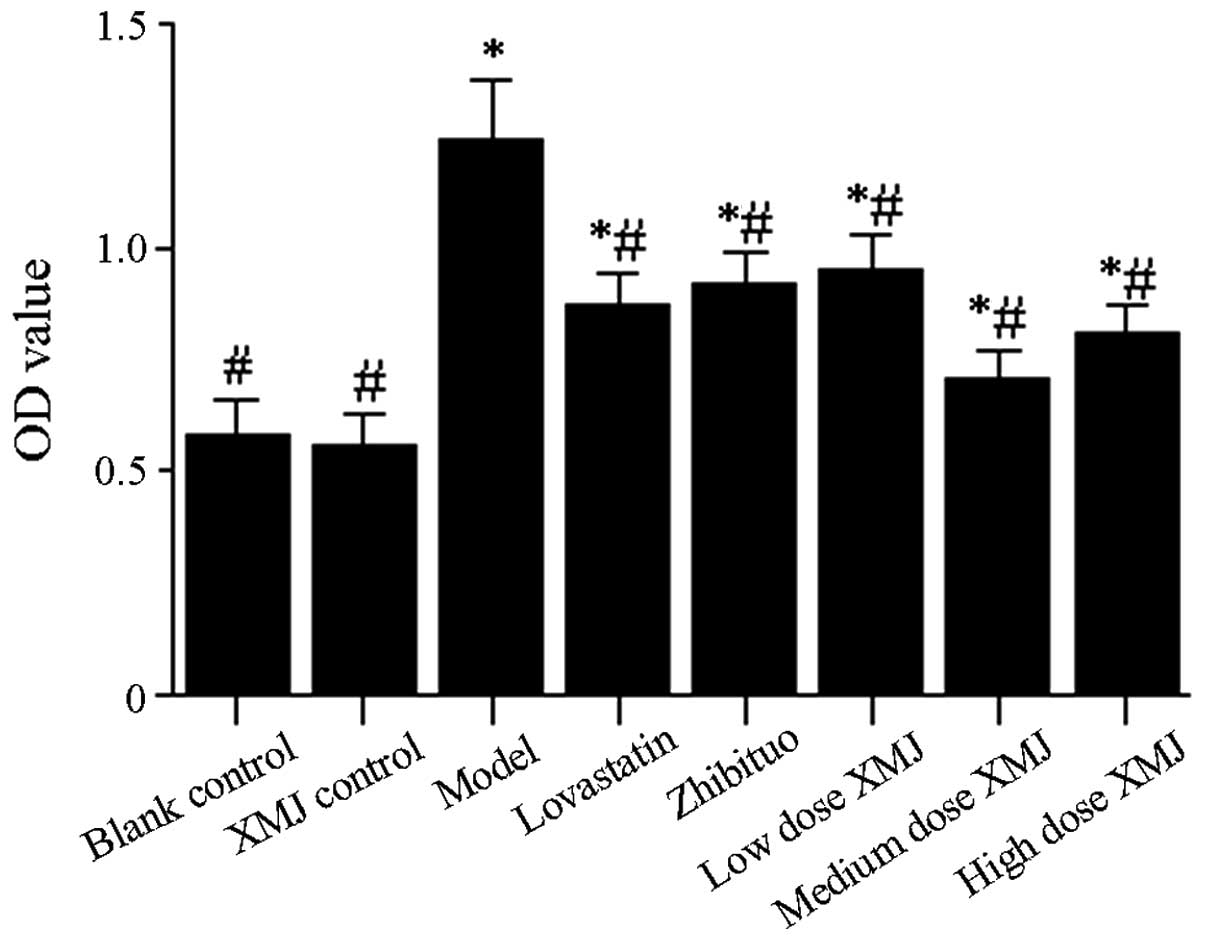
Inhibitory effects of XMJ on
H2O2-induced HASMC proliferation
Cells that proliferate after HASMC treatment are
damaged and gradually migrated to the vascular intima from the
tunica media vasorum, thus inducing plaque formation (17). Excessive proliferation of HASMCs is
also an important factor in high blood pressure (18). The results showed that HASMCs in
the model group were significantly more proliferative after 24 h of
interaction than the cells in the normal group (Fig. 2, P<0.05). The results showed
that HASMCs in the model group were significantly more
proliferative after 24 h of interaction than the cells in the
normal group, proliferative HASMCs in the middle-dose XMJ group,
low-dose XMJ group and high-dose XMJ group were significantly
inhibited after 24 h of interaction in a dose-dependent manner.
XMJ anti-inflammatory effects
Inflammation induces atherosclerotic plaque
formation and occurs during the development of AS. Therefore, we
examined the inflammatory factors MMP-2 and TIMP-2 in the model
group. The results showed that the levels of both factors were
significantly decreased in the model group compared with those in
the normal group (P<0.05); however, the levels of both factors
increased significantly after treatment with different
concentrations of XMJ compared with those in the model group
(P<0.05). The most significant effect was observed in the
middle-dose XMJ group. The levels of both factors also increased in
the zhibituo and lovastatin groups; however, the effect was not
significant compared with those in the XMJ groups (Table I).
 | Table IXMJ anti-inflammatory effects. |
Table I
XMJ anti-inflammatory effects.
| Groups | TIMP-2 (pg/l) | MMP-2 (μg/l) | NF-κB (ng/l) |
|---|
| Blank control |
1987.36±125.39a | 0.682±0.09a | 44.98±7.89a |
| XMJ control |
1875.41±115.47a | 0.675±0.08a | 46.66±8.47a |
| Model |
1120.39±157.14b | 0.543±0.07b |
165.98±12.47b |
| Lovastatin |
1354.39±124.17a,b | 0.587±0.06a,b |
110.35±11.27a,b |
| Zhibituo |
1368.27±124.77a,b | 0.588±0.08a,b | 78.98±8.74a,b |
| Low-dose XMJ |
1452.33±102.47a,b | 0.622±0.07a,b | 79.54±6.57a,b |
| Middle-dose
XMJ |
1688.69±149.38a,b | 0.636±0.08a,b | 63.21±4.57a,b |
| High-dose XMJ |
1657.69±123.96a,b | 0.654±0.07a,b | 62.17±6.46a,b |
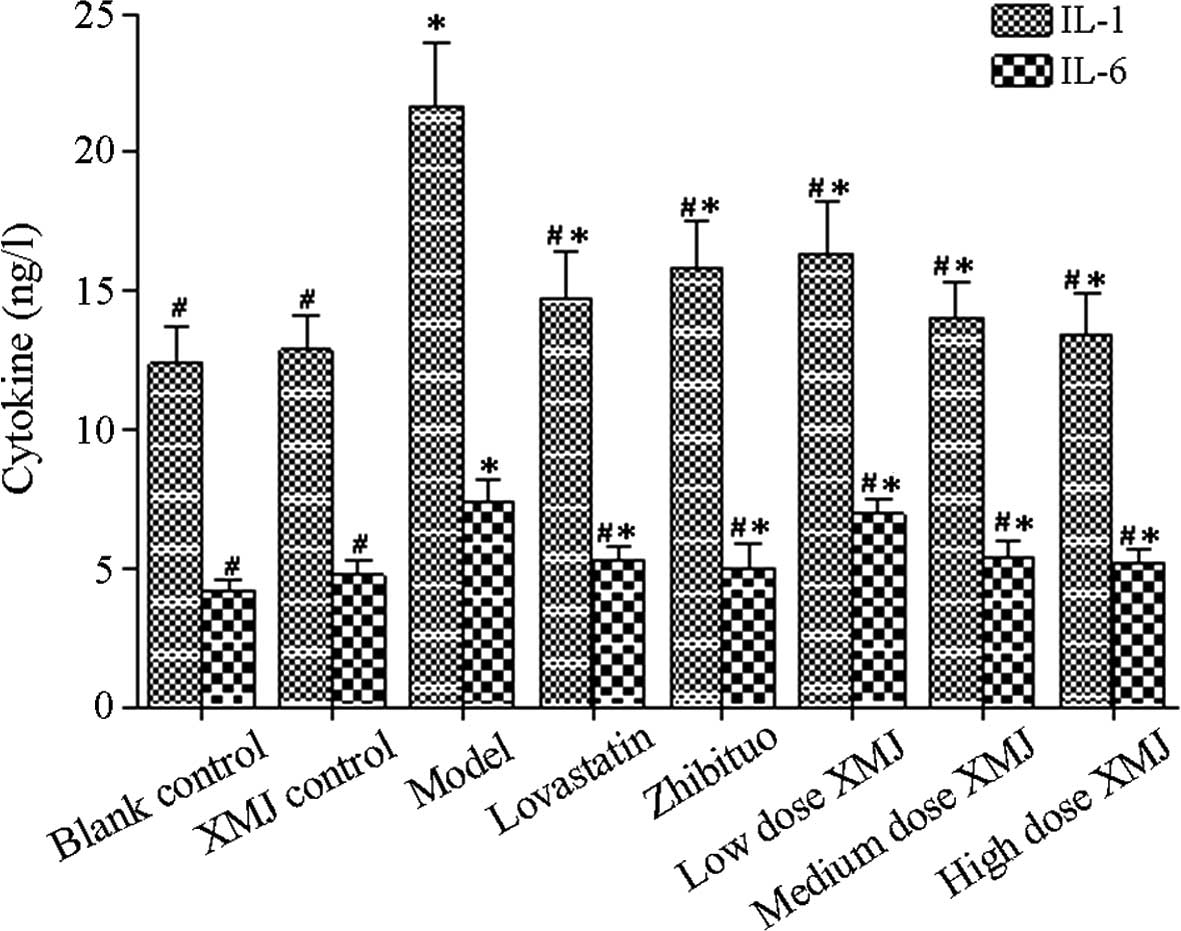
NF-κB promotes the release of various
inflammatory factors
The activation mechanism of the NF-κB pathway and
its effect on related inflammatory factor content (including IL-1
and IL-6 levels; Fig. 3) were
investigated. The results showed that the NF-κB content in the
model group was significantly increased compared with that in the
normal group (P<0.05), thus indicating that the NF-κB pathway
was significantly inhibited. However, the NF-κB content decreased
significantly after treatment with different concentrations of XMJ
compared with those in the model group (P<0.05). This result
indicates that inhibition of NF-κB pathway activation by XMJ
reduced the inflammatory response. The NF-κB content decreased
significantly in the zhibituo group compared with that in the model
group (P<0.05). No significant difference was observed between
the zhibituo and XMJ groups. The NF-κB content also decreased in
the lovastatin group compared with that in the model group
(P<0.05). However, the effect of the lovastatin group was
greater than that of the XMJ and zhibituo groups (Table I).
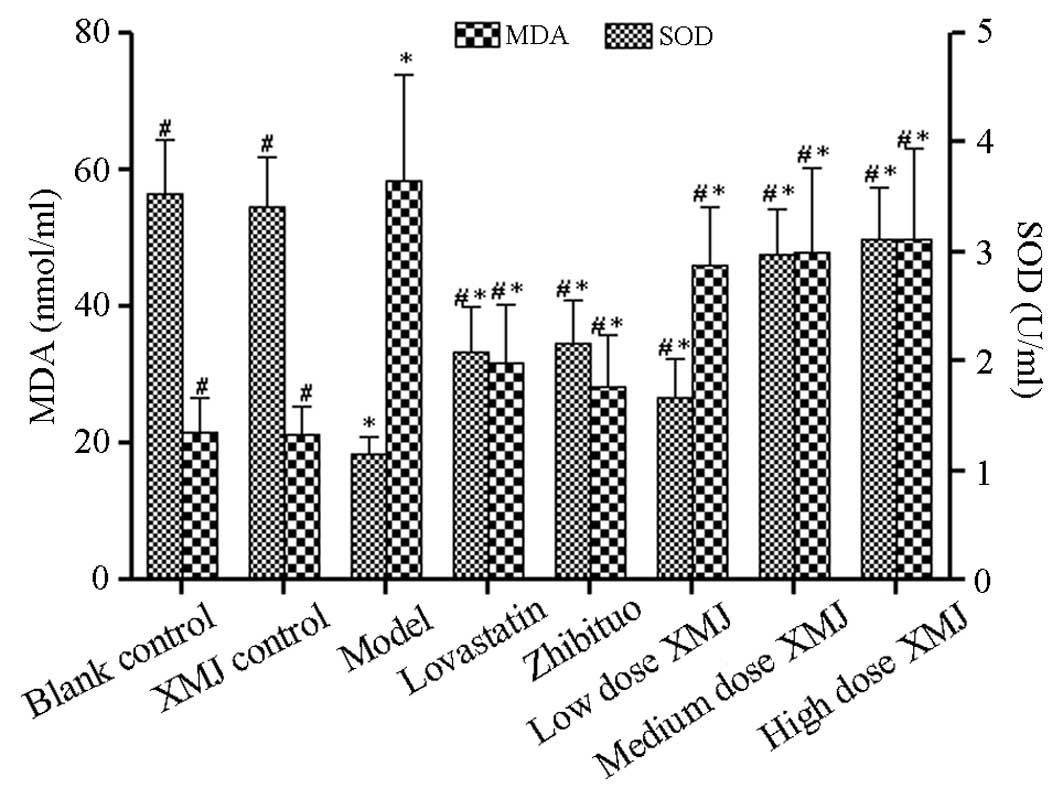
XMJ antioxidant effects
Lipid peroxidation induces plaque formation during
AS development (19). Plaque
formation may be controlled if lipid peroxidation is inhibited
effectively. SOD is a well-known and important antioxidant enzyme.
Therefore, we first detected the SOD activity (Fig. 4). The SOD activity increased
significantly in a concentration-dependent manner after treatment
with various concentrations of XMJ. The SOD activity was
significantly different in the high-dose XMJ group from that in the
model group (P<0.05). This result showed that XMJ may
effectively promote the synthesis of antioxidant molecules such as
SOD by HASMCs, thus effectively inhibiting the occurrence of
oxidation. The MDA content, which is an important indicator of
oxidative stress, was also measured. The MDA content in the model
group increased significantly compared with that in the normal
group, but decreased significantly after treatment with various
concentrations of XMJ (P<0.05). The MDA content in the zhibituo
and XMJ groups was significantly decreased compared with that in
the model group (P<0.05), thus indicating that XMJ exerts
antioxidant effects by inhibiting MDA synthesis.
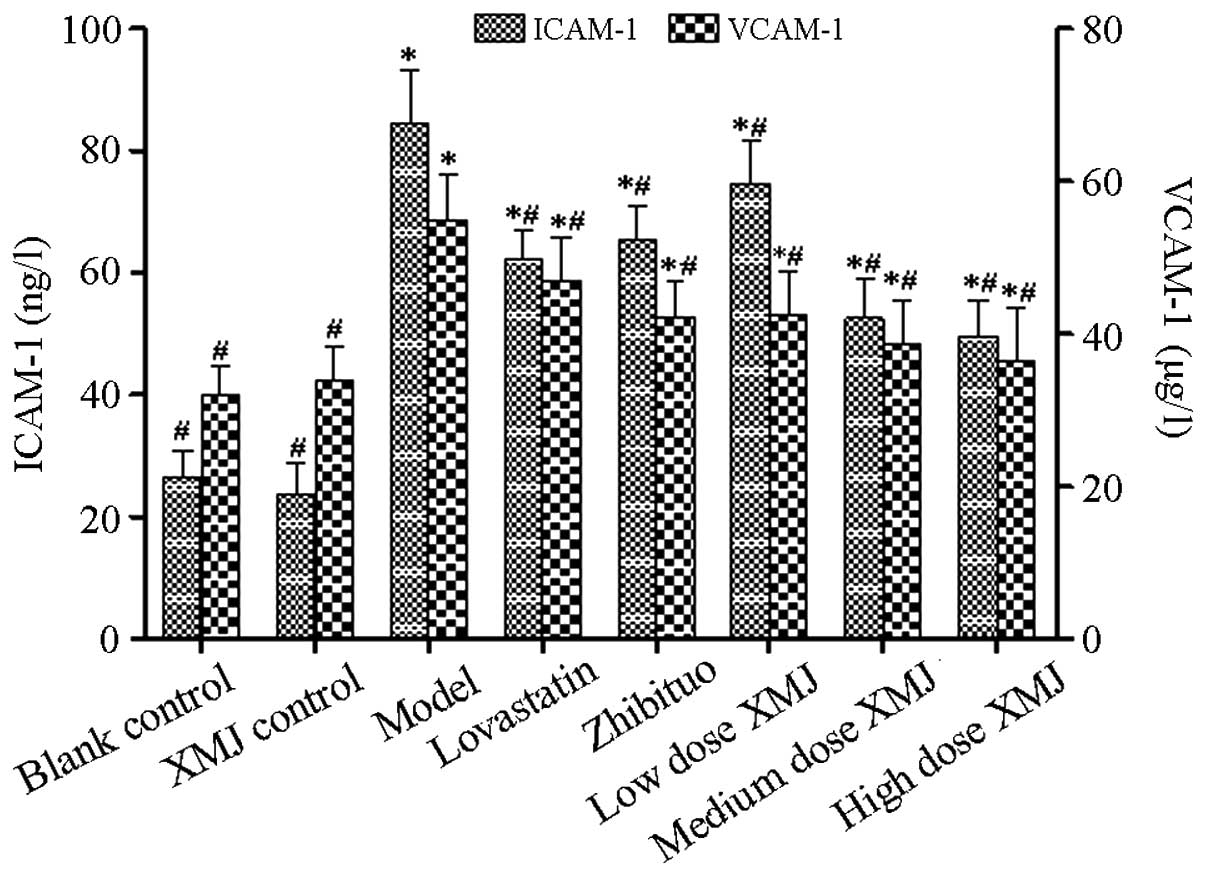
Anti-adhesion effects of XMJ inhibit
HASMC migration
The proliferation and adhesion capacity of HASMCs
from the tunica media to the tunica intima is enhanced in injured
HASMCs (20). Therefore, the
expression of adhesion factors ICAM-1 and VCAM-1 was detected
(Fig. 5). The results showed that
the levels of the two adhesion factors were significantly increased
in the model group compared with those in the normal group
(P<0.05), but decreased significantly in a
concentration-dependent manner after treatment with different
concentrations of XMJ compared with those in the model group
(P<0.05). The ICAM-1 content in the zhibituo and lovastatin
groups decreased significantly (P<0.05) as did the VCAM-1
content. These results showed that XMJ reduced the adhesion and
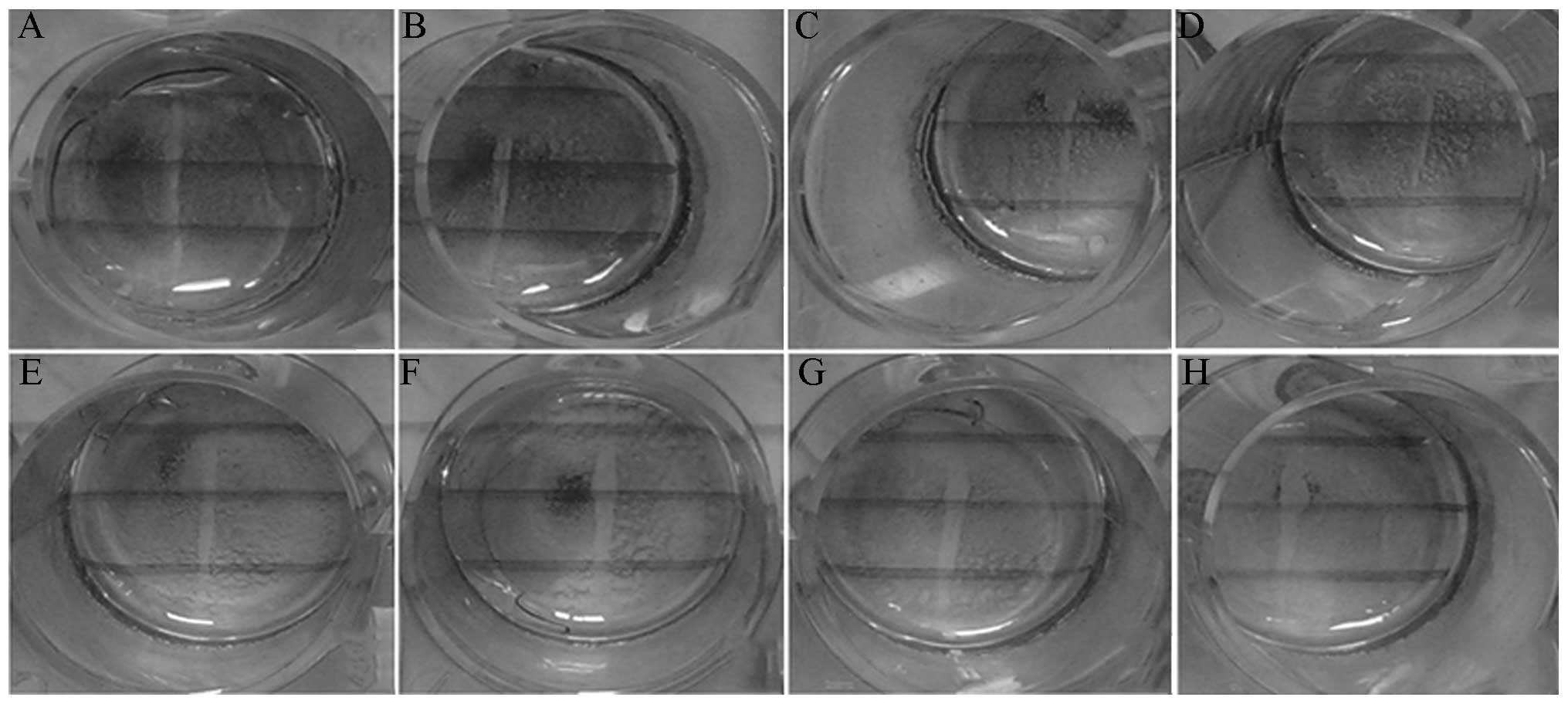
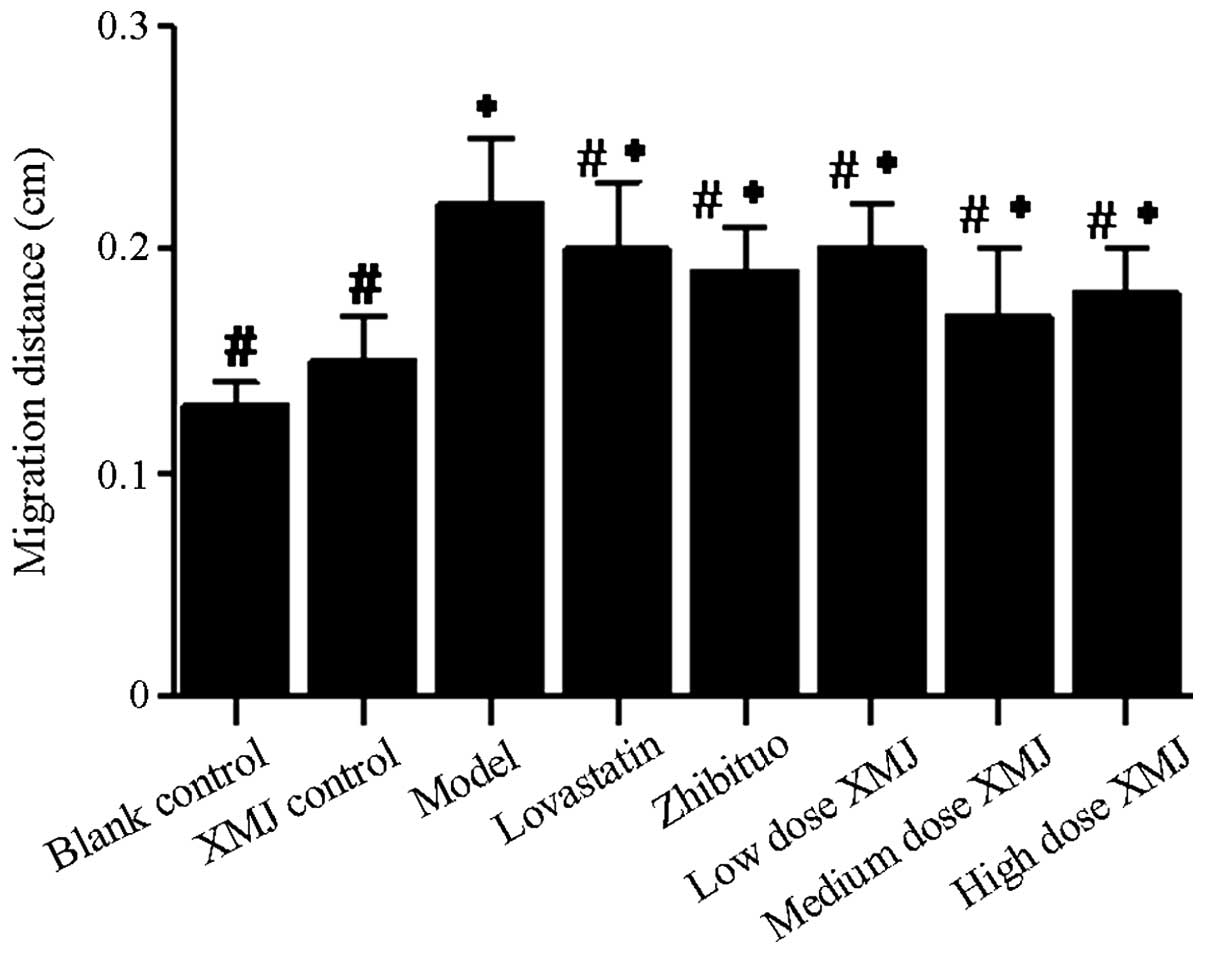
migration of HASMCs from the tunica media to the tunica intima. The
scratch experiment showed that the scratch width narrowed
significantly in the model group, and that the cell migration
ability became stronger (Fig. 6).
The scratch width widened significantly after treatment with
different concentrations of XMJ. Cells were shed from the cell wall
due to the reduction in cell adhesion. No significant difference
was observed between the scratch distances in the zhibituo and
lovastatin groups compared with those in the model group (Fig. 7).
Discussion
Contractile SMCs are present in the tunica media,
the cytoplasms contain numerous myofilaments, but fewer organelles
(21,22). The main function of SMCs is to
regulate vascular tone. Inflammation and trauma reduce the
specificity of telescopic protein expression, inducing a
proliferative state in SMCs, increasing their migration and
proliferation and promoting damage repair processes (23,24).
The overexpression of the repair process in damaged blood vessels
induces the development of vascular diseases such as AS, vascular
stenosis and hypertension (25–27).
During the development of AS lesions, VSMCs confer proliferative
effects with migrative functions and promote the endometrial repair
of vascular intima damage, thus resulting in restenosis and
atherosclerotic plaque formation and growth (28–30).
Therefore, the prevention of VSMC proliferation and aggregation may
contribute to the prevention of restenosis and atherosclerotic
plaque formation.
The results of the present study indicated that XMJ
inhibited HASMC proliferation by inhibiting IL-1 and IL-6
expression. A reduction in the number of SMCs effectively decreases
the tension of blood vessels and prevents vascular stenosis
(31). AS is a chronic
inflammatory disease. XMJ promoted TIMP-2 and MMP-2 expression by
activating the NF-κB pathway. This may reduce the
H2O2-induced HASMC injury and inhibit further
cell damage by reducing the inflammatory response. This is likely
to delay the development of disease and promote healing. SOD is
important as an antioxidant. Increased SOD synthesis helps to
reduce oxidative stress in the AS process (32). VCAM-1 and ICAM-1 are important in
the migration of SMCs from the tunica media to the tunica intima
(33). XMJ reduced the adhesion of
HASMCs by inhibiting HASMC synthesis, thus effectively inhibiting
HASMC migration. The results showed that XMJ promotes cell repair
by inhibiting the proliferation, inflammatory response, adhesion
and antioxidant mechanisms of HASMC and is likely to reduce plaque
formation in AS. XMJ may play a protective role in AS. Further
studies are required to determine whether the inhibition of
intercellular adhesion expression causes plaque shedding and
cardiovascular and cerebrovascular diseases.
Acknowledgements
This study was supported by Major Research Projects
of the Department of Science and Technology of Henan Province
(China) (no. 121100910300).
References
|
1
|
Gupta DK, Kwong RY and Pfeffer MA:
Cardiovascular imaging in clinical practice: what does late
gadolinium enhance? JAMA. 309:929–930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou CH, Tsai WC, Wang MC, et al: Effects
of deranged glucose homeostasis on peripheral arterial stiffness
index in patients with pre-diabetes mellitus. Int Heart J.
54:27–32. 2013.PubMed/NCBI
|
|
3
|
Yang C, Li D, Mennett R, et al: The impact
of pulmonary hypertension on outcomes of patients with low left
ventricular ejection fraction: a propensity analysis. J Heart Valve
Dis. 21:767–773. 2012.PubMed/NCBI
|
|
4
|
Bernat R, Szavits-Nossan J, Trbović A,
Kapov-Svilicić K, Sesto I and Sipić T: Relationship of genetic
markers for atherosclerosis and long-term outcome after
percutaneous coronary intervention with stenting. Coll Antropol.
36:1385–1390. 2012.PubMed/NCBI
|
|
5
|
Ahimastos AA, Walker PJ, Askew C, et al:
Effect of ramipril on walking times and quality of life among
patients with peripheral artery disease and intermittent
claudication: a randomized controlled trial. JAMA. 309:453–460.
2013. View Article : Google Scholar
|
|
6
|
Velic A, Laturnus D, Chhoun J, Zheng S,
Epstein P and Carlson E: Diabetic basement membrane thickening does
not occur in myocardial capillaries of transgenic mice when
metallothionein is overexpressed in cardiac myocytes. Anat Rec
(Hoboken). 296:480–487. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahali D, Mondal S and Sadhu P:
Percutaneous transluminal coronary angioplasty in a patient in
cardiogenic shock due to recent anterior wall MI with history of
prior inferior wall MI 15 days back. J Indian Med Assoc.
110:325–326. 2012.
|
|
8
|
Parthasarathy S, Santanam N, Ramachandran
S and Meilhac O: Oxidants and antioxidants in atherogenesis. An
appraisal. J Lipid Res. 40:2143–2157. 1999.PubMed/NCBI
|
|
9
|
Bernal-Mizrachi C, Gates AC, Weng S, et
al: Vascular respiratory uncoupling increases blood pressure and
atherosclerosis. Nature. 435:502–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar
|
|
11
|
Qin C and Liu Z: In atherogenesis, the
apoptosis of endothelial cell itself could directly induce
over-proliferation of smooth muscle cells. Med Hypotheses.
68:275–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li WJ, Nie SP, Peng XP, et al: Ganoderma
atrum polysaccharide improves age-related oxidative stress and
immune impairment in mice. J Agric Food Chem. 60:1413–1418. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baeza I, De Castro NM, Arranz L and De la
Fuente M: Soybean and green tea polyphenols improve immune function
and redox status in very old ovariectomized mice. Rejuvenation Res.
13:665–674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanderson P, Elsom RL, Kirkpatrick V, et
al: UK food standards agency workshop report: diet and immune
function. Br J Nutr. 103:1684–1687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marko MG, Ahmed T, Bunnell SC, et al:
Age-associated decline in effective immune synapse formation of
CD4(+) T cells is reversed by vitamin E supplementation. J Immunol.
178:1443–1449. 2007.PubMed/NCBI
|
|
16
|
Shao K, Chen W, Li S, et al: Effect of Xin
Mai Jia formula on rat with Atherosclerosis. Lishizhen Medicine and
Materia Medica Research. 22:2480–2481. 2011.(In Chinese).
|
|
17
|
Shai SY, Sukhanov S, Higashi Y, Vaughn C,
Kelly J and Delafontaine P: Smooth muscle cell-specific
insulin-like growth factor-1 overexpression in Apoe-/- mice does
not alter atherosclerotic plaque burden but increases features of
plaque stability. Arterioscler Thromb Vasc Biol. 30:1916–1924.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Parsons KK, Chi L, Malakauskas SM
and Le TH: Glutathione S-transferase-micro1 regulates vascular
smooth muscle cell proliferation, migration, and oxidative stress.
Hypertension. 54:1360–1368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luchtefeld M, Grothusen C, Gagalick A,
Jagavelu K, Schuett H, Tietge UJ, Pabst O, Grote K, Drexler H,
Förster R and Schieffer B: Chemokine receptor 7 knockout attenuates
atherosclerotic plaque development. Circulation. 122:1621–1628.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu L, Qin L, Zhang H, He Y, Chen H, Pober
JS, Tellides G and Min W: AIP1 prevents graft arteriosclerosis by
inhibiting interferon-γ-dependent smooth muscle cell proliferation
and intimal expansion. Circ Res. 109:418–427. 2011.PubMed/NCBI
|
|
21
|
Rudijanto A: The role of vascular smooth
muscle cells on the pathogenesis of atherosclerosis. Acta Med
Indones. 39:86–93. 2007.PubMed/NCBI
|
|
22
|
Kinnear C, Chang WY, Khattak S, et al:
Modeling and rescue of the vascular phenotype of Williams-Beuren
syndrome in patient induced pluripotent stem cells. Stem Cells
Transl Med. 2:2–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheung C, Bernardo AS, Trotter MW,
Pedersen RA and Sinha S: Generation of human vascular smooth muscle
subtypes provides insight into embryological origin-dependent
disease susceptibility. Nat Biotechnol. 30:165–173. 2012.
View Article : Google Scholar
|
|
24
|
Yang G, Pei Y, Teng H, Cao Q and Wang R:
Specificity protein-1 as a critical regulator of human
cystathionine gamma-lyase in smooth muscle cells. J Biol Chem.
286:26450–26460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O’Sullivan JF, Martin K and Caplice NM:
Microribonucleic acids for prevention of plaque rupture and
in-stent restenosis: ‘a finger in the dam’. J Am Coll Cardiol.
57:383–389. 2011.PubMed/NCBI
|
|
26
|
Daniel JM and Sedding DG: Circulating
smooth muscle progenitor cells in arterial remodeling. J Mol Cell
Cardiol. 50:273–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou MT, Chang SN, Ke C, et al: The
proliferation and differentiation of placental-derived multipotent
cells into smooth muscle cells on fibrillar collagen. Biomaterials.
31:4367–4375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marsboom G and Archer SL: Pathways of
proliferation: new targets to inhibit the growth of vascular smooth
muscle cells. Circ Res. 103:1047–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Villiers JA, Houreld N and Abrahamse H:
Adipose derived stem cells and smooth muscle cells: implications
for regenerative medicine. Stem Cell Rev. 5:256–265.
2009.PubMed/NCBI
|
|
30
|
Jacob T, Clouden N, Hingorani A and Ascher
E: The effect of cotinine on telomerase activity in human vascular
smooth muscle cells. J Cardiovasc Surg (Torino). 50:345–349.
2009.PubMed/NCBI
|
|
31
|
Acilan C, Serhatli M, Kacar O, Adiguzel Z,
Tuncer A, Hayran M and Baysal K: Smooth muscle cells isolated from
thoracic aortic aneurysms exhibit increased genomic damage, but
similar tendency for apoptosis. DNA Cell Biol. 31:1523–1534. 2012.
View Article : Google Scholar
|
|
32
|
Fukai T and Ushio-Fukai M: Superoxide
dismutases: role in redox signaling, vascular function, and
diseases. Antioxid Redox Signal. 15:1583–1606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kazama K, Usui T, Okada M, Hara Y and
Yamawaki H: Omentin plays an anti-inflammatory role through
inhibition of TNF-α-induced superoxide production in vascular
smooth muscle cells. Eur J Pharmacol. 686:116–123. 2012.PubMed/NCBI
|