Introduction
Natural dietary compounds have been widely and
safely consumed for centuries and have potential applications in
pharmacology and cancer therapy (1). Fucoidan is a naturally occurring
polysaccharide compound present in brown algae, including Fucus
vesiculosus, Cladosiphon okamuranus and Laminaria
saccharina(2,3). Numerous intensive studies have
identified its biological activities, including antioxidative,
immunomodulatory, antiviral, antithrombotic and anticoagulant
effects (4–6). In addition, a number of studies
support that the use of fucoidan as a supplement provides
protection against various cancers (7–9).
However, the anticancer effects of fucoidan in mucoepidermoid
carcinoma (MEC) cells have yet to be studied.
Mitogen-activated protein kinases (MAPKs) are
involved in cellular proliferation, differentiation and apoptosis
(10), and the dynamic balance
between extracellular signal-regulated kinase (ERK), c-Jun
NH2-terminal kinase (JNK) and p38 MAPK contributes to
the determination of cell fate (11). Previous studies have also
demonstrated that MAPKs have essential roles in modulating the
function of mitochondrial pro- and anti-apoptotic proteins
(12,13). Myeloid cell leukemia-1 (Mcl-1), an
anti-apoptotic member of the Bcl-2 family, has a pivotal role in
protecting cells against apoptosis and is overexpressed in various
human cancers (14). It is also
important in cell survival regulatory pathways, suggesting the
vital role of Mcl-1 in the regulation of apoptosis (15). Thus, MAPKs and Mcl-1 may be
potential molecular targets for apoptotic cell death in cancer
cells.
In the present study, the effects of fucoidan and
its molecular mechanisms in the MC3 MEC cell line were
investigated.
Materials and methods
Reagents
Fucoidan (from Fucus vesiculosus) and
4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma (St.
Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), 100× antibiotic solution, trypsin and
D-phosphate-buffered saline (PBS) were obtained from WelGENE Inc.
(Daegu, Republic of Korea). The poly (ADP-ribose) polymerase
antibody was obtained from BD Biosciences (San Diego, CA, USA).
Actin antibody was purchased from Santa Cruz Biotechnology Inc.
(Santa Cruz, CA, USA). Antibodies for phospho-ERK, total ERK,
phospho-JNK, total JNK, phospho-p38, total p38, Mcl-1, cleaved
caspase-3 and cleaved poly ADP ribose polymerase (PARP) were
purchased from Cell Signaling Technology, Inc. (Denver, MA, USA).
The pan caspase inhibitor, z-VAD, was obtained from R&D Systems
(Minneapolis, MN, USA).
Cell culture and chemical treatments
MC3 MEC cells were obtained from Professor Wu
Junzheng (Fourth Military Medical University, Xi'an, China). Cells
were cultured in DMEM supplemented with 10% FBS and 100 U/ml each
of penicillin and streptomycin in a humidified atmosphere of 5%
CO2 at 37ºC. An equal number of cells were seeded and
allowed to attach to the well plate. The cells were pretreated with
a pan caspase inhibitor, z-VAD (10 μM) 1 hr before fucoidan
treatment. When the cells reached 50–60% confluence, they were
treated with fucoidan (25, 50 and 100 μg/ml) dissolved in 0.1%
dimethyl sulfoxide (DMSO; vehicle control).
Cell proliferation assay
Cell proliferation was determined by cell counting
using a Neubauer's chamber (hemocytometer, Neubauer dual count
chamber; Thermo Fisher Scientific Inc., Waltham, MA, USA). MC3 MEC
cells were exposed to DMSO or fucoidan for 48 h. Following the
period of exposure, cell were stained with trypan blue (0.04%) and
then counted. Each experiment was carried out in triplicate and the
results are expressed as the mean ± standard deviation.
DAPI staining
The apoptotic effects of fucoidan on MC3 MEC cells
were measured using a fluorescent nuclear dye, DAPI. MC3 MEC cells
were seeded and treated with varied concentrations (25, 50 and 100
μg/ml) of fucoidan, harvested by trypsinization and resuspended in
PBS. The cells were fixed in 100% methanol at room temperature (RT)
for 10 min, deposited on slides and then stained with DAPI solution
(2 mg/ml). The DAPI-stained cell morphology was observed under a
fluorescence microscope (Microscope Axio Imager. M2; Carl Zeiss Co.
Ltd., Seoul, Korea).
Western blot analysis
Whole cell lysates were extracted with lysis buffer
and protein concentrations were measured using a DC Protein Assay
(Bio-Rad, Hercules, CA, USA). Samples containing equal
concentrations of protein were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred to
Immun-Blot™ polyvinylidene fluoride membranes (Bio-Rad). The
membranes were blocked with 5% skimmed milk in Tris-buffered saline
with Tween for 1 h 30 min at RT and maintained overnight at 4ºC
with primary antibodies. Membranes were then incubated with
horseradish peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology Inc.) at RT for 1 h 30 min. Antibody-bound proteins
were detected using enhanced chemiluminescence (ECL) western
blotting luminol reagent (Santa Cruz Biotechnology Inc.).
Statistical analysis
Data were assessed for statistical significance
using a Student's t-test. P<0.05 compared to that of the vehicle
control was considered to indicate a statistically significant
difference.
Results
Fucoidan inhibits cell proliferation and
induces apoptosis in MC3 MEC cells
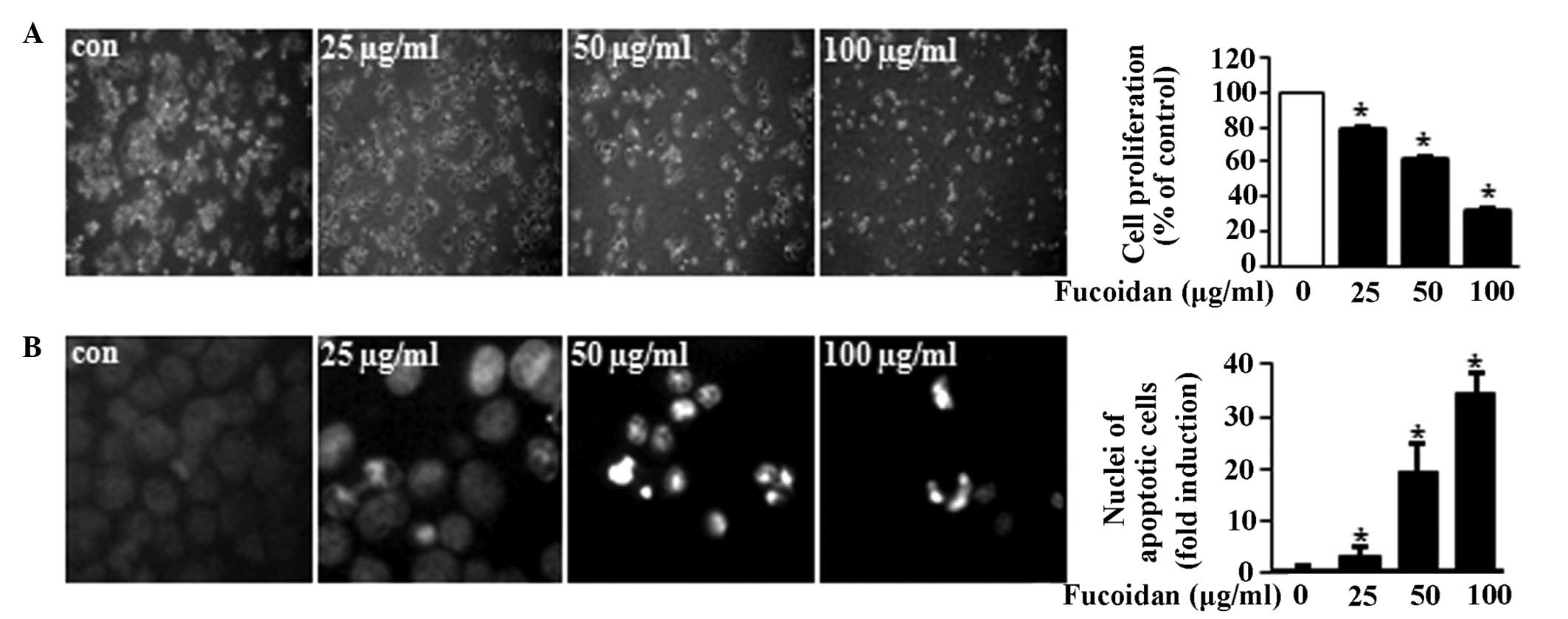
To investigate the anticancer effects of fucoidan,
the growth-inhibitory effects of fucoidan in the MC3 MEC cell line
were first assessed. Cells were treated with DMSO or fucoidan (25,
50 and 100 μg/ml) for 48 h. The results demonstrated that fucoidan
induced morphological changes of the MC3 MEC cells and the
proliferation of the cells was significantly reduced in a
concentration-dependent manner (Fig.
1A). Then, whether the growth-inhibitory effects of fucoidan
were associated with apoptotic cell death was investigated. As
shown in Fig. 1B, cells treated
with fucoidan exhibited nuclear fragmentation and chromatin
condensation in a concentration-dependent manner. The results
demonstrated that fucoidan inhibited cell growth and induced
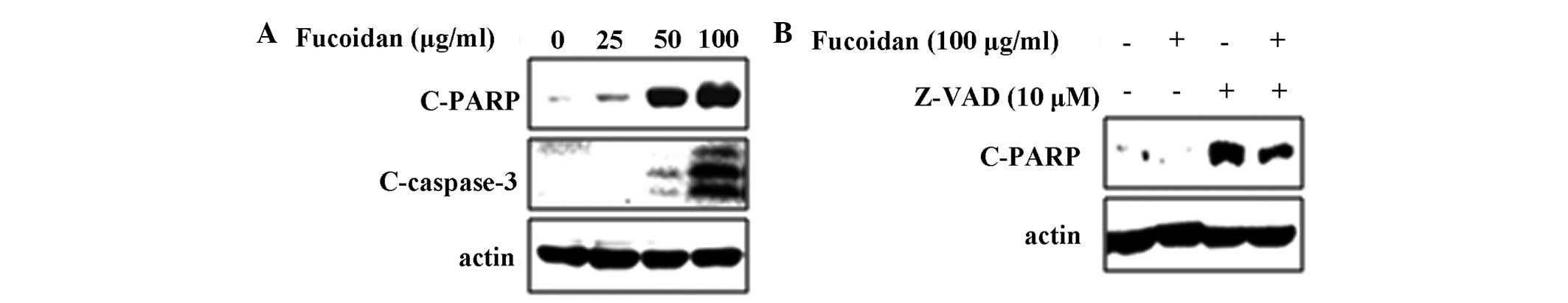
apoptosis in MC3 MEC cells. The apoptotic activity of fucoidan was
then determined by evaluating the levels of PARP cleavage and the
activation of caspase 3. As shown in Fig. 2A, fucoidan-treated MC3 MEC cells
demonstrated increased cleavage of PARP and caspase-3. To
investigate the involvement of caspase 3 in fucoidan-induced
apoptosis, a pan caspase inhibitor, z-VAD, was used. The results
showed that the cleavage of PARP induced by fucoidan was partially
blocked in the presence of z-VAD, suggesting that fucoidan-induced
apoptosis is mediated by caspase activation (Fig. 2B).
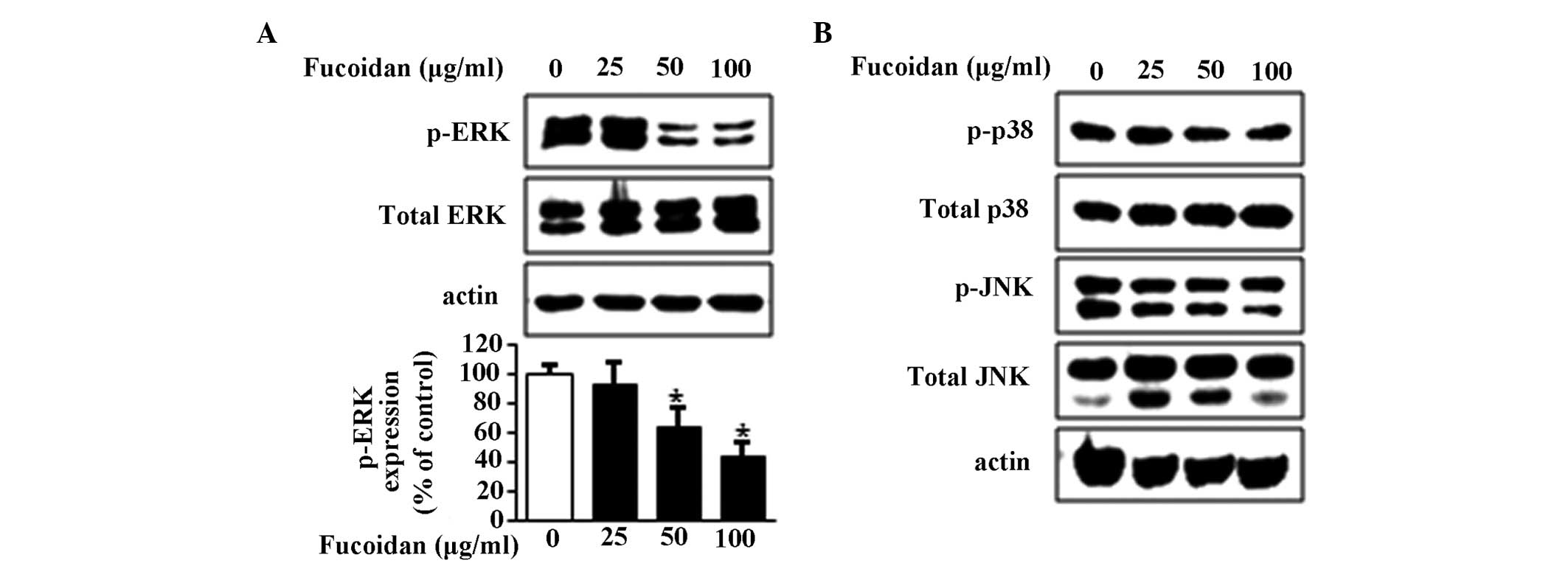
Fucoidan decreases phosphorylation of
ERK1/2 but does not change phospho-p38 and phospho-JNK levels in
MC3 MEC cells
The MAPK family is positively associated with
apoptotic cell death (16,17) and the MAPK signaling pathway is
frequently dysregulated in neoplastic transformation (18). It has also been indicated that
activation of the ERK1/2 pathway is commonly associated with
survival; by contrast, the JNK1/2 and p38 MAPK pathway is
associated with apoptosis (19).
In the present study, the effects of fucoidan on the
phosphorylation of ERK1/2, p-38 and JNK were examined, and the
results showed that fucoidan downregulated the phosphorylation of
ERK1/2 in a concentration-dependent manner (Fig. 3A), but did not alter the
phosphorylation or total expression levels of p38 and JNK (Fig. 3B). Therefore, ERK1/2 may be
important in fucoidan-induced apoptosis.
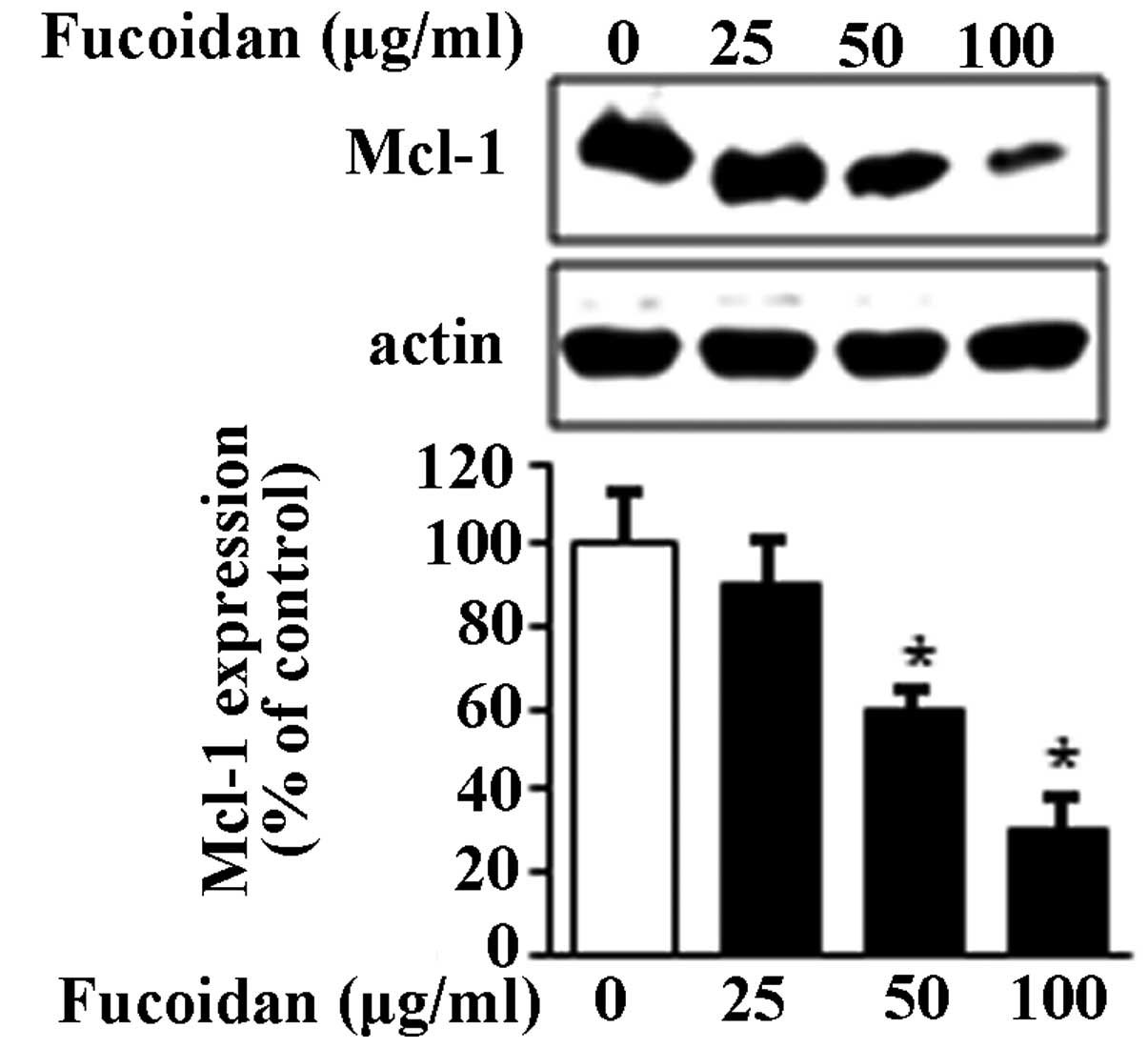
Fucoidan downregulates Mcl-1, a
downstream target of ERK1/2
A number of anti-apoptotic effector proteins have
been identified downstream of ERK1/2 signaling, including Mcl-1
(20,21). Thus, whether fucoidan treatment
affects the Mcl-1 protein in MC3 cells was investigated using
western blot analysis. The results showed that the expression of
Mcl-1 protein significantly decreased with fucoidan treatment in a
concentration-dependent manner (Fig.
4). These results indicated that the expression of Mcl-1 may be
regulated by the ERK1/2 pathway and subsequently induce apoptosis
in MC3 MEC cells.
Discussion
Fucoidan is a potent inducer of apoptosis in various
cancer cell lines (5,22). A previous study has shown that
fucoidan induced extrinsic or intrinsic apoptotic signals in
different cancer cell types via the altered expression or
activities of mitochondria-associated proteins, cell cycle
regulatory proteins, proteases and transcription factors (9). However, the molecular mechanisms by
which fucoidan initiates apoptosis in MC3 MEC cells have not been
characterized. In the present study, the aim was to investigate the
in vitro anti-cancer effects of fucoidan in MC3 MEC cells.
The results demonstrated that fucoidan inhibited cell growth and
induced apoptosis in MC3 MEC cells, which was indicated by
decreased cell proliferation, nuclear fragmentation, chromatin
condensation, cleaved PARP and activated caspase 3. In addition, a
pan caspase inhibitor, z-VAD, blocked fucoidan-induced apoptosis
suggesting that this effect is caspase-dependent.
MAPK family members appear to be important in the
regulation of cell survival (23).
For example, ERK1/2 activation has been shown to promote the
anti-apoptotic functions of Bcl-2 and cell survival in neuronal
PC12 cells, whereas the activation of JNKs results in cell death
via the apoptotic signaling pathway (24). Previous studies have identified
that fucoidan induces apoptotic cell death by activating the ERK1/2
pathway (25–27); however, additional studies have
also demonstrated that fucoidan is able to inactivate the ERK
pathway for apoptosis (1,9,28).
This suggests that the role of the ERK pathway in fucoidan-induced
apoptosis remains controversial. Therefore, in the present study,
the effects of fucoidan on the ERK1/2 signaling pathway were
investigated. The results showed a concentration-dependent
suppression of ERK1/2 phosphorylation. To eliminate the involvement
of additional MAPK family members, such as JNK and p38, their
expression levels were also evaluated. The results indicated that
they were not altered by fucoidan, suggesting that the inactivation
of ERK1/2 by fucoidan results in the induction of apoptosis.
The ERK pathway promotes cancer cell survival
through inhibition of the apoptotic cascade by controlling the
expression or activity of Bcl-2 family members (28,29).
The fact that the Bcl-2 family and the ERK signaling pathway were
both implicated in the control of cell survival suggests that
ERK-stimulated enhancement of cell survival may be mediated through
its effects on the expression of Bcl-2 or other Bcl-2 family
members (28). Mcl-1 is an
anti-apoptotic protein that is highly expressed in malignant tumors
and has been implicated in resistance to chemotherapy (30). It has also been identified that ERK
is an important regulator of Mcl-1 stability (31). Thus, in the present study, the
effects of fucoidan on Mcl-1 were investigated, and the results
showed that Mcl-1 expression was reduced by fucoidan in a
concentration-dependent manner. These results suggest that fucoidan
may have induced apoptosis through inactivation of the ERK pathway
and the inhibition of Mcl-1.
In conclusion, to the best of our knowledge, this
study demonstrated for the first time that fucoidan is able to
induce apoptotic cell death in MC3 human MEC cells and this is
associated with concentration-dependent inactivation of the ERK1/2
pathway to regulate Mcl-1 protein. These results suggest that
fucoidan may be a promising dietary compound for the treatment of
MEC.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
no. 2012003731) and research funds of Chonbuk National University,
2013.
References
|
1
|
Zhang Z, Teruya K, Yoshida T, Eto H and
Shirahata S: Fucoidan extract enhances the anti-cancer activity of
chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer
cells. Mar Drugs. 11:81–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cumashi A, Ushakova NA, Preobrazhenskaya
ME, et al: A comparative study of the anti-inflammatory,
anticoagulant, antiangiogenic, and antiadhesive activities of nine
different fucoidans from brown seaweeds. Glycobiology. 17:541–552.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye J, Li Y, Teruya K, et al:
Enzyme-digested fucoidan extracts derived from seaweed Mozuku of
Cladosiphon novae-caledoniae kylin inhibit invasion and
angiogenesis of tumor cells. Cytotechnology. 47:117–126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Zhang Q, Zhang Z and Li Z:
Antioxidant activity of sulfated polysaccharide fractions extracted
from Laminaria japonica. Int J Biol Macromol. 42:127–132.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alekseyenko TV, Zhanayeva SY, Venediktova
AA, et al: Antitumor and antimetastatic activity of fucoidan, a
sulfated polysaccharide isolated from the Okhotsk Sea Fucus
evanescens brown alga. Bull Exp Biol Med. 143:730–732. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maruyama H, Tamauchi H, Iizuka M and
Nakano T: The role of NK cells in antitumor activity of dietary
fucoidan from Undaria pinnatifida sporophylls (Mekabu).
Planta Med. 72:1415–1417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Teruya K, Eto H and Shirahata S:
Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism
involving the ROS-dependent JNK activation and
mitochondria-mediated pathways. PLoS One. 6:e274412011. View Article : Google Scholar
|
|
8
|
Koyanagi S, Tanigawa N, Nakagawa H, Soeda
S and Shimeno H: Oversulfation of fucoidan enhances its
anti-angiogenic and antitumor activities. Biochem Pharmacol.
65:173–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aisa Y, Miyakawa Y, Nakazato T, et al:
Fucoidan induces apoptosis of human HS-sultan cells accompanied by
activation of caspase-3 and down-regulation of ERK pathways. Am J
Hematol. 78:7–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schroeter H, Boyd CS, Ahmed R, et al:
c-Jun N-terminal kinase (JNK)-mediated modulation of brain
mitochondria function: new target proteins for JNK signalling in
mitochondrion-dependent apoptosis. Biochem J. 372:359–369. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aoki H, Kang PM, Hampe J, et al: Direct
activation of mitochondrial apoptosis machinery by c-Jun N-terminal
kinase in adult cardiac myocytes. J Biol Chem. 277:10244–10250.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun NK, Huang SL, Chang TC and Chao CC:
Sorafenib induces endometrial carcinoma apoptosis by inhibiting
Elk-1-dependent Mcl-1 transcription and inducing
Akt/GSK3β-dependent protein degradation. J Cell Biochem.
114:1819–1831. 2013.PubMed/NCBI
|
|
15
|
Yang-Yen HF: Mcl-1: a highly regulated
cell death and survival controller. J Biomed Sci. 13:201–204. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death - apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aquilano K, Baldelli S, Rotilio G and
Ciriolo MR: trans-Resveratrol inhibits
H2O2-induced adenocarcinoma gastric cells
proliferation via inactivation of MEK1/2-ERK1/2-c-Jun signalling
axis. Biochem Pharmacol. 77:337–347. 2009.PubMed/NCBI
|
|
19
|
Rahman MS, Yamasaki A, Yang J, Shan L,
Halayko AJ and Gounni AS: IL-17A induces eotaxin-1/CC chemokine
ligand 11 expression in human airway smooth muscle cells: role of
MAPK (Erk1/2, JNK, and p38) pathways. J Immunol. 177:4064–4071.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sawatzky DA, Willoughby DA, Colville-Nash
PR and Rossi AG: The involvement of the apoptosis-modulating
proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute
inflammation in vivo. Am J Pathol. 168:33–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun HL, Tsai AC, Pan SL, et al: EPOX
inhibits angiogenesis by degradation of Mcl-1 through ERK
inactivation. Clin Cancer Res. 15:4904–4914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Philchenkov A, Zavelevich M, Imbs T,
Zvyagintseva T and Zaporozhets T: Sensitization of human malignant
lymphoid cells to etoposide by fucoidan, a brown seaweed
polysaccharide. Exp Oncol. 29:181–185. 2007.PubMed/NCBI
|
|
23
|
Párrizas M, Saltiel AR and LeRoith D:
Insulin-like growth factor 1 inhibits apoptosis using the
phosphatidylinositol 3′-kinase and mitogen-activated protein kinase
pathways. J Biol Chem. 272:154–161. 1997.
|
|
24
|
Anderson P: Kinase cascades regulating
entry into apoptosis. Microbiol Mol Biol Rev. 61:33–46.
1997.PubMed/NCBI
|
|
25
|
Jin JO, Song MG, Kim YN, Park JI and Kwak
JY: The mechanism of fucoidan-induced apoptosis in leukemic cells:
involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol
Carcinog. 49:771–782. 2010.PubMed/NCBI
|
|
26
|
Hyun JH, Kim SC, Kang JI, et al: Apoptosis
inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol
Pharm Bull. 32:1760–1764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boo HJ, Hyun JH, Kim SC, et al: Fucoidan
from Undaria pinnatifida induces apoptosis in A549 human
lung carcinoma cells. Phytother Res. 25:1082–1086. 2011.
|
|
28
|
Boucher MJ, Morisset J, Vachon PH, Reed
JC, Lainé J and Rivard N: MEK/ERK signaling pathway regulates the
expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of
human pancreatic cancer cells. J Cell Biochem. 79:355–369. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar
|
|
30
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Domina AM, Vrana JA, Gregory MA, Hann SR
and Craig RW: MCL1 is phosphorylated in the PEST region and
stabilized upon ERK activation in viable cells, and at additional
sites with cytotoxic okadaic acid or taxol. Oncogene. 23:5301–5315.
2004. View Article : Google Scholar : PubMed/NCBI
|