Introduction
Spinal cord injury (SCI) occurs predominantly in
young people as a result of traffic or sports-related accidents and
leads to severe neurological deficits, such as paraplegia and
quadriplegia. SCI is usually accompanied by the formation of cystic
cavities surrounded by glial scars, which severely impede the
regeneration of severed axons (1).
The poor recovery of the central nervous system, a delicate tissue
that is unable to tolerate toxic conditions, is generally
attributed to the hostile local environment created at the trauma
site. Two major barriers to repair that have been identified
include the local inflammatory response, acknowledged for its
neurotoxic potential, and the creation of glial scars, which has
been demonstrated to impair regeneration (1–3).
Glial scars are composed of extracellular matrices and various
types of cells; astrocytes, in particular, are important in glial
scar formation. Astrocytes upregulate the expression of glial
fibrillary acidic protein (GFAP) (4,5), as
well as re-expressing vimentin (Vim) and secreting a number of
extracellular matrix proteins following SCI. In particular,
chondroitin sulfate proteoglycans (CSPGs) are considered to be the
primary component of extracellular matrix proteins (6–8).
Although reactive astrocytes may exert a number of beneficial
effects by regulating local immune responses and promoting tissue
repair (9,10), results from numerous studies have
indicated that glial scarring is one of the major factors hindering
spontaneous axonal regeneration, and that the suppression of glial
scarring may reduce tissue damage and improve
morphological/functional recovery (11–14).
For these reasons, interventions designed to attenuate astrogliosis
represent valuable therapeutic agents for the management of
SCI.
The activation of extracellular signal-regulated
kinase (ERK) by mitogen-activated protein kinase (MAPK)/ERK (MEK)
through phosphorylation is an important step in signal transduction
for cell processes that transfer chemical signals from the cell
surface to the nucleus (15,16).
It has been demonstrated that the inhibition of the ERK signaling
pathway provided neuroprotection in cell models of mechanical
trauma (17). Furthermore, Lu
et al(18) revealed that in
a rat model of spinal cord injury, MEK/ERK inhibition reduced
microglial activation, cytokine production and inflammatory cell
infiltration. The MEK inhibitor U0126 inhibited ERK phosphorylation
and the migration of astrocytes across a wound, and the migration
of human astrocytes following injury has been indicated to be
partly initiated by the activation of the MEK/ERK signaling pathway
(19). Inhibiting ERK
phosphorylation with U0126 has been shown to significantly
attenuate apoptotic neuronal loss and improve neurological function
(20). The MEK/ERK signal
transduction pathway may be critical in the formation of the
microenvironment in SCI. However, it has not been fully
investigated whether inhibitors of MEK are able to regulate glial
scar formation and improve functional recovery following SCI.
In the present study, the ability of an inhibitor of
MEK1/2 to affect astrocytic scar formation following SCI was
examined. The results in this study indicated that U0126 inhibited
astrocyte proliferation, as well as the expression of CSPGs, in
vivo. In addition, an improvement in hindlimb motor function
was observed. Therefore, this study demonstrated a therapeutic
potential for inhibitors of MEK in the regulation of glial scar
formation and the promotion of axonal regeneration and functional
recovery following SCI.
Materials and methods
Animals and surgical procedures
All experimental procedures were performed in
accordance with protocols approved by the Governmental Animal Care
Committee of the Medical College of Xiamen University (Zhangzhou,
China) and conformed to the National Institute of Health guidelines
on the ethical use of animals. Prior to surgery, the animals were
anesthetized with chloral hydrate (400 mg/kg,
intraperitoneally; Beyotime Institute of Biotechnology, Haimen,
China). During surgery, the rats were placed on a warming pad to
maintain a body temperature of 37.0±0.5°C. Following injury, the
animals were returned to individual cages with sufficient water and
food and were then treated with an intramuscular injection of
penicillin (The 175th Hospital of PLA, Zhangzhou, China) at a dose
of 200,000 U/day, for three days.
Adult female Sprague Dawley rats (weight, 240–260 g)
were randomly assigned into three experimental groups: sham injury
(group I, n=30), SCI (group II, n=30) and U0126 treatment (group
III, n=30). U0126 was obtained from Beyotime Institute of
Biotechnology. The rats were obtained from Experimental Animal
Center of Xiamen University. The traumatic SCI model was
established by the weight-drop technique, as described in a
previous study (21). Briefly,
following anesthetization, a T12 spinal laminectomy was conducted
to expose the spinal cord and a moderate-intensity weight-drop (10
g × 7.0 cm) was performed using an impactor with a diameter of 2.5
mm (Xiamen University). The rats in the sham surgery group
underwent a similar experimental procedure to that used for SCI
induction, with the exception of the weight-drop step; saline was
administered to rats in the sham injury and SCI groups through
pumps, as described in the following section.
Bladders were manually pressed twice daily until
spontaneous voiding occurred and food and water were freely
accessible at a lowered height in the cages. U0126 was administered
to the rats in group III (n=30) at a dose of 20 μg/day by
intraperitoneal (ip) injection within 1 h subsequent to SCI until 9
days post-injury. The experimental control rats (group II, n=30)
were treated with ip injections of saline instead of U0126. The
normal control rats without SCI (group I, n=30) were maintained
throughout the experiment.
Behavioral assessments
The rats were tested for locomotor deficits at 1, 3,
5, 7 and 14 days (n=30, per group) and 21 and 28 days (n=15, per
group) subsequent to SCI with the open field locomotor test,
developed by Basso et al(22). This Basso, Beattie and Bresnahan
(BBB) locomotor rating scale was conducted by two observers, who
were blinded to the experimental procedures of each rat. The BBB
rating scale is a 21-point system based on operationally defined
behavioral features, which follow the recovery progression from
complete paralysis to normal locomotion. The rating scale ranges
from 0 to 21, with a score of 0 indicating complete hind limb
paralysis and a score of 21 denoting completely normal locomotor
function.
Somatosensory-evoked potentials
(SEPs)
The rats were anesthetized and 1 min later an
incision was made along the midline of the back. The cranium bone
was cleaned by removing the tissue under the skin. A standard
dental drill was used to drill five burr holes into the exposed
area of the cranium. Four holes were located on the somatosensory
cortex corresponding to the hind and forelimbs in each hemisphere.
On each hemisphere, the forelimb recording sites were located 0.2
mm posterior to the bregma and 3.8 mm laterally from the bregma,
and the hindlimb recording sites were located 2.5 mm posterior to
the bregma, and 2.8 mm laterally from the bregma. A fifth hole,
drilled on the right frontal bone and situated 2 mm from the
sagittal and coronal sutures, served as the intracranial reference.
Transcranial screw electrodes were then screwed into the holes,
such that they made very light contact with the dura mater. The
distal end of each electrode was inserted into one of the slots of
an electrode pedestal. Sub-dermal needle electrode pairs (MedCom
Asia, Inc., Guangzhou, China) were used to electrically stimulate
the tibial nerves of the left and right hind limbs. An isolated
constant current stimulator (Shanghai Haishen Medical Electronic
Instrument Co., Ltd., Shanghai, China) was used for the electrical
stimulation of the limbs. A Microsoft Windows-based personal
computer was interfaced with the stimulator and a neurological
monitoring system (Model M-800; MedCom Asia, Inc.) was used to set
the stimulation parameters and trigger the stimulator. The values
of P1 latency, N1 latency and P1-N1 amplitude were collected one
day prior to surgery, on the day of surgery and 14 and 28 days
postoperatively, and were subject to statistical comparisons.
Tissue processing, staining and
histopathology
On days 14 and 28 post-injury, three rats from each
group were sacrificed for immunohistological staining. Sections of
the spinal cords encompassing the injured sites were dissected and
fixed by immersion in 4% formaldehyde for 24 h, and cryoprotected
in 10% sucrose at 4°C until they sank. The spinal cords were then
embedded in optimum cutting temperature (OCT) compound (Beyotime
Institute of Biotechnology), frozen and cut into 3-μm cryostat
sections in the horizontal plane. The tissue sections were stained
with a Hematoxylin and Eosin (H&E) Staining kit (Beyotime
Institute of Biotechnology) to assess the morphology of the injury
site. Eight to ten sections were stained for GFAP or Vim to
identify reactive astrocytes. The immunohistochemical staining was
performed using a Histonstain®-Plus kit (Invitrogen Life
Technologies, Carlsbad, CA, USA), in accordance with the
manufacturer’s instructions. The images were captured using a FV
300 confocal microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed with the
Statistical Package for Social Sciences (SPSS) version 13.0 for
Windows (SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U test was
used to compare the BBB score and positive cell count, and SEP was
analyzed using one-way analysis of variance (ANOVA). P<0.05 was
considered to indicate a statistically significant difference. Data
are presented as the mean ± standard error of the mean (SEM).
Results
Behavioral test
The BBB locomotor scale was used to evaluate all
rats prior to the electrophysiological evaluation. Table I shows the mean BBB scores for the
three groups over the time-course of the experiment. All rats were
healthy prior to surgery and exhibited BBB scores of 21 (data not
shown). In the sham injury group, there was no significant
difference between the hindlimb movement scores prior to and
following SCI and the movement appeared normal throughout the
observation period (BBB 21 points). The SCI and U0126-treated
groups showed motor function improvements one day subsequent to
injury, although the U0126-treated group exhibited better total
recovery than the control group by day 28, scoring an average of
16.50±1.08 points. The control group scored 12.00±1.70 points on
day 28 (P<0.05). A score of 10 indicated only occasional
weight-supported plantar steps and no front-hind limb coordination,
while a score of 14 meant consistent weight-supported plantar steps
and consistent front-hind limb coordination (22). Thus, it was noteworthy that the
U0126 group, and not the control group, exceeded this
threshold.
 | Table IBBB score results of each group at
different times. |
Table I
BBB score results of each group at
different times.
| Time-point | Group I (score) | Group II (score) | Group III
(score) |
|---|
| Day of injury
(n=12) | 21.00±0.00 | 00.00±0.00a | 00.00±0.00a |
| 1 day post-injury
(n=12) | 21.00±0.00 | 1.65±0.67a | 1.45±0.89a |
| 3 days post-injury
(n=12) | 21.00±0.00 | 3.50±0.69a | 3.45±0.69a |
| 5 days post-injury
(n=12) | 21.00±0.00 | 5.70±0.80a | 5.20±1.15a |
| 7 days post-injury
(n=12) | 21.00±0.00 | 7.80±0.76a | 8.15±1.04a |
| 14 days post-injury
(n=12) | 21.00±0.00 | 9.65±1.50a | 13.70±1.26a,b |
| 21 days post-injury
(n=6) | 21.00±0.00 | 10.40±1.51a | 14.10±1.52a,b |
| 28 days post-injury
(n=6) | 21.00±0.00 | 12.00±1.70a | 16.50±1.08a,b |
SEPs
Tables II and
III show the latency and
amplitude of the SEPs, respectively. The day subsequent to injury,
the mean SEP latency was observed to be notably extended, while the
amplitudes were observed to have decreased markedly in group II and
the U0126-treated group. In the U0126-treated group 14 days after
injury, the mean SEP latency had decreased and the SEP amplitude
had increased compared with the corresponding values on the day
subsequent to injury. The difference between the these two groups
was statistically significant (P<0.05). The mean SEP amplitudes
of the U0126-treated group were consistently higher than those of
group II. The difference between the two groups was revealed to be
statistically significant for the majority of the weekly recordings
(P<0.05). As Table II shows,
the SEP latencies were shorter in the U0126-treated group than in
group II and the difference between the SEP latencies of these
groups was statistically significant on days 14 and 28 post-injury
(P<0.05).
 | Table IISEP latency at different times in
each group (msec; n=15). |
Table II
SEP latency at different times in
each group (msec; n=15).
| Group | Before injury | 1 day
post-injury | 14 days
post-injury | 28 days
post-injury |
|---|
| Group I | 13.54±0.39 | 13.62±0.39 | 13.60±0.45 | 13.58±0.45 |
| Group II | 13.57±0.46 | 23.36±0.36a | 20.41±0.34a | 17.43±0.44a |
| Group III | 13.57±0.29 | 23.53±0.42a | 19.72±0.43a,b | 16.86±0.55a,b |
 | Table IIISEP amplitude at different times in
each group (μV; n=15). |
Table III
SEP amplitude at different times in
each group (μV; n=15).
| Group | Before injury | 1 day
post-injury | 14 days
post-injury | 28 days
post-injury |
|---|
| Group I | 5.98±0.22 | 6.02±0.25 | 6.02±0.42 | 5.99±0.30 |
| Group II | 5.97±0.25 | 1.71±0.28a | 3.38±0.50a | 3.79±0.41a |
| Group III | 5.96±0.23 | 1.74±0.32a | 3.84±0.33a,b | 4.19±0.11a,b |
Histological assessments
Visual study
Following injury, diffuse hyperemia and edema were
immediately observed in the dorsal region of the spinal cord in
groups II and III; at 14 and 28 days post-SCI, scar formation was
visible on the outside of the endorhachis of the lesion region and
notable conglutination with the endorhachis was apparent. In
addition, the spinal cord was atrophic, with a reduced diameter. In
group I, at 14 and 28 days post-SCI, a small amount of scar
formation was observed in the outside of the endorhachis of the
lesion region and conglutination with the endorhachis was apparent.
This may have been due to the stimulus of the surgery when opening
the vertebral plate. However, there was no evident edema in the
spinal cord and the posterior central blood vessel and the
structure of the spinal cord were clearly visible.
H&E staining
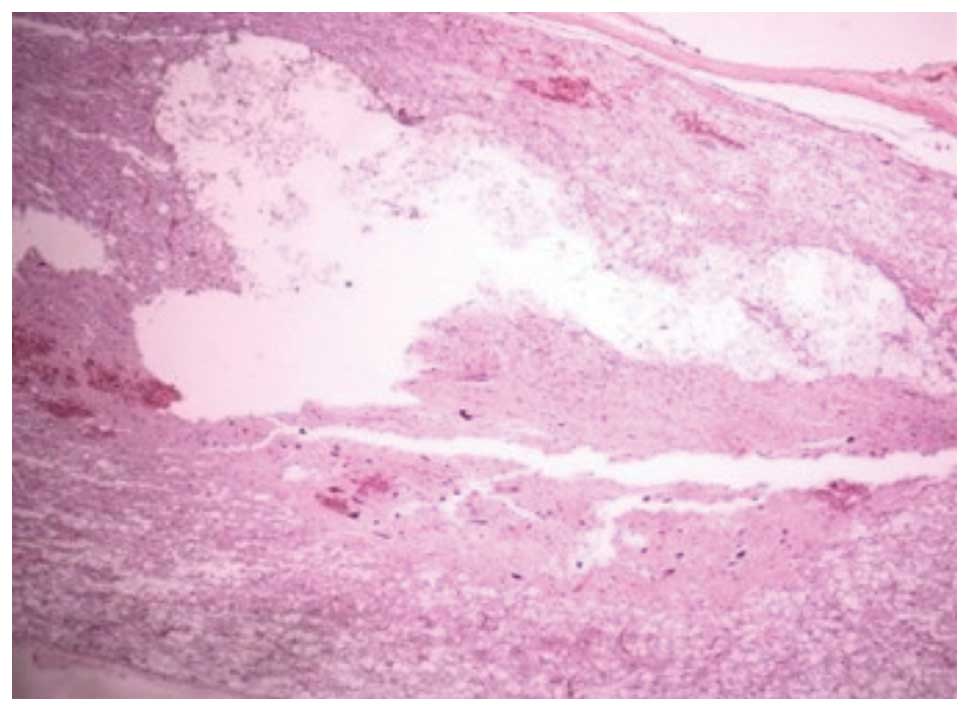
At 14 days post-SCI, H&E staining in group II
showed that a little hemorrhagic focus was apparent in the gray and
white matter of the spinal cord and that the structure of the
spinal cord was badly destroyed, with neurons dissolved and
liquefied in the gray matter. This caused a large liquefied and
necrotic area, forming a cystic space. In addition, a large number
of swollen axons and vacuoles were observed in the white matter and
the nerve fibers were disorganized. At 28 days, the hemorrhagic
focus in the gray and white matter was almost completely absorbed
and the structure of the spinal cord was destroyed further, with
neurons dissolved and liquefied in the gray matter, forming a large
number of vacuolar structures. In addition, there was a reduction
in the inflammatory infiltration, and the formation of a pyknotic
glial scar, surrounded by a large number of glial cells, was
observed (Fig. 1). At 14 days
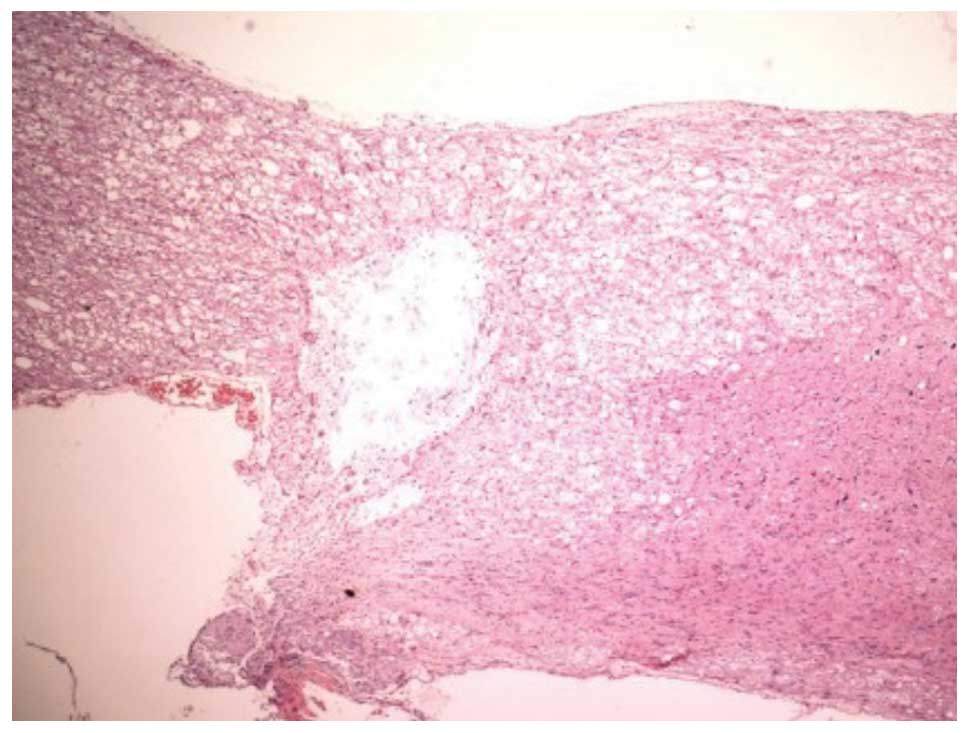
post-SCI, the H&E staining in group III also showed that the
structure of the spinal cord was destroyed, with the infiltration
of inflammatory cells, dehydration and disintegration of the
neurons, hyperplastic and hypertrophic gliocytes and the formation
of cystic space. However, the degree and area of the damage was
reduced compared with those in group II. At 28 days post-SCI, the
H&E staining in group III showed that the infiltration of
inflammatory cells was reduced and that the size of the formed scar
was smaller than that in group II (Fig. 2). The H&E staining in group I
at 14 and 28 days post-SCI showed that there were no evident
changes in most of the structure of the spinal cord; the structure
of the neurons was clearly visible, the outer limits of the gray
and white matter were marked and there were no cystic spaces.
However, a small amount of hemorrhaging was observed in the spinal
cord of a few rats and there was a slight gathering of gliocytes.
This may have been associated with a reduction in the stability of
the spine and the subsequent injury of the spinal cord for the
resection of the vertebral plate.
GFAP immunohistochemical results
The numbers of GFAP-positive cells in each group on
days 14 and 28 are listed in Table
IV. In the GFAP immunohistochemical staining, the cytoplasm of
the positive cells was brown and radially formed, spider-like
projections were observed. At 14 and 28 days post-SCI, the staining
in the sham injury group showed a small volume of positive cells,
with a relatively sparse density. The nerve structure was visible.
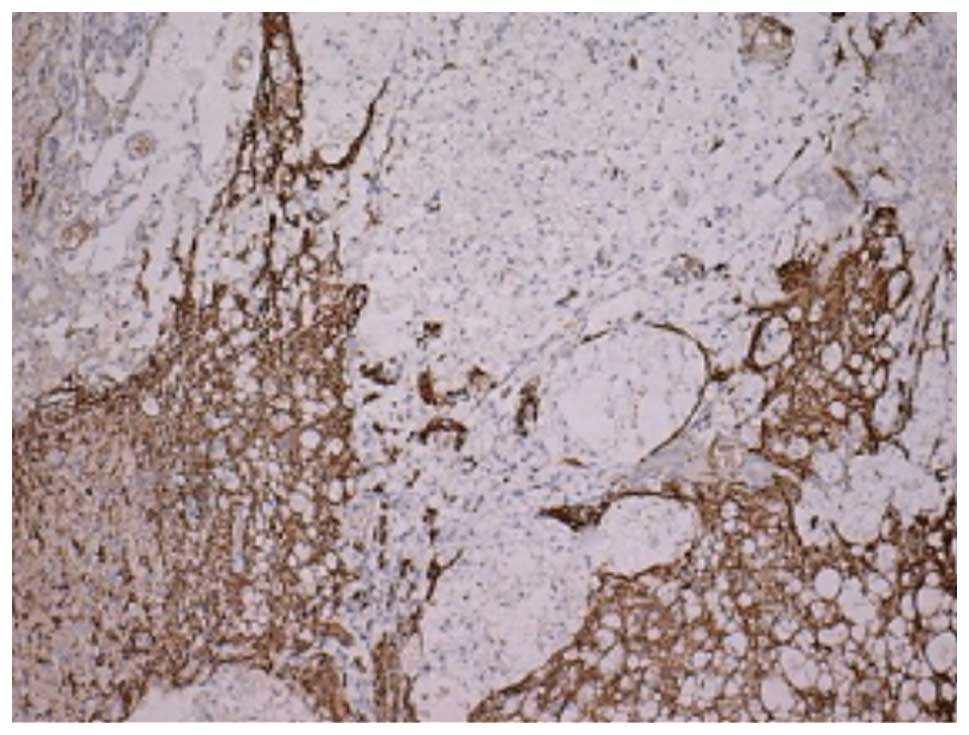
At 14 days post-SCI, the number of GFAP-positive cells in group II
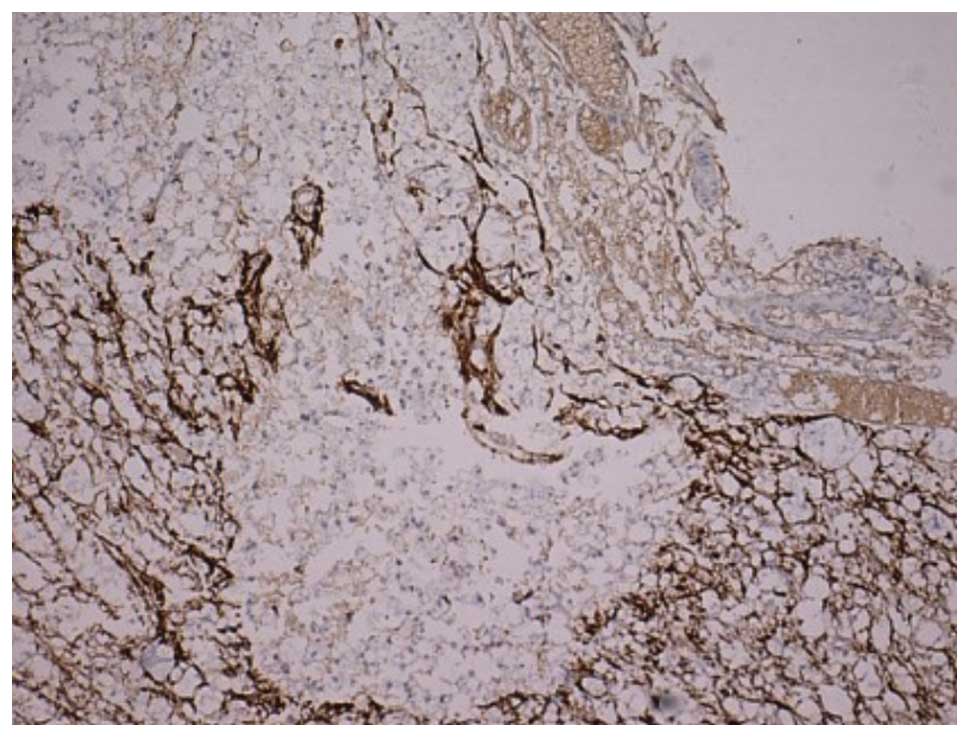
was increased. As shown in Fig. 3,
the cells were deeply stained, showing hypertrophy and neurite
extension. A number of positive cells surrounded the cystic cavity.
Astrocyte proliferation and hypertrophy were also observed near the
injury, although the extent of the proliferation was less than the
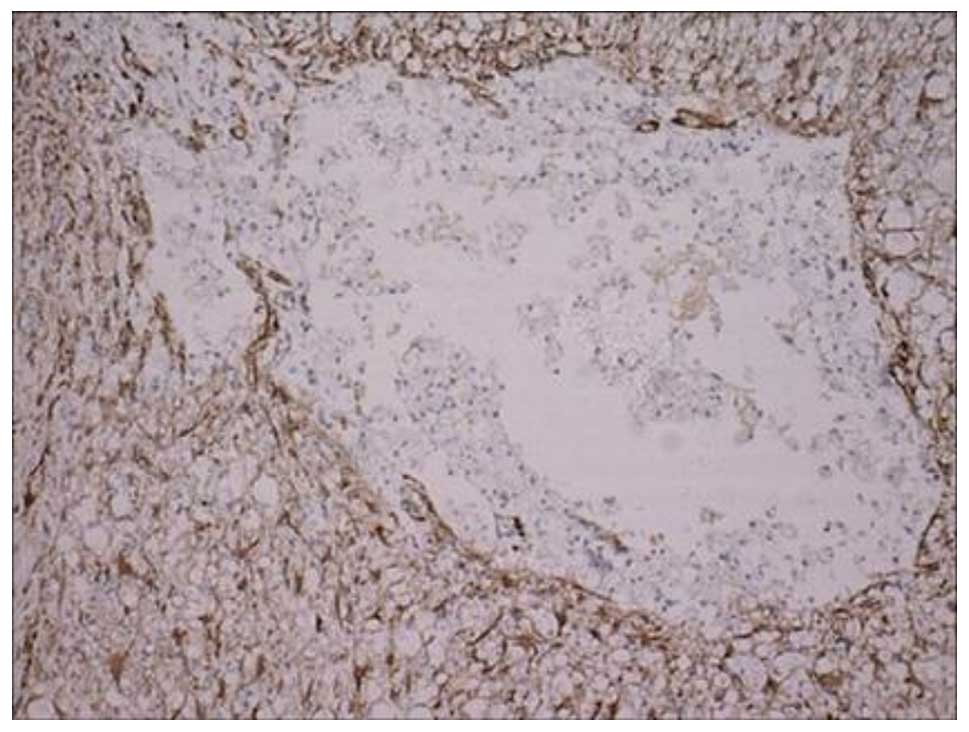
injury area. At 28 days post-SCI, the number of GFAP-positive cells
in group II had significantly reduced compared with that at 14 days
post-SCI. However, the prominence of the positive cells was thicker
and longer, woven into reticulate structure and formed a dense
glial scar (Fig. 4). As Table IV shows, there were more
GFAP-positive cells in group II than in the U0126-treated group and
the difference between the two groups was statistically significant
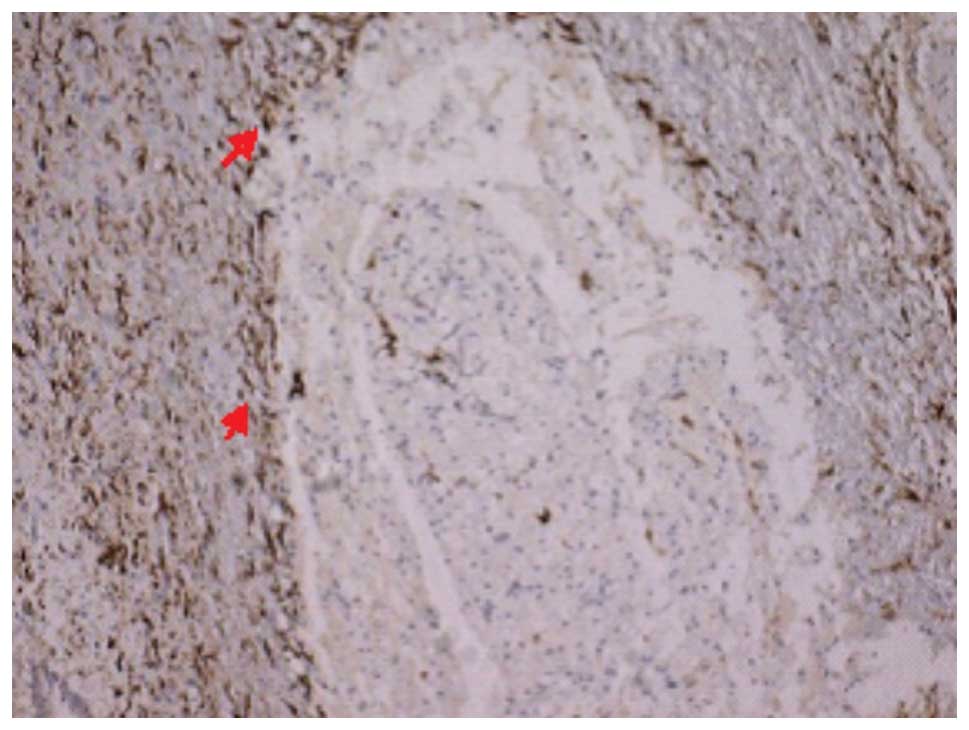
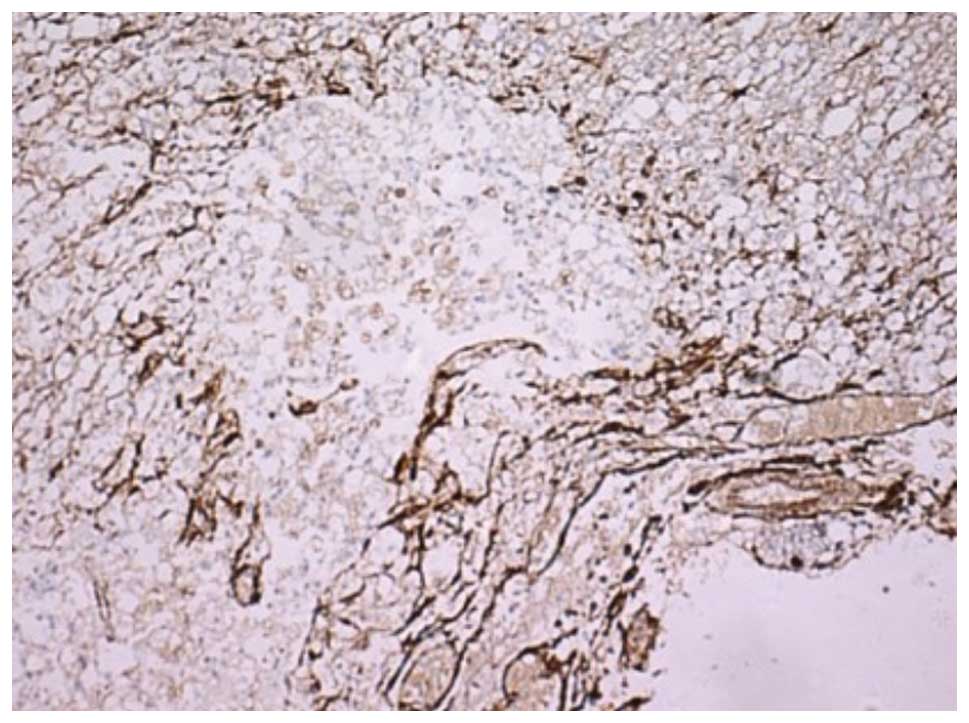
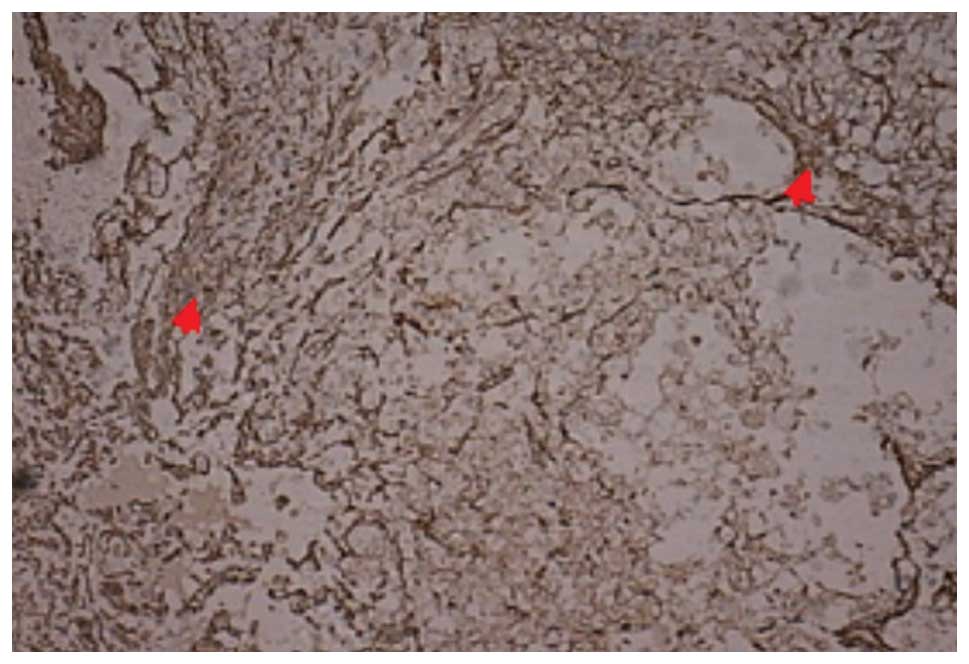
(P<0.05). As shown in Figs. 5
and 6, respectively, at 14 and 28
days post-SCI, the number of GFAP-positive cells in group III was
significantly reduced and the GFAP-positive cells became smaller,
with a reduced prominence and pale staining. In addition, the scope
of the glial scar was smaller.
 | Table IVGFAP-positive cells at different
times in each group (n=15). |
Table IV
GFAP-positive cells at different
times in each group (n=15).
| Group | 14 days post-injury
(n) | 28 days post-injury
(n) |
|---|
| Group I | 27.82±1.29 | 27.1±1.66 |
| Group II | 143.56±1.09a | 110.68±9.41a |
| Group III | 133.56±3.31a,b | 102.44±6.93a,b |
Vim immunohistochemical results
The number of the Vim-positive cells in each group
on days 14 and 28 are listed in Table
V. The Vim immunohistochemical staining revealed that there
were no positive cells in group I, and that the structural
integrity of the neurons was retained. In groups II and III, the
Vim immunohistochemical staining showed that the cytoplasm of
positive cells contained brown particles and radially formed,
spider-like projections were observed. At 14 days subsequent to
SCI, the number of Vim-positive cells in group II was increased and
a number of positive cells were observed to surround the cystic
cavity (Fig. 7). At 28 days
subsequent to SCI, the number of Vim-positive cells in group III
was significantly lower than that in group II (Fig. 8) and the difference between the two
groups was statistically significant (P<0.05).
 | Table VVim-positive cells at different times
in each group (n=15). |
Table V
Vim-positive cells at different times
in each group (n=15).
| Group | 14 days post-injury
(n) | 28 days post-injury
(n) |
|---|
| Group I | 0.00±0.00 | 0.00±0.00 |
| Group II | 93.82±4.48a | 72.96±4.16a |
| Group III | 89.32±6.50a,b | 66.44±4.46a,b |
Discussion
Traumatic SCI is a devastating ailment that leaves
the majority patients with permanent neurological deficits. At
present, the treatment for patients with SCI centers on the
surgical stabilization of the initial injury to prevent further
loss of neurological function, followed by aggressive
rehabilitation. A previous study has demonstrated the ability of
the nervous system to adapt to SCI from a functional (neuronal
plasticity) and a structural (neuronal remodeling) standpoint
(23). However, the formation of a
glial scar following SCI has been suggested to create a
microenvironment that is unfavorable for continued axonal
regeneration and neurological recovery (24,25).
Following SCI, astrocytes become hypertrophic, proliferate and show
an overexpression of GFAP and the re-expression of Vim.
Hypertrophic astrocytes are the major cellular component of the
glial scar (1). Increasingly,
studies have shown that microglial activation is one of the major
causes of secondary damage subsequent to SCI, and that the
suppression of microglial activation may reduce tissue damage and
improve morphological/functional recovery (26,27).
The data presented in this study have provided novel insights into
the unusual role of MEK/ERK signaling in astrocyte activation.
The MAPK family includes ERK, p38MAPK and c-Jun
N-terminal kinase (JNK) (28). The
initiation of the ERK/MAPK cascade involves the activation of three
kinases: Ras, Raf and MEK, and the ERK/MAPK pathway has
traditionally been considered to be important in cell proliferation
and differentiation (29–32). As mentioned previously,
phosphorylated ERK may be expressed in neurons, microglia and
astrocytes, with particularly persistent expression levels in
astrocytes (33). This study aimed
to explore whether the MEK/ERK signaling pathway participated in
the process of glial scar formation. In this study, the MEK
inhibitor U0126 was used to treat rats with SCI. Cells positive for
GFAP and Vim, two markers used to identify reactive astrocytes
(34), were detected as indicators
of astrocyte proliferation. Following SCI, it was observed that
there was an overexpression of GFAP and a re-expression of vimentin
in the SCI group compared with the sham group. However, the GFAP
and Vim expression was significantly reduced in the U0126 treatment
group, and in this group, the volume of positive cells was reduced,
the glial scar was smaller than that in the SCI group and the
positive cell density was sparser. It has been demonstrated that
the U0126 MEK inhibitor downregulates the expression of GFAP and
Vim and that it may be possible to inhibit the formation of glial
scars by reducing the proliferation of astrocytes. The results of
the present study suggested that there is potential for Vim to be
used as the indicator of gliosis following SCI, as Vim failed to be
expressed in the sham injury group. This was consistent with a
previous study (35).
It has been suggested that the MEK/ERK signaling
pathway participates in the inflammatory reaction and the process
of triggering the negative factors of SCI (18,36),
which has been considered to involve cell apoptosis (37,38).
By blocking this signaling pathway, the inflammatory reaction and
apoptotic regeneration are inhibited and neurological recovery is
stimulated. The current study demonstrates the potential benefits
of treatment with MEK inhibitors following SCI and the effect of
the treatment on the preservation of the ascending somatosensory
pathway using SEP monitoring. The results showed improvements in
SEP amplitudes lasting for several weeks. This was also accompanied
by increased motor behavioral scores and histological preservation,
indicative of neuroprotection. Importantly, early enhancements of
SEP amplitudes in the U0126-treated rats indicated that the
benefits lay in the preservation of somatosensory conductivity
following injury.
We propose that the MEK/ERK signaling pathways may
be involved in the formation of glial scars. Furthermore, this
study has underlined the potential of U0126 for improving the
functional outcome following SCI. Future studies are required to
detail the pathophysiological events that activate the ERK/MAPK
pathway and spinal cells, in order to advance the understanding of
the role of ERK phosphorylation and spinal cells in the mechanisms
underlying SCI.
References
|
1
|
Silver J and Miller JH: Regeneration
beyond the glial scar. Nat Rev Neurosci. 5:146–156. 2004.
View Article : Google Scholar
|
|
2
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shechter R, Raposo C, London A, Sagi I and
Schwartz M: The glial scar-monocyte interplay: a pivotal resolution
phase in spinal cord repair. PLoS One. 6:e279692011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferraguti F, Corti C, Valerio E, Mion S
and Xuereb J: Activated astrocytes in areas of kainate-induced
neuronal injury upregulate the expression of the metabotropic
glutamate receptors 2/3 and 5. Exp Brain Res. 137:1–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Widestrand A, Faijerson J, Wilhelmsson U,
et al: Increased neurogenesis and astrogenesis from neural
progenitor cells grafted in the hippocampus of GFAP−/−
Vim−/− mice. Stem Cells. 25:2619–2627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cafferty WB, Yang SH, Duffy PJ, Li S and
Strittmatter SM: Functional axonal regeneration through astrocytic
scar genetically modified to digest chondroitin sulfate
proteoglycans. J Neurosci. 27:2176–2185. 2007. View Article : Google Scholar
|
|
7
|
Massey JM, Hubscher CH, Wagoner MR, et al:
Chondroitinase ABC digestion of the perineuronal net promotes
functional collateral sprouting in the cuneate nucleus after
cervical spinal cord injury. J Neurosci. 26:4406–4414. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jefferson SC, Tester NJ and Howland DR:
Chondroitinase ABC promotes recovery of adaptive limb movements and
enhances axonal growth caudal to a spinal hemisection. J Neurosci.
31:5710–5720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Faulkner JR, Herrmann JE, Woo MJ, Tansey
KE, Doan NB and Sofroniew MV: Reactive astrocytes protect tissue
and preserve function after spinal cord injury. J Neurosci.
24:2143–2155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rolls A, Shechter R and Schwartz M: The
bright side of the glial scar in CNS repair. Nat Rev Neurosci.
10:235–241. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stirling DP, Khodarahmi K, Liu J, et al:
Minocycline treatment reduces delayed oligodendrocyte death,
attenuates axonal dieback, and improves functional outcome after
spinal cord injury. J Neurosci. 24:2182–2190. 2004. View Article : Google Scholar
|
|
12
|
Tian DS, Xie MJ, Yu ZY, et al: Cell cycle
inhibition attenuates microglia induced inflammatory response and
alleviates neuronal cell death after spinal cord injury in rats.
Brain Res. 1135:177–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pekny M and Nilsson M: Astrocyte
activation and reactive gliosis. Glia. 50:427–434. 2005. View Article : Google Scholar
|
|
14
|
Okada S, Nakamura M, Katoh H, et al:
Conditional ablation of Stat3 or Socs3 discloses a dual role for
reactive astrocytes after spinal cord injury. Nat Med. 12:829–834.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kolch W: Meaningful relationships: the
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J. 351:289–305. 2000. View Article : Google Scholar
|
|
16
|
Ji RR and Woolf CJ: Neuronal plasticity
and signal transduction in nociceptive neurons: implications for
the initiation and maintenance of pathological pain. Neurobiol Dis.
8:1–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori T, Wang X, Jung JC, et al:
Mitogen-activated protein kinase inhibition in traumatic brain
injury: in vitro and in vivo effects. J Cereb Blood Flow Metab.
22:444–452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu K, Cho CL, Liang CL, et al: Inhibition
of the MEK/ERK pathway reduces microglial activation and
interleukin-1-beta expression in spinal cord ischemia/reperfusion
injury in rats. J Thorac Cardiovasc Surg. 133:934–941. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim JH, Gibbons HM, O’Carroll SJ, Narayan
PJ, Faull RL and Dragunow M: Extracellular signal-regulated kinase
involvement in human astrocyte migration. Brain Res. 20:1–13. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu K, Liang CL, Liliang PC, et al:
Inhibition of extracellular signal-regulated kinases 1/2 provides
neuroprotection in spinal cord ischemia/reperfusion injury in rats:
relationship with the nuclear factor-kappaB-regulated
anti-apoptotic mechanisms. J Neurochem. 114:237–246. 2010.
|
|
21
|
Black P, Markowitz RS, Damjanov I, et al:
Models of spinal cord injury: Part 3. Dynamic load technique.
Neurosurgery. 22:51–60. 1988.PubMed/NCBI
|
|
22
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weightdrop device versus transection. Exp
Neurol. 139:244–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yiu G and He Z: Glial inhibition of CNS
axon regeneration. Nat Rev Neurosci. 7:617–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parry PV and Engh JA: Promotion of
neuronal recovery following experimental SCI via direct inhibition
of glial scar formation. Neurosurgery. 70:N10–N11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okada S, Nakamura M, Mikami Y, et al:
Blockade of interleukin-6 receptor suppresses reactive astrogliosis
and ameliorates functional recovery in experimental spinal cord
injury. J Neurosci Res. 76:265–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park YM, Lee WT, Bokara KK, et al: The
multifaceted effects of agmatine on functional recovery after
spinal cord injury through Modulations of BMP-2/4/7 expressions in
neurons and glial cells. PLoS One. 8:e539112013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qu WS, Tian DS, Guo ZB, et al: Inhibition
of EGFR/MAPK signaling reduces microglial inflammatory response and
the associated secondary damage in rats after spinal cord injury. J
Neuroinflammation. 23:1782012.PubMed/NCBI
|
|
28
|
Ji RR, Gereau RW IV, Malcangio M and
Strichartz GR: MAP kinase and pain. Brain Res Rev. 60:135–248.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kloos RT, Ringel MD, Knopp MV, et al:
Phase II trial of sorafenib in metastatic thyroid cancer. J Clin
Oncol. 27:1675–1684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cruz CD, Charrua A, Vieira E, Valente J,
Avelino A and Cruz F: Intrathecal delivery of resiniferatoxin (RTX)
reduces detrusor overactivity and spinal expression of TRPV1 in
spinal cord injured animals. Exp Neurol. 214:301–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Greco S, Muscella A, Elia MG, Romano S,
Storelli C and Marsigliante S: Mitogenic signalling by B2
bradykinin receptor in epithelial breast cells. J Cell Physiol.
201:84–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jones MK, Wang H, Peskar BM, et al:
Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs:
insight into mechanisms and implications for cancer growth and
ulcer healing. Nat Med. 5:1418–1423. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhuang ZY, Gerner P, Woolf CJ and Ji RR:
ERK is sequentially activated in neurons, microglia, and astrocytes
by spinal nerve ligation and contributes to mechanical allodynia in
this neuropathic pain model. Pain. 114:149–159. 2005. View Article : Google Scholar
|
|
34
|
Pekny M and Pekna M: Astrocyte
intermediate filaments in CNS pathologies and regeneration. J
Pathol. 204:428–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dahl D, Ruegger DC, Bignami A, et al:
Vimentin, the 57000 molecular weight protein of fibroblast
filaments, is the major cytoskeletal component in immature glia.
Eur J Cell Biol. 24:191–196. 1998.PubMed/NCBI
|
|
36
|
Genonvese T, Esposito E, Mazzon E, et al:
Evidence for the role of mitogen-activated protein kinase signaling
pathways in the development of spinal cord injury. J Pharmacol Exp
Ther. 325:100–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stoica BA, Movsesyan VA, Knoblach SM and
Faden AI: Ceramide induces neuronal apeptosis through
mitogen-activated protein kinases and causes release of multiape
mitochondrial proteins. Mol Cell Neurosci. 29:355–371. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu Z, Wang BR, Wang X, Kuang F, Duan XL
and Jiao XY: ERK1/2 and p38 mitogen-activated protein kinase
mediate iNOS-induced spinal neuron degeneration after acute
traumatic spinal cord injury. Life Sci. 79:1895–1905. 2006.
View Article : Google Scholar : PubMed/NCBI
|