1. Introduction
Human papillomaviruses (HPVs) are small,
double-stranded DNA viruses. HPV infection occurs on the surface of
the epithelium in the basal layer, and the life cycle of the virus
is distinguished according to the differentiation program of the
infected cells. The HPV family encompasses ~200 categories
(1), of which 40 categories have
been isolated from the genital tract (2). On the basis of HPV clinical
associations, HPVs may be divided into two types: high- or
low-risk. High-risk HPVs participate in the development of cervical
neoplasia in persistently infected females (3). Of these, HPV-16, -18, -31 and -35 are
the most prevalent, and are commonly associated with lesions that
may progress to high-grade intraepithelial neoplasia and ultimately
to carcinoma. The other types of HPV, including HPV-6 and -11, are
low-risk and have been shown to be predominantly associated with
benign lesions, which seldom progress to cancer (4). HPV DNA has been detected in <99.7%
of all cases of cervical cancer. Moreover, infection with HPV-16
and -18 accounts for >50% of all cervical cancer (5), and cervical cancer accounts for
one-fifth of all cancer-associated mortalities among females
diagnosed each year, making it the second most common type of
cancer in females worldwide (6).
In addition, a number of low-risk types may be transmitted sexually
and may cause genital condyloma (7). All types of HPV share a common
genomic structure and encode eight proteins, including six early
proteins: E1, E2, E4, E5, E6 and E7, and two late proteins. In
particular, E5, E6 and E7 oncoproteins of the high-risk strains are
considered to be antiapoptotic oncoproteins, and the main
contributors to malignant transformation (8). E2 and E7 are also considered to be
proapoptotic proteins. Therefore, there is a close correlation
between apoptosis and the regulation of the three oncoproteins, E5,
E6 and E7.
2. E6 oncoprotein
The HPV E6 oncoprotein is a relatively small
protein. With regard to HPV-16, the E6 oncoprotein is comprised of
150 amino acids, containing two
CX2C-X29-CX2C zinc-like fingers
joined by an interdomain linker of 36 amino acids (9,10).
For the high-risk E6 genes, the truncated E6 (encoding residues
1–41 HPV-16 E6) is able to inactivate the functions of full-length
E6 by binding to the interface of the C- and N-terminal halves of
the E6 protein (11,12). Notably, E6 protein has no enzymatic
activities and the majority of the activities are considered to be
triggered by protein-protein interactions (13). The first and most common protein
that interacts with E6 is E6-associated protein (E6AP), a ubiquitin
ligase (14). The ubiquitin
cascade functions to target proteins for proteasomal degradation by
adding multiple ubiquitin monomers to the protein destined to be
destroyed. Therefore the E6, E6AP and target proteins form a
complex, which leads to ubiquitination of the target protein and
subsequent proteasome-mediated degradation (15). One of the main targets of E6
protein is the tumor suppressor p53, which is a DNA site-specific
transcription factor and one of the key signaling coordinators in
the cell following genotoxic or cytotoxic stress (16). The E6 protein is able to bind to
p53 with the aid of E6AP and prevent p53 from inducing apoptosis by
targeting it for degradation via the ubiquitin-proteasome pathway
(Fig. 1).
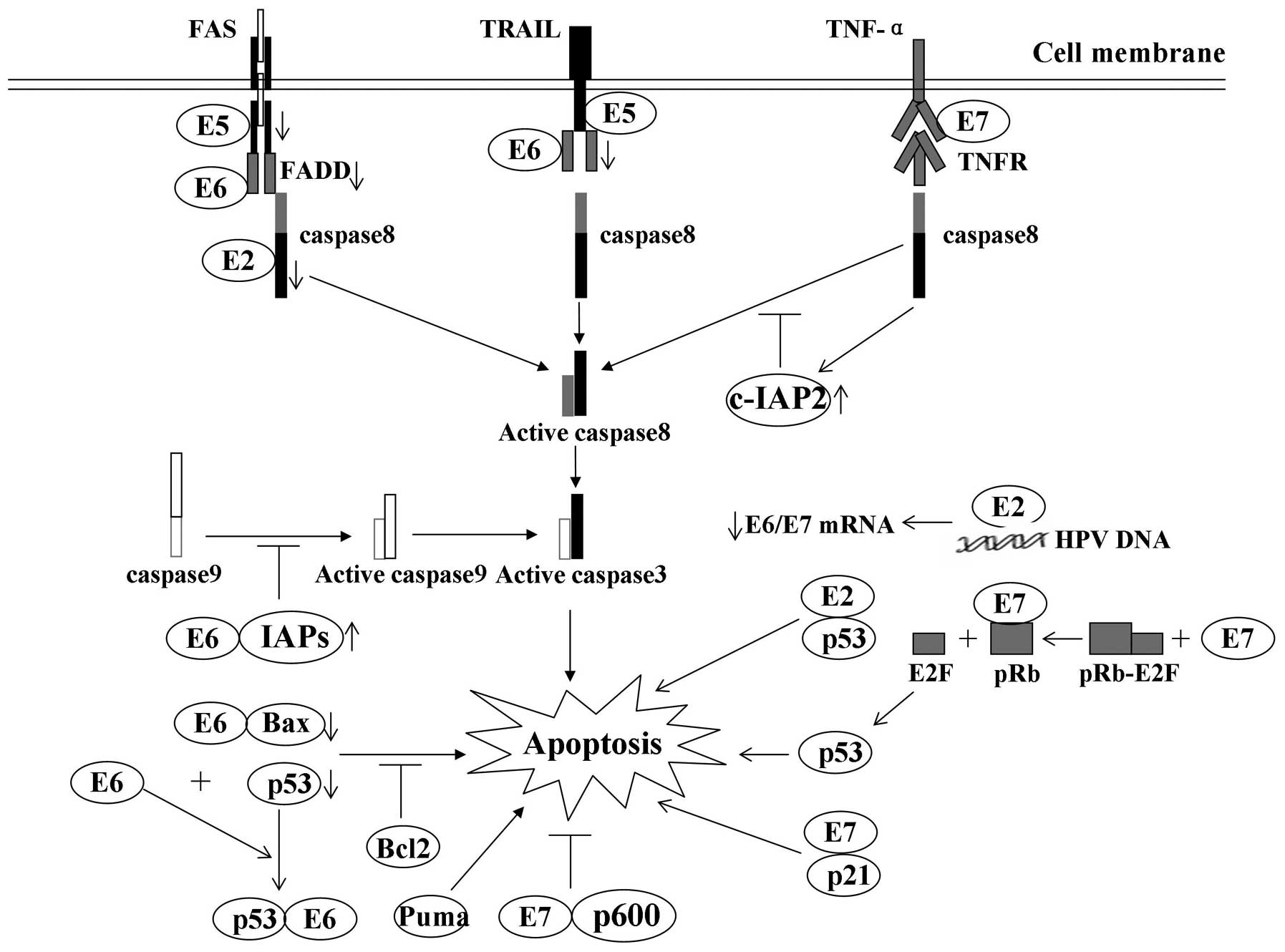 | Figure 1Modulation of apoptosis by HPV early
proteins: A number of the interactions of viral oncoproteins with
cellular proteins and their effects on apoptosis. E2 protein is
able to induce apoptosis by downregulating the transcription of
E6/E7 mRNA and by binding with p53, respectively. E5 impairs the
formation of the death-inducing signaling complex triggered by FasL
and TRAIL. E6 protein inhibits apoptosis by targeting proapoptotic
proteins, including p53 and Bax, for proteolytic degradation. E6
protein also protects cells from death receptor-induced apoptosis
by blocking apoptotic signal transduction and inducing the
expression of IAPs. E7 oncoprotein-expressing cells are usually
predisposed to undergo apoptosis, in part due to degradation of the
antiapoptotic protein pRb and E2F-1 binding. Downwards arrow,
degradation of cellular proteins; inhibition arrow, inhibition
status of the pathway; upwards arrow, upregulation of proteins.
HPV, human papillomavirus; FasL, Fas ligand; TNF, tumor necrosis
factor; TRAIL, TNF-related apoptosis-inducing ligand; IAP,
inhibitor of apoptosis; TNFR, TNF receptor; FADD, Fas-associated
protein with death domain. |
The E6 oncoprotein is involved in two pathways
associated with apoptosis (Fig.
1), including p53 inactivation and blocking apoptosis (17). Firstly, p53 inactivation may
trigger the E6-induced apoptosis inhibition. p53 rapidly initiates
the signaling pathway for DNA repair and apoptosis when it is
activated, while the E6 proteins impair the p53-triggered signaling
pathways and the cell death. In addition to the cytotoxic damage,
the improper stimulation of DNA synthesis, such as HPV infection,
may also activate p53 and induce apoptosis. HPVs stimulate DNA
synthesis in the infected cells against the normal cell response of
activating p53. The most important mechanism of p53 inactivation by
high-risk HPVs is by inducing p53 degradation via the
ubiquitin-proteasome pathway (18). Additionally, E6 proteins in
high-risk HPV may also inhibit p53 activation by blocking the
alternate reading frame p14 (p14/ARF) pathway (19), and by interacting with a histone
acetyltransferase, hADA3 (20).
Secondly, inhibition of apoptosis may be triggered by the E6
oncoprotein. In addition to p53-mediated apoptosis, p53-independent
apoptosis is also able to eliminate abnormal cells, while E6 is
capable of blocking apoptosis in cells and mice lacking p53
(21). Different stresses may
trigger two major apoptotic pathways, the intrinsic and extrinsic
pathway. Notably, the E6 protein is able to disturb these pathways
and prevent cell death under endogenous and exogenous stress
(22).
The intrinsic apoptotic pathway participates in
apoptosis associated with the cell nucleus, which includes DNA
damage, oxidative stress, starvation and other stimuli. These
stresses, involving mitochondria and endoplasmic reticulum (ER),
activate a series of pathways and change the balance between pro-
and anti-apoptotic signals when the balance is upset. When the cell
senses intrinsic stress, proapoptotic BH3-only proteins become
activated and abrogate the function of antiapoptotic proteins. This
allows for the formation of pores in the mitochondrial membrane,
comprised of proapoptotic Bax or Bak, and the release of
mitochondrial inner membrane proteins, including cytochrome
c, apoptosis-inducing factor (AIF), endonuclease G,
Smac/Diablo and Htr/Omi. The activation of these proteins may
result in the cleavage of caspase 3 and 7, and ultimately lead to
cell death. For the inhibition of HPV E6 oncoproteins, E6 is able
to block the apoptotic pathway by indirect interaction with the
protein Bak (23). The extrinsic
apoptotic signaling pathway may be activated, as a part of the host
response, and ‘death receptors’ on the cell surface may be induced
by extracellular signals during HPV infection (24). The death receptors include tumor
necrosis factor (TNF) receptor-1 (TNFR-1), Fas/CD95 and TNF-related
apoptosis-inducing ligand (TRAIL) receptor (DR4 and DR5), and
belong to the TNFR family. The receptors stimulate caspases 8 and
10, leading to the formation of the death-inducing signaling
complex (DISC). The DISC also activates the downstream executioner
caspases, such as caspases 3 and 7, and triggers apoptosis. In
addition to the TNF pathway, it has also been shown that HPV-16 E6
is capable of inhibiting apoptosis stimulated by Fas and TRAIL
pathways.
Although the extrinsic and intrinsic pathways have
individual roles, the pathways are not isolated. Caspase 8 is
activated during intrinsic apoptotic signaling via an amplification
loop mediated by caspases 3 and 7. Similarly, mitochondrial
signaling may be triggered during activation of the extrinsic
cascade, via caspase 8-mediated cleavage of the BH3-only protein
Bid. Therefore, the E6 protein targets intrinsic and extrinsic
signaling, protecting the infected cells from multiple apoptotic
stimuli and cross-activation between the two pathways. HPV-16 E6
has also been reported to either increase levels of c-Myc in
E6-expressing cells or to exert no effect on c-Myc levels; thus,
the overall role that c-Myc may have in E6-mediated cytoprotection
has yet to be fully elucidated (25).
3. E5 oncoprotein
Of the HPV oncoproteins E5 is the smallest, and, for
HPV-16, consists of 83 amino acids (26). The detection of this protein has
proved difficult due to its extreme hydrophobicity, membrane
localization and low levels of expression. Chang et
al(27) analyzed HPV-16 E5
protein expression using immunohistochemistry. Since the high-risk
HPV E5 gene is able to integrate into the human genome during
malignant progression, the E5 gene is rarely detected in cervical
tumors. The HPV-16/18 E5 mRNA and protein are expressed in
anogenital low-grade squamous intraepithelial lesions (ISIL), which
protect the infected cells from apoptosis during HPV infection. The
HPV-16 E5 protein is primarily localized to the ER, but is also
detected in the Golgi apparatus and nuclear membrane (28,29).
By contrast, HPV-6 E5 is mainly localized to the Golgi apparatus
(28) and the HPV-11 E5 protein is
localized primarily in the nucleus (30). However, HPV E5 DNA synthesis may be
enhanced following the transfection of HPV-16 E5 into primary human
keratinocytes cultured in serum-starved medium (31). E5 protects the cells from apoptosis
through two main pathways: inhibition of death receptor-mediated
apoptosis and ER stress-induced apoptosis (31).
HPV E5 inhibits death receptor-mediated apoptosis in
human keratinocytes. HPV E5 is capable of downregulating the total
amount of Fas receptor and decreasing Fas location, as well as
altering the formation of DISC induced by TRAIL; thus, E5 is able
to impair Fas ligand (FasL)- and TRAIL-mediated apoptosis (32) (Fig.
1). The exogenous proteins may activate the cellular defense
and disturb the ER homeostasis, so as to induce ER stress (33,34).
HPV-16 E5 protein suppresses three main proteins in the ER stress
pathway, including cyclooxygenase-2 (COX-2), X-box binding protein
1 (XBP-1) and inositol-requiring enzyme-1a (IRE1α) (35,36).
Therefore, the E5 protein downregulates COX-2, XBP-1 and IRE1a,
which is beneficial to viral persistence. However, the
downregulation of ER stress response genes by HPV-16 E5 in primary
genital keratinocytes suggests that the inhibition of this ER
stress pathway is an event favorable to viral replication and
persistence (35). In cervical
cancer cells, EP4 protein may be activated by HPV-16 E5, which
activates protein-kinase A. Protein kinase A is responsible for
antiapoptotic effects, such as mediating
ubiquitin-proteasome-mediated Bax degradation. In addition, the
activated EP4 may also enhance the expression of vascular
endothelial growth factor (VEGF), so as to lead to tumor
immortalization in cervical carcinoma (37).
4. E7 oncoprotein
Oncoprotein E7 is a small acidic polypeptide
composed of approximately 100 amino acids (38) that shares functional similarities
with other viral oncoproteins, specifically adenovirus E1A and SV40
large T antigen (39).
Ohlenschläger et al(40)
revealed that the N-terminal domain of E7 protein is unfolded,
while the C-terminal domain is a tightly packed zinc-binding fold.
The N-terminus of E7 contains two conserved regions (CRs), CR1 and
CR2. The E7 protein is also the major HPV oncoprotein and its
expression is sufficient to immortalize primary human epithelial
cells at a low frequency. The main target of E7 is retinoblastoma
protein (pRb) and its associated proteins, p107 and p130. E7
oncoprotein in high-risk HPVs is necessary for viral pathogenesis
and cellular transformation (8).
The present review primarily discusses the modulation of apoptosis
by HPV E7 oncoprotein.
The HPV-16 E7 oncoprotein induces p53-dependent and
-independent apoptosis. E7 leads to antiapoptotic pRb degradation
via a mechanism that involves association with and reprogramming of
the cullin 2 ubiquitin ligase complex, indicating that E7 may
trigger apoptosis (Fig. 1). It has
been indicated that the C-terminal of the E7 protein contains a
low-affinity pRb binding site, which interacts with pRb (41). p53 is also required for apoptosis
in the retina of transgenic mice expressing this oncogene (42). There are also other pathways that
trigger apoptosis involving the E7 protein. In the first pathway,
for the low- or high-risk HPV, E7 protein activates apoptosis in
NIH3T3 cells through a conserved Leu-X-Cys-X-Glu (LXCXE) motif in
second chromodomain (CD2) of the E7 structure binding to the pRb.
However, the binding ability of low-risk HPV E7 is ~10-fold lower
than that of the high-risk HPV E7. In the second pathway, E7 and
p21 form a complex which activates cathepsin B, an apoptotic
mediator, in U2OS cells. In the third pathway, E7 protein initiates
TRAIL and TNF-α-induced apoptosis in primary human keratinocytes
(43). In the last pathway, it is
likely that E7-induced apoptosis is associated with the interaction
between E7 and E2F1. The complex is able to trigger E2F1-driven
transcription, which contributes to increased apoptosis. Notably,
HPV E7 is also associated with E2F6 and blocks its ability to be a
transcriptional repressor (38).
In addition, E2F1 stabilizes p53 through the induction of the
p19ARF protein, which functions by binding directly to Mdm-2 and
preventing p53 degradation.
The HVP E7 oncoprotein may also inhibit apoptosis
and cytokine-mediated cell death depending on the cell and the
viral types (38). For example, it
was reported that HPV16 E7 was able to interact with and abrogate
the growth-inhibitory activities of cyclin-dependent kinase
inhibitor (CKI)sp21 and CKIsp27 to antagonize the activation of p53
(38). CKIsp21 and CKIsp27 have
been implicated in TGF-β-mediated inhibition of growth. HPV 16 E7
is able to inactivate CKIsp21 and CKIsp27, and abrogate
TGF-β-mediated growth inhibition (44). High- or low-risk HPV E7 are capable
of interacting with the 600 kDa pRb-associated factor, p600
(45). The conjugation of E7 and
p600 may protect detached cells from apoptosis, contributing to
viral transformation (46). HPV-16
E7 protein inhibits TNF-α-mediated apoptosis in normal human
fibroblasts by upregulating the expression of the inhibitor of
apoptosis (IAP) protein, c-IAP2, and by a mechanism involving the
suppression of caspase 8 activation. Siva-1 is a type of
proapoptotic cellular factor that is capable of binding to the
antiapoptotic protein Bcl-XL. The HPV-16 E7 interferes with the
binding of Siva-1 and Bcl-XL, so the released Bcl-XL is able to
fully exert its antiapoptotic function (47).
5. Interaction of the oncoproteins
The HPV full-length E2 protein is involved in
activating or repressing the transcription of E6/E7. The E2 protein
serves either as an activator or repressor of transcription,
depending on the context of E2 binding sites within the promoter
region (particularly for the P97 promoter) (48). The E6 and E7 oncoproteins determine
the transformational extent of infected epithelial cells (49). The E6 and E7 genes are located in
the same open reading frame (ORF) and are transcribed as a single
bicistronic E6/E7 transcript from early promoter P97 [nucleotide
(nt)31–97] (50). There is also an
enhancer (nt7535–7862), located at the 5′ end of the P97 promoter
in the long control region (LCR), which controls the expression of
the E6/E7 transcript (49,50). E2 protein differentially regulates
E6/E7 expression by binding to the ACCGN4CGGT
palindromic sequence in four binding sites (E2BSs) in the LCR (such
as P97 and the enhancer) (51).
The modulation of the E2 protein to the E6/E7 transcription depends
on its binding ability to the E2BSs genome structure (49). The binding of E2 (HPV-16 and -18)
to promoter and enhancer is able to upregulate P97 activity. The
activated P97 activates the transcription of E6, which binds to
p53. Therefore, the interaction of E6 and P97 results in a decrease
in the half-life of p53 within cells, and further inhibits
apoptosis. In addition, HPV E2 protein stabilizes p53 and maintains
apoptosis in HeLa cells (52).
However, the expression of HPV-31 E2 in normal human epidermal
keratinocytes (NHK) cells appears to destabilize p53. The E2F
protein is released from pRb-E2F complexes when E7 binds to pRb.
The members of the E2F family of transcription factors activate
E2F1 expression, which may induce apoptosis in serum-starved cells
(53). The repression of E7
transcription by E2 may reduce the level of the episomal E2F,
decreasing the level of apoptosis. In SiHa cells, the expression of
HPV-16 E2 protein may increase the activity of E2F (28). Furthermore, overexpression of
HPV-31 E2 in NHK cells may also induce an increase in E2F1 mRNA
expression (54). HPV-16 and -18
E2 proteins have been shown to activate transcription of HPV-16 E6
and E7 oncogenes (55); however,
there are numerous other factors that affect the repression and
activation of E6 and E7 oncoproteins. For example, the binding
ability of E2 to E2BSs may be inhibited by E2BSs methylation in its
5′CpG islands (56). Moreover, the
promoter and enhancer are regulated by cellular and viral proteins.
Binding of octamer-binding factor 1 (Oct 1) and nuclear factor 1
(NF 1) to the LCR may disturb E2 interaction with P97, and lead to
downregulation of P97 activity (57). In addition, HPVs exist as an
integrated form in the infected cells, which may affect the
progression of cervical cancer (58). The integrated form of HPV usually
appears in the E1/E2 gene, leading to the inhibition of the
E2-mediated regulation of E6/E7 oncoprotein (59). The HPV episomal form differentially
methylates the P97 promoter and LCR (60). In conclusion, in order to avoid
E2-induced apoptosis, the HPV genome modulates the survival of
infected cells through the activities of E6 and E7 (8).
6. Conclusion
HPV prevents host-triggered apoptosis through p53
inactivation, apoptosis blocking, downregulation of TNF-R1 and a
sustained expression of inhibitors of apoptosis. The primary
mediators of the HPV-induced effects are the E5, E6 and E7
oncoproteins. With the exception of the function of blocking
apoptosis, E5 protein may cooperate with E6 and E7 to immortalize
cells, and play an inhibitory role in apoptosis. The studies
discussed here suggest that the oncoproteins E5, E6 and E7 are
important molecular targets for the prevention of the development
of premalignant intraepithelial lesions and their progression to
cancer.
References
|
1
|
Nichols AC, Palma DA, Chow W, et al: High
frequency of activating PIK3CA mutations in human
papillomavirus-positive orpharyngeal cancer. JAMA Otolaryngol Head
Neck Surg. 139:617–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan CH, Filippova M and Duerksen-Hughes
P: Modulation of apoptotic pathways by human papillomaviruses
(HPV): mechanisms and implications for therapy. Viruses.
4:3831–3850. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaspersen MD, Larsen PB, Ingerslev HJ, et
al: Identification of multiple HPV types on spermatozoa from human
sperm donors. PLoS One. 6:e180952011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schlecht NF, Kulaga S, Robitaille J, et
al: Persistent human papillomavirus infection as a predictor of
cervical intraepithelial neoplasia. JAMA. 286:3106–3114. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alam MS, Ali A, Mehdi SJ, et al: HPV
typing and its relation with apoptosis in cervical carcinoma from
Indian population. Tumor Biol. 33:17–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pisani P, Bray F and Parkin DM: Estimates
of the world-wide prevalence of cancer for 25 sites in the adult
population. Int J Cancer. 97:72–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
zur Hausen H: Papillomaviruses in the
causation of human cancers - a brief historical account. Virology.
384:260–265. 2009.PubMed/NCBI
|
|
8
|
Garnett TO and Duerksen-Hughes PJ:
Modulation of apoptosis by human papillomavirus (HPV) oncoproteins.
Arch Virol. 151:2321–2335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mischo A, Ohlenschläger O, Hortschansky P,
Ramachandran R and Görlach M: Structural insights into a wildtype
domain of the oncoprotein E6 and its interaction with a PDZ domain.
PLoS One. 8:e625842013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai Q, Lv L, Shao Q, Li X and Dian A:
Human papillomavirus early proteins and apoptosis. Arch Gynecol
Obstet. 287:541–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani F and Banks L: The human
papillomavirus E6 protein and its contribution to malignant
progression. Oncogene. 20:7874–7887. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nominé Y, Masson M, Charbonnier S, et al:
Structural and functional analysis of E6 oncoprotein: insights in
the molecular pathways of human papillomavirus-mediated
pathogenesis. Mol Cell. 21:665–678. 2006.PubMed/NCBI
|
|
13
|
Ristriani T, Nominé Y, Masson M, Weiss E
and Travé G: Specific recognition of four-way DNA junctions by the
C-terminal zinc-binding domain of HPV oncoprotein E6. J Mol Biol.
305:729–739. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huibregtse JM, Scheffner M and Howley PM:
A cellular protein mediates association of p53 with the E6
oncoprotein of human papillomavirus type 16 or 18. EMBO J.
10:4129–4135. 1991.PubMed/NCBI
|
|
15
|
Scheffner M, Huibregtse JM, Vierstra RD
and Howley PM: The HPV-16 E6 and E6-AP complex functions as a
ubiquitin-protein ligase in the ubiquitination of p53. Cell.
75:495–505. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murray-Zmijewski F, Slee EA and Lu X: A
complex barcode underlies the heterogeneous response of p53 to
stress. Nat Rev Mol Cell Biol. 9:702–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Howie HL, Katzenellenbogen RA and Galloway
DA: Papillomavirus E6 proteins. Virology. 384:324–334. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blanchette P and Branton PE: Manipulation
of the ubiquitin-proteasome pathway by small DNA tumor virus.
Virology. 384:317–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khoronenkova SV and Dianov GL: The
emerging role of Mule and ARF in the regulation of base exicision
repair. FEBS Lett. 585:2831–2835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar A, Zhao Y, Meng G, et al: Human
papillomovirus oncoperotein E6 inactivates the transcriptional
coactivator human ADA3. Mol Cell Biol. 22:5801–5812. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aylon Y and Oren M: p53: guardian of
ploidy. Mol Oncol. 5:315–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Contreras-Paredes A, De la Cruz-Hernández
E, Martínez-Ramírez I, Dueñas-González A and Lizano M: E6 variants
of human papillomavirus 18 differentially modulate the protein
kinase B/phosphatidylinositol 3-kinase (akt/PI3K) signaling
pathway. Virology. 383:78–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Underbrink MP, Howie HL, Bedard KM, Koop
JI and Galloway DA: The E6 proteins from multiple human
betapapillomavirus types degrade Bak and protect keratinocytes from
apoptosis after UVB irradiation. J Virol. 82:10408–10417. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gewies A: Introduction to apoptosis.
ApoReview. 1–26. 2003.
|
|
25
|
Gewin L and Galloway DA: E box-dependent
activation of telomerase by human papillomavirus type 16 E6 does
not require induction of c-Myc. J Virol. 75:7198–7201. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Venuti A, Paolini F, Nasir L, et al:
Papillomavirus E5: the smallest oncoprotein with many functions.
Mol Cancer. 10:1402011. View Article : Google Scholar
|
|
27
|
Chang JL, Tsao YP, Liu DW, Huang SJ, Lee
WH and Chen SL: The expression of HPV-16 E5 protein in squamous
neoplastic changes in the uterine cervix. J Biomed Sci. 8:206–213.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conrad M, Bubb VJ and Schlegel R: The
human papillomavirus type 6 and 16 E5 proteins are
membrane-associated proteins which associated with the
16-kilodalton pore-forming protein. J Virol. 67:6170–6178.
1993.PubMed/NCBI
|
|
29
|
Borzacchiello G, Roperto F, Campo MS and
Venuti A: 1st international workshop on papillomavirus E5 oncogene
- a report. Virology. 408:135–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen SL and Mounts P: Transforming
activity of E5a protein of human papillomavirus type 6 in NIH 3T3
and C127 cells. J Virol. 64:3226–3233. 1990.PubMed/NCBI
|
|
31
|
Hu L, Potapova TA, Li S, et al: Expression
of HPV16 E5 produces enlarged nuclei and polyploidy through
endoreplication. Virology. 405:342–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kabsch K and Alonso A: The human
papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated
apoptosis in HaCaT cells by different mechanisms. J Virol.
76:12162–12172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Shi Q, Xu K, et al: Familial CJD
associated PrP mutants within transmembrane region induced Ctm-PrP
retention in ER and triggered apoptosis by ER stress in SH-SY5Y
cells. PLoS One. 6:e146022011. View Article : Google Scholar
|
|
34
|
Xu K, Wang X, Shi Q, et al: Human prion
protein mutants with deleted and inserted octarepeats undergo
different pathways to trigger cell apoptosis. J Mol Neurosci.
43:225–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sudarshan SR, Schlegel R and Liu XF: The
HPV-16 E5 protein represses expression of stress pathway genes
XBP-1 and COX-2 in genital keratinocytes. Biochem Biophys Res
Commun. 399:617–622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Condjella R, Liu X, Suprynowicz F, et al:
The canine papillomavirus E5 protein signals from the endoplasmic
reticulum. J Virol. 83:12833–12841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oh JM, Kim SH, Lee YI, et al: Human
papillomavirus E5 protein induces expression of the EP4 subtype of
prostaglandin E2 receptor in cyclic AMP response element-dependent
pathways in cervical cancer cells. Carcinogenesis. 30:141–149.
2009.
|
|
38
|
McLaughlin-Drubin ME and Münger K: The
human papillomavirus E7 oncoprotein. Virology. 384:335–344. 2009.
View Article : Google Scholar
|
|
39
|
Toscano-Garibay JD, Benitez-Hess ML and
Alvarez-Salas LM: Isolation and characterization of an RNA aptamer
of the HPV-16 E7 oncoprotein. Arch Med Res. 42:88–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ohlenschläger O, Seiboth T, Zengerling H,
et al: Solution structure of the partially folded high-risk human
papillomavirus 45 oncoprotein E7. Oncogene. 25:5953–5959.
2006.PubMed/NCBI
|
|
41
|
Liu X, Clements A, Zhao K and Marmorstein
R: Structure of human Papillomavirus E7 oncoprotein and its
mechanism for inactivation of the retinoblastoma tumor suppressor.
J Biol Chem. 281:578–586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghim S, Jenson AB, Bubier JA, Silva KA,
Smith RS and Sundberg JP: Cataracts in transgenic mice caused by a
human papillomavirus type 18 E7 oncogene driven by KRT1–14. Exp Mol
Pathol. 85:77–82. 2008.PubMed/NCBI
|
|
43
|
Zimmermann M, Koreck A, Meyer N, et al:
TNF-like weak inducer of apoptosis (TWEAK) and TNF-alpha cooperate
in the induction of keratinocyte apoptosis. J Allergy Clin Immunol.
127:200–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pardali K and Moustakas A: Actions of
TGF-β as tumor suppressor and pro-metastatic factor in human
cancer. Biochim Biophys Acta. 1775:21–62. 2007.
|
|
45
|
DeMasi J, Huh KW, Nakatani Y, Münger K and
Howley PM: Bovine papillomavirus E7 transformation function
correlates with cellular p600 protein binding. Proc Natl Acad Sci
USA. 102:11486–11491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
DeMasi J, Chao MC, Kumar AS and Howley PM:
Bovine papillomavirus E7 oncoprotein inhibits anoikis. J Virol.
81:9419–9425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Severino A, Abbruzzese C, Manente L, et
al: Human papillomavirus-16 E7 interacts with Siva-1 and modulates
apoptosis in HaCaT human immortalized keratinocytes. J Cell
Physiol. 212:118–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wells SI, Francis DA, Karpova AY,
Dowhanick JJ, Benson JD and Howley PM: Papillomavirus E2 induces
senescence in HPV-positive cells via pRB- and p21(CIP)-dependent
pathway. EMBO J. 19:5762–5771. 2000. View Article : Google Scholar
|
|
49
|
Mazumder Indra D, Singh RK, Mitra S, Dutta
S, et al: Genetic and epigenetic changes of HPV16 in cervical
cancer differentially regulate E6/E7 expression and associate with
disease progression. Gynecol Oncol. 123:597–604. 2011.PubMed/NCBI
|
|
50
|
Tang S, Tao M, McCoy JP Jr and Zheng ZM:
The E7 oncoprotein is translated from spliced E6*I transcripts in
high-risk human papillomavirus type 16-or type 18-positive cervical
cancer cell lines via translation reinitiation. J Virol.
80:4249–4263. 2006.
|
|
51
|
Dell G and Gaston K: Human papillomavirus
and their role in cervical. Cell Mol Life Sci. 58:1923–1942. 2001.
View Article : Google Scholar
|
|
52
|
Webster K, Parish J, Pandya M, Stern PL,
Clarke AR and Gaston K: The human papillomavirus (HPV) 16 E2
protein induces apoptosis in the absence of other HPV proteins and
via a p53-dependent pathway. J Biol Chem. 275:87–94. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu X and Levine AJ: p53 and E2F-1
cooperate to mediate apoptosis. Proc Natl Acad Sci USA.
91:3602–3606. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Frattini MG, Hurst SD, Lim HB, Swaminathan
S and Laimins LA: Abrogation of a mitotic checkpoint by E2 proteins
from oncogenic human papillomaviruses correlates with increased
turnover of the p53 tumor suppressor protein. EMBO J. 16:318–331.
1997. View Article : Google Scholar
|
|
55
|
Bouvard V, Storey A, Pim D and Banks L:
Characterization of the human papillomavirus E2 protein: evidence
of trans-activation and trans-repression in cervical keratinocytes.
EMBO J. 13:5451–5459. 1994.PubMed/NCBI
|
|
56
|
Kim K, Gamer-Hamrick PA, Fisher C, Lee D
and Lambert PF: Methylation patterns of papillomavirus DNA, its
influence on E2 function, and implications in viral infection. J
Virol. 77:12450–12459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dong XP, Stubenrauch F, Beyer-Finkler E
and Pfister H: Prevalence of deletions of YY1-binding sites in
episomal HPV 16 DNA from cervical cancers. Int J Cancer.
58:803–808. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pett M and Coleman N: Integration of
high-risk human papillomavirus: a key event in cervical
carcinogenesis? J Pathol. 212:356–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Arisa-Pulido H, Peyton CL, Joste NE,
Vargas H and Wheeler CM: Human papillomavirus type 16 integration
in cervical carcinoma in situ and in invasive cervical cancer. J
Clin Microbiol. 44:1755–1762. 2006. View Article : Google Scholar
|
|
60
|
Bhattacharjee B and Sengupta S: CpG
methylation of HPV 16 LCR at E2 binding site proximal to P97 is
associated with cervical cancer in presence of intact E2. Virology.
354:280–285. 2006. View Article : Google Scholar : PubMed/NCBI
|















