Introduction
Postpartum hemorrhage (PPH) refers to >500 ml
blood loss within 24 h following vaginal delivery, >1,000 ml
following cesarean delivery, or the requirement for a blood
transfusion within 24 h of delivery (1,2). PPH
is reported to occur in ~5% of all deliveries, and the risk is
significantly greater with cesarean delivery than vaginal delivery
(3,4). In China, PPH is the most common
serious obstetric complication and the leading cause of maternal
mortality, accounting for 49.9% of maternal deaths (5). The leading cause of PPH is uterine
atony, followed by retained placenta and injury to the genital
tract (1,3). Risk factors for PPH include fetal
macrosomy, prolonged labor, multiple pregnancies, polyhydramnios,
uterine myoma, placenta previa, grand multiparity and uterine
infection (1,3).
The majority of maternal deaths due to PPH may be
avoided, and the key lies in early diagnosis and proper treatment.
However, PPH is one of the most challenging complications faced by
clinicians. Active management of the third stage of labor includes
the administration of uterotonic agents following the cesarean
section or during the third stage of labor for vaginal delivery,
and studies have shown that it may reduce the incidence of PPH
(1,6–9).
Oxytocin is the most commonly used uterotonic agent for the
prevention of PPH, and has been demonstrated to reduce blood loss
following delivery (1,8). However, oxytocin has a half-life of
<10 min and thus must be administered by continuous intravenous
infusion (10). Furthermore,
saturation of uterine receptors may occur, and excessive dosages
are capable of producing water toxicity due to its antidiuretic
effect (10). Other uterotonic
agents have been studied, and have been shown to reduce PPH,
including carbetocin, a long-acting synthetic oxytocin analogue,
ergot alkaloids (such as ergonovine, syntometrine) and
prostaglandins (such as misoprostol and carboprost) (11–17).
Carboprost tromethamine (Hemabate) is the synthetic
15-methyl analogue of prostaglandin F2α, and has been
reported to be 84–96% effective in the treatment of persistent
hemorrhage due to uterine atony (18). However, since its introduction,
there have been few studies of its effectiveness for the prevention
and treatment of PPH, and only one specifically examining its use
following cesarean delivery (16,17,19–21).
The purpose of this study was to compare carboprost
with oxytocin for the prevention of PPH in high-risk females
undergoing cesarean delivery.
Patients and methods
Patients
Pregnant females at a high risk of PPH who were
scheduled to undergo a cesarean section at the Jinan Maternity and
Child Care Hospital (Jinan, China) between December, 2010 and May,
2012 were included in this study. Patients with coagulation
disorders or contraindications for receiving prostaglandin drugs
were excluded from the study. The patients were randomly divided
into three groups and received different uterotonics (oxytocin,
carboprost and oxytocin plus carboprost) during cesarean section,
following the delivery of the infant. This study was approved by
the Institutional Review Board of the Jinan Maternity and Child
Care Hospital, and all patients provided written informed consent
for participation in the study.
Interventions
In the oxytocin group, patients received a
myometrial injection of 20 units oxytocin (Shanghai Harvest
Pharmaceutical Co., Ltd., Shanghai, China) during the cesarean
section, immediately following the delivery of the infant, and
subsequently a continuous intravenous infusion of 20 units oxytocin
diluted in 1,000 ml saline or Ringer’s solution. In the carboprost
group, patients received a myometrial injection of 0.25 mg
carboprost tromethamine (Pharmacia & Upjohn Company, Kalamazoo,
MI, USA) during the cesarean section, immediately following the
delivery of the infant. The injection was repeated every 15 min, as
required, until a maximum total dose of 2 mg had been administered.
In the oxytocin plus carboprost tromethamine group, patients
received a myometrial injection of 0.25 mg carboprost tromethamine
and continuous intravenous infusion of 20 units oxytocin diluted in
1,000 ml saline or Ringer’s solution during the cesarean section,
immediately following the delivery of the infant.
In cases in which uterine bleeding was not able to
be effectively controlled by the aforementioned methods and the
blood loss was ≥1,000 ml, other methods of control, including the
administration of additional uterotonic agents, uterine artery
ligation, uterine gauze packing or blood transfusions, were
used.
Data recorded and compared included the volume of
blood lost during surgery and within 2 h of surgery, preoperative
and postoperative hemoglobin levels, and the other methods used to
control bleeding, if required.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation or median with range, depending on the normality
of the data distribution. Categorical variables are expressed by
frequencies and percentages. The differences among the three groups
were detected using analysis of variance (ANOVA) or a
Kruskal-Wallis test for continuous variables, and using a Fisher’s
exact test for categorical variables, as appropriate. In addition,
the differences prior to and following delivery were examined using
the paired Student’s t-test or Wilcoxon signed ranks test. For all
analyses, a two-sided P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS 15.0 statistical software (SPSS Inc., Chicago,
IL, USA).
Results
Patients
A total of 117 females at increased risk of PPH who
received a cesarean delivery between December, 2010 and May, 2012
were included in this study. The patients were between 19 and 40
years of age and 35 and 40 weeks gestation. There were 29 cases of
twins, 12 cases of polyhydramnios, 23 cases of placenta previa and
53 cases of fetal macrosomia. There were 37 patients in the
oxytocin group, 36 in the carboprost group and 44 in the oxytocin
plus carboprost group. No significant differences were identified
in maternal age, gravidity and parity, gestational age at delivery
and reason for cesarean delivery among the three groups (all
P>0.05, Table I).
 | Table IPatient demographic data. |
Table I
Patient demographic data.
| Variables | Oxytocin group
(n=37) | Carboprost group
(n=36) | Oxytocin + carboprost
group (n=44) | P-value |
|---|
| Age
(years)a | 28.65±4.77 | 27.92±4.29 | 27.05±3.54 |
0.230d |
| Reason for cesarean
deliveryb | | | |
0.928e |
| Twin pregnancy | 11 (29.7) | 7 (19.4) | 11 (25.0) | |
| Hydramnios | 3 (8.1) | 5 (13.9) | 4 (9.1) | |
| Placenta previa | 6 (16.2) | 7 (19.4) | 10 (22.7) | |
| Macrosomia | 17 (45.9) | 17 (47.2) | 19 (43.2) | |
| Gestational age
(weeks)a | 37.99±1.22 | 37.37±1.33 | 37.99±1.39 |
0.068d |
|
Gravidac | 2 (1–6) | 2 (1–5) | 2 (1–4) |
0.518f |
| Parac | 1 (1–3) | 1 (1–3) | 1 (1–3) |
0.696f |
Blood loss and interventions
Blood loss was ≥1,000 ml in 13 patients from the
oxytocin group, four patients from the carboprost group, and three
patients from the oxytocin plus carboprost group. In the oxytocin
group, the oxytocin dose was increased or other drugs were also
administered in 12 cases, bilateral uterine artery ligation was
performed in six cases, uterine gauze packing was adopted in two
cases and a blood transfusion was performed in four cases (data not
shown). In the carboprost group the dosage was increased or other
drugs were also administered in three cases, bilateral uterine
artery ligation was performed in three cases and uterine gauze
packing was adopted in two cases. In the oxytocin plus carboprost
group, the carboprost dose was increased or other drugs were also
administered in two cases, bilateral uterine artery ligation was
performed in two cases and uterine gauze packing was adopted in one
case.
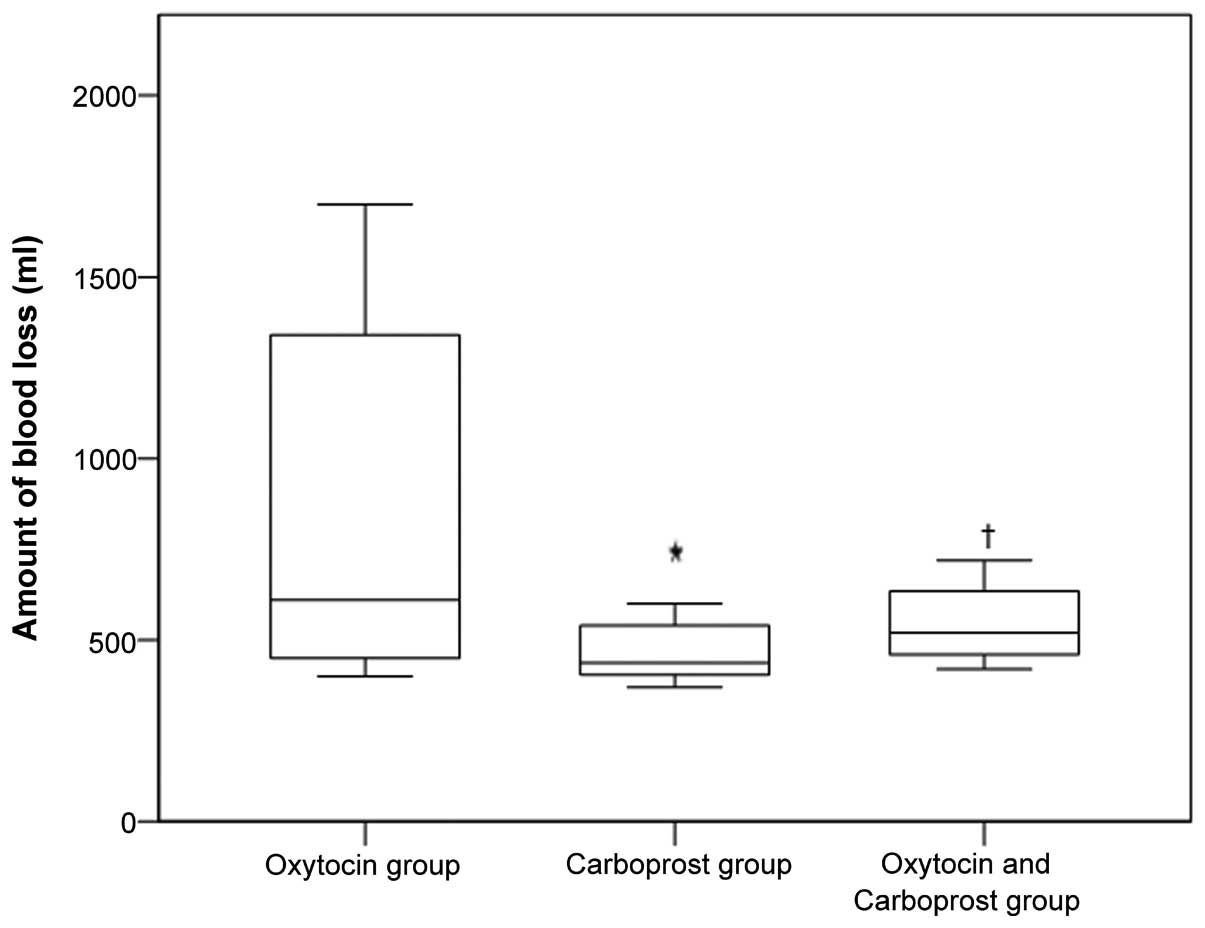
The median blood loss in the oxytocin, carboprost,
and oxytocin plus carboprost groups was 610, 438 and 520 ml,
respectively (Fig. 1). The median
blood loss in the carboprost group was significantly lower than
that in the oxytocin and oxytocin plus carboprost groups (both
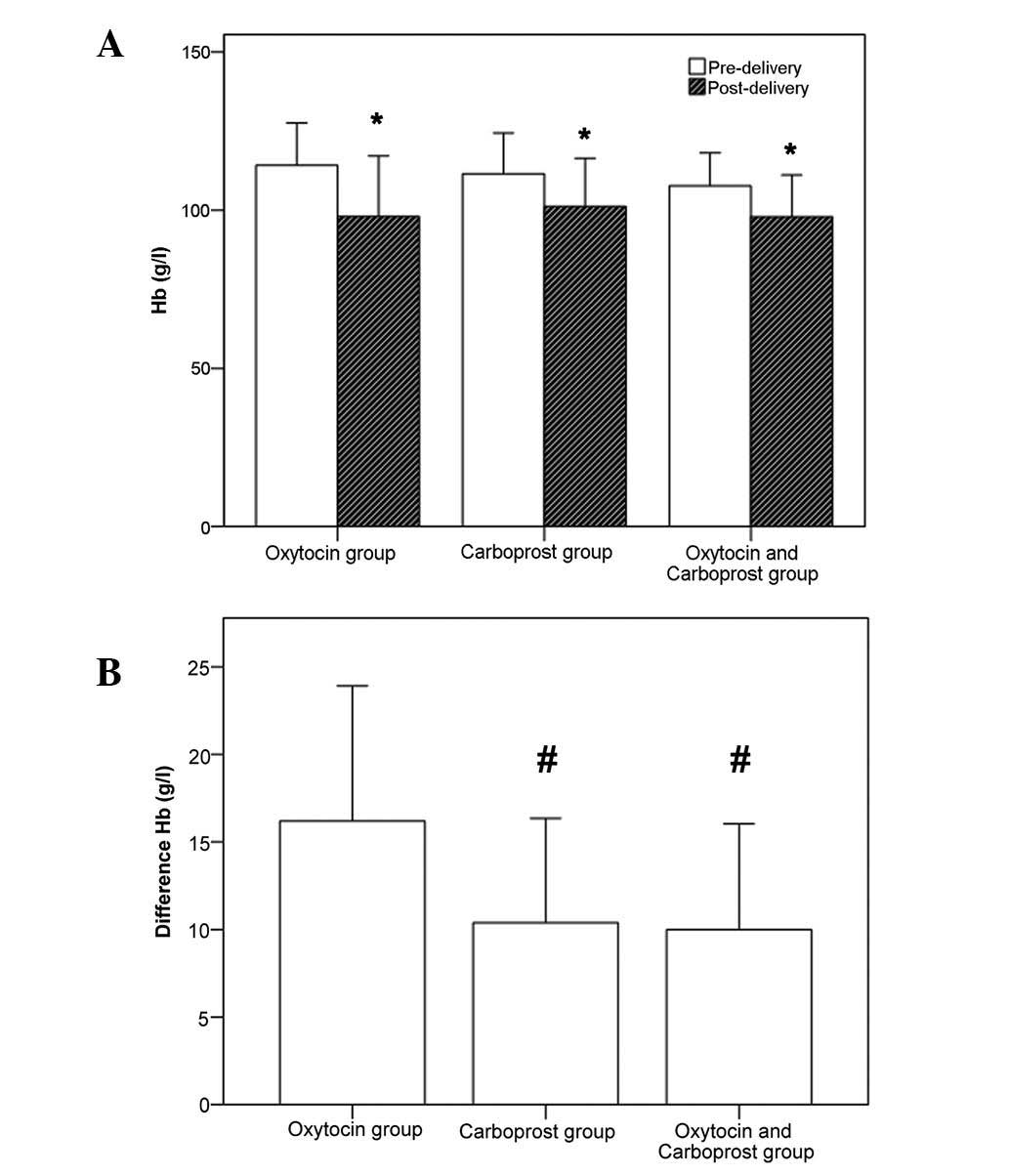
P<0.05). The change in hemoglobin levels prior to and following
delivery in the three groups is shown in Fig. 2. In all three groups, the
hemoglobin level following delivery was significantly lower than it
had been prior to delivery (all P<0.01, Fig. 2A). Furthermore, there was a
significant difference in the reduction in hemoglobin levels among
the three groups (P<0.001, Fig.
2B). Patients in the carboprost and oxytocin plus carboprost
groups had a significantly smaller reduction in hemoglobin levels
compared with the oxytocin group.
Adverse events
The adverse events that occurred in the three groups
are summarized in Table II.
Nausea and vomiting were the most common adverse events. Vomiting
occurred in eight (22.2%) patients in the carboprost group, as
compared with two (5.4%) in the oxytocin group and two (4.5%) in
the oxytocin plus carboprost group, and the difference was
statistically significant (P=0.036).
 | Table IIAdverse events. |
Table II
Adverse events.
| Variables | Oxytocin group
(n=37) | Carboprost group
(n=36) | Oxytocin + carboprost
group (n=44) | P-value |
|---|
| Nausea | 2 (5.4) | 6 (16.7) | 4 (9.1) | 0.274 |
| Vomiting | 2 (5.4) | 8 (22.2)a | 2 (4.5)b | 0.036c |
| Fever | 2 (5.4) | 3 (8.3) | 0 (0.0) | 0.096 |
| Diarrhea | 0 (0.0) | 1 (2.8) | 1 (2.3) | 0.758 |
| Headache | 0 (0.0) | 1 (2.8) | 0 (0.0) | 0.297 |
| Elevated blood
pressure | 0 (0.0) | 4 (11.1) | 2 (4.5) | 0.113 |
Discussion
The results of the present study demonstrated that
carboprost was more effective at preventing PPH than oxytocin in
patients at a high risk of PPH undergoing cesarean delivery. The
side-effects observed in the three groups were similar, with the
exception of vomiting, which was more common in the patients who
received carboprost.
To date, PPH remains a leading cause of maternal
morbidity and mortality in China, as well as in many underdeveloped
countries (2,5). The main causes of PPH are uterine
atony, residual trophoblastic tissue, genital tract trauma and
clotting disorders. Of these, uterine atony is the most common and
is apparent in 70–90% of all cases of PPH (1,2). PPH
within 2 h of delivery accounts for ~90% of the total number of
cases (1,2).
Oxytocin is the most commonly used drug for the
prevention and treatment of excessive bleeding following delivery
(8). The most significant benefit
of oxytocin is rapid action without causing elevated blood pressure
or tetanic uterine contractions. Studies have demonstrated that the
routine prophylactic use of oxytocin may reduce the need for
additional uterotonics (8,22). However, the use of oxytocin is
limited by the dose. Myometrial oxytocin receptor saturation may
affect its effectiveness, and excessive dosages may result in
coronary artery contraction, hypotension and antidiuretic
effect-induced water intoxication (8,23).
Therefore, other uterotonics may be required in patients at
high-risk of PPH. While it is clear that uterotonics are capable of
reducing blood loss during the third stage of labor and preventing
PPH, the most effective uterotonic in in the case of cesarean
versus vaginal delivery and in certain other circumstances has not
been elucidated (1,7).
Carboprost tromethamine is the synthetic 15-methyl
analogue of prostaglandin F2α. It may be administered
via intramuscular injection at a dose of 0.25 mg, and may be
repeated every 15 min until a maximum total dose of 2 mg has been
administered (24). Carboprost has
been reported to be 84–96% effective in the treatment of persistent
hemorrhage due to uterine atony, and may avoid the need for
surgical intervention (18).
However, few studies have examined its use for the prevention of
PPH. Vaid et al(16)
compared prophylactic sublingual misoprostol, intramuscular
methylergometrine and intramuscular carboprost for the active
management of the third stage of labor, and observed that the three
drugs were equally effective in the prevention of PPH, although
diarrhea was more common in the patients who received carboprost.
In a similar study, Abdel-Aleem et al(19) compared carboprost and
methylergometrine in 150 females who were randomly assigned to
receive one of the two drugs, and observed that the duration of the
third stage of labor and mean blood loss were significantly less in
the carboprost group. Oleen and Mariano (20) reported that carboprost effectively
controlled bleeding in 208 out of 237 (87.8%) cases of PPH.
Carboprost may cause prostaglandin-like
side-effects, including nausea, vomiting, diarrhea, headaches,
hypertension and bronchial asthma caused by the contraction of
smooth muscles (24). It may also
act on the thermoregulatory center, increasing the basal body
temperature (24). Patients may
experience hot flashes, sweating and increased irritability. Lamont
et al(17) compared
carboprost and syntometrine for the prevention of PPH and revealed
that although the two drugs were as effective in the prevention of
PPH, diarrhea occurred in 21% of the patients who received
carboprost, compared with only 0.8% of the patients who received
syntometrine. Despite the aforementioned potential side-effects,
serious side-effects are rare and self-limited (16). The present results demonstrated
that vomiting was relatively common in the patients who received
carboprost; however, it was readily managed.
There are limitations to the present study that
should be considered. This was a single center study with a
relatively small study group; thus, the results may not be
generalizable to other patient populations.
In conclusion, the results of this study
demonstrated that carboprost was more effective than oxytocin in
preventing PPH in high-risk patients undergoing cesarean delivery.
The drug was well-tolerated with minimal adverse effects.
Carboprost may be considered to be suitable drug for the active
management of the third stage of labor in this patient
population.
Abbreviations:
|
PPH
|
postpartum hemorrhage
|
References
|
1
|
Leduc D, Senikas V, Lalonde AB, et al:
Active management of the third stage of labour: prevention and
treatment of postpartum hemorrhage. J Obstet Gynaecol Can.
31:980–993. 2009.PubMed/NCBI
|
|
2
|
World Health Organization. WHO
recommendations for the prevention of postpartum haemorrhage. World
Health Organisation: Department of Making Pregnancy Safer; Geneva,
Switzerland: 2007
|
|
3
|
Carroli G, Cuesta C, Abalos E and
Gulmezoglu AM: Epidemiology of postpartum haemorrhage: a systematic
review. Best Pract Res Clin Obstet Gynaecol. 22:999–1012. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wedisinghe L, Macleod M and Murphy DJ: Use
of oxytocin to prevent haemorrhage at caesarean section - a survey
of practice in the United Kingdom. Eur J Obstet Gynecol Reprod
Biol. 137:27–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su Y, Xu Z and Jiang S: Practical
Obstetrics. 2nd edition. Shandong Science and Technology Press;
Jinan, Shandong, China: pp. 4862004
|
|
6
|
Begley CM, Gyte GM, Devane D, McGuire W
and Weeks A: Active versus expectant management for women in the
third stage of labour. Cochrane Database Syst Rev.
11:CD0074122011.
|
|
7
|
Gizzo S, Patrelli TS, Gangi SD, et al:
Which uterotonic is better to prevent the postpartum hemorrhage?
Latest news in terms of clinical efficacy, side effects, and
contraindications: a systematic review. Reprod Sci. 20:1011–1019.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roach MK, Abramovici A and Tita AT: Dose
and duration of oxytocin to prevent postpartum hemorrhage: a
review. Am J Perinatol. Dec 3–2012.(Epub ahead of print).
|
|
9
|
Oladapo OT, Akinola OI, Fawole AO, et al:
Active management of third stage of labor: evidence versus
practice. Acta Obstet Gynecol Scand. 88:1252–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rath W: Prevention of postpartum
haemorrhage with the oxytocin analogue carbetocin. Eur J Obstet
Gynecol Reprod Biol. 147:15–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higgins L, Mechery J and Tomlinson AJ:
Does carbetocin for prevention of postpartum haemorrhage at
caesarean section provide clinical or financial benefit compared
with oxytocin? J Obstet Gynaecol. 31:732–739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Bonis M, Torricelli M, Leoni L, et al:
Carbetocin versus oxytocin after caesarean section: similar
efficacy but reduced pain perception in women with high risk of
postpartum haemorrhage. J Matern Fetal Neonatal Med. 25:732–735.
2012.PubMed/NCBI
|
|
13
|
Gibbins KJ, Albright CM and Rouse DJ:
Postpartum hemorrhage in the developed world: whither misoprostol?
Am J Obstet Gynecol. 208:181–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cordovani D, Balki M, Farine D, et al:
Carbetocin at elective Cesarean delivery: a randomized controlled
trial to determine the effective dose. Can J Anaesth. 59:751–757.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su LL, Chong YS and Samuel M: Carbetocin
for preventing postpartum haemorrhage. Cochrane Database Syst Rev.
4:CD0054572012.
|
|
16
|
Vaid A, Dadhwal V, Mittal S, et al: A
randomized controlled trial of prophylactic sublingual misoprostol
versus intramuscular methyl-ergometrine versus intramuscular
15-methyl PGF2alpha in active management of third stage of labor.
Arch Gynecol Obstet. 280:893–897. 2009. View Article : Google Scholar
|
|
17
|
Lamont RF, Morgan DJ, Logue M and Gordon
H: A prospective randomised trial to compare the efficacy and
safety of hemabate and syntometrine for the prevention of primary
postpartum haemorrhage. Prostaglandins Other Lipid Mediat.
66:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buttino L Jr and Garite TJ: The use of 15
methyl F2 alpha prostaglandin (Prostin 15M) for the control of
postpartum hemorrhage. Am J Perinatol. 3:241–243. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdel-Aleem H, Abol-Oyoun EM, Moustafa SA,
et al: Carboprost trometamol in the management of the third stage
of labor. Int J Gynaecol Obstet. 42:247–250. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oleen MA and Mariano JP: Controlling
refractory atonic postpartum hemorrhage with Hemabate sterile
solution. Am J Obstet Gynecol. 162:205–208. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ezeh UO and Pearson M: The control of
blood loss at cesarean section with intramyometrial prostaglandin
F2 alpha analog versus intravenous synthetic oxytocin. Am J Obstet
Gynecol. 173:353–354. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nordström L, Fogelstam K, Fridman G,
Larsson A and Rydhstroem H: Routine oxytocin in the third stage of
labor: a placebo controlled randomized trial. Br J Obstet Gynecol.
104:781–786. 1997.PubMed/NCBI
|
|
23
|
Güngördük K, Asicioglu O, Celikkol O,
Olgac Y and Ark C: Use of additional oxytocin to reduce blood loss
at elective caesarean section: A randomised control trial. Aust N Z
J Obstet Gynaecol. 50:36–39. 2010.PubMed/NCBI
|
|
24
|
Carboprost [Package insert]. Kirkland.
Quebec, Canada: Pfizer; 2004
|