Introduction
Osteoarthritis (OA), which involves the dysfunction
of adult articular cartilage, is the most common form of joint
disease with manifestations of damaged articular cartilage,
chondro-osteophyte formation and thickening of subchondral bone,
and may result in arthralgia, joint deformation and limited
mobility in patients. OA is the second leading cause of long-term
disability in adults (1).
Previous studies have shown that the synthetic
activity of articular chondrocytes is dependent on insulin-like
growth factor-1 (IGF-1), transforming growth factor-β (TGF-β) and
bone morphogenetic protein (2–4), and
controlled by interleukin-1β (IL-1β), tumor necrosis factor-α
(TNF-α) and nitric oxide (NO) (5).
OA often results from an imbalance in the catabolic and anabolic
activity of cartilage and results in cartilage degradation.
Numerous scientists have studied the imbalance of cartilage
metabolism, but the signaling pathways involved remain unclear
(5).
Nuclear factor of activated T cells 1 (NFAT1), also
named NFATc2/NFATp, is a member of the NFAT family and was
initially identified as a regulator of cytokine expression during
the immune response (6). Early
studies demonstrated that tumor-like proliferation appeared in the
articular cartilage and peripheral joint tissues of adult mice
deficient in NFAT1. However, gene mutation analysis identified that
NFAT1 was not a tumor suppressor (6). A histopathological study found that
mature NFAT1-deficient mice demonstrated manifestations of OA, such
as articular chondrocytes decomposing to form clusters, bone bud
formation and sub-chondral bone thickening (7). The manifestations of OA in mice are
similar to those in humans (8,9). The
primary aim of the present study was to clarify the role of NFAT1
in OA pathology.
Subjects and methods
Subjects
Twelve articular cartilage samples were collected
from seven patients with OA and five additional patients who
required joint-replacement surgery following a traffic accident.
Cartilage tissues were collected from the First Affiliated Hospital
of Soochow University (Suzhou, China) and preserved in liquid
nitrogen within 6 h following the surgery to enable the analysis of
NFAT1, IL-1β and TNF-α expression by western blotting. This study
was conducted in accordance with the declaration of Helsinki and
with approval from the Ethics Committee of the First Affiliated
Hospital of Soochow University (Suzhou, China). Written informed
consent was obtained from all participants.
Isolation and culture of cartilage
cells
Articular cartilage tissues from the healthy human
knee were cut into 1–2 cm pieces with a scalpel, digested with
trypsin (100 g/l) for 30 min followed by hyaluronidase (1 mg/ml;
Sigma-Aldrich, Gillingham, UK) for 15 min and then incubated in
collagen enzyme B (Roche Diagnostics Ltd., West Sussex, UK) for
12–15 h at 37ºC. The acquired cells were filtered through a 200
mesh copper screen. The cartilage cells were washed twice with
phosphate-buffered saline (PBS) and cultured in Dulbecco’s modified
Eagle’s medium (DMEM)/F12 (10% fetal bovine serum, 100 U/ml
penicillin and 100 μg/ml streptomycin) to a concentration of
2×105cells/ml. Chondrocytes were identified by
morphological observation following the method of Li et
al(10).
Luciferase reporter assays
An equal number of chondrocytes (1×105
per well) were seeded in each well of a 6-well plate. The cells
were allowed to attach for 24 h. The cells were then transfected
with a pNFAT1-luc plasmid (5 μg/ml, provided by Dr Weilin King of
Shanghai Jiao Tong University, Xuhui, China) using Lipofectamine
2000 reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Cells transfected
with an empty pGL3 vector and PBS were used as the control.
Following transfection (24 h), the cells were incubated in 10 ng/ml
IL-1β for 36 h. Then, cell extracts were analyzed for luciferase
activity using the dual-luciferase reporter assay system (Promega
Corp., Madison, WI, USA). The experiment was repeated three times
and results were expressed as the mean ± standard error of the
mean.
RNAi-mediated gene silencing
An equal number of chondrocytes (1×105
per well) were seeded into each well of a 6-well plate. The cells
were allowed to attach for 24 h to 70–80% confluence. The cells
were then transfected with siRNA targeting NFAT1 (5 μg/ml;
5′-CAGCGGAGTCCAAGGTTGTGTTCAT-3′) using Lipofectamine 2000 reagent.
The control cells were transfected with scrambled siRNA in the
presence of PBS. After 24 h, the cells were incubated at 37ºC with
IL-1β (10 ng/ml) for 36 h. The supernatant was collected and TNF-α,
MMP-1, MMP-3 and MMP-9 were detected by enzyme-linked immunosorbent
assay (ELISA). The ELISA kits were obtained from R&D Systems
Inc., Minneapolis, MN, USA (TNF-α), Amersham Pharma Biotech,
Cambridge, England (MMP-1 and MMP-3) and R&D Systems Inc.
(MMP-9). All of the experiments for each sample were performed in
triplicate. Simultaneously, the total protein content was extracted
using cell lysis buffer [0.3% NP-40, 1 mM EDTA, 50 mM Tris-Cl (pH
7.4), 2 mM EGTA, 1% Triton X-100, 150 mM NaCl, 25 mM NaF, 1 mM
Na3VO3 and 10 μg/ml PMSF] and the expression
of NFAT1 and β-actin were analyzed by western blotting according to
the manufacturer’s instructions.
Statistical analysis
Data were analyzed using SPSS software, version 11.0
(SPSS, Inc., Chicago, IL, USA) and are expressed as the mean ±
standard error. Student’s t-test was performed for comparisons
between two groups. The significant level was α=0.05 and P<0.05
was considered to indicate a statistically significant
difference.
Results
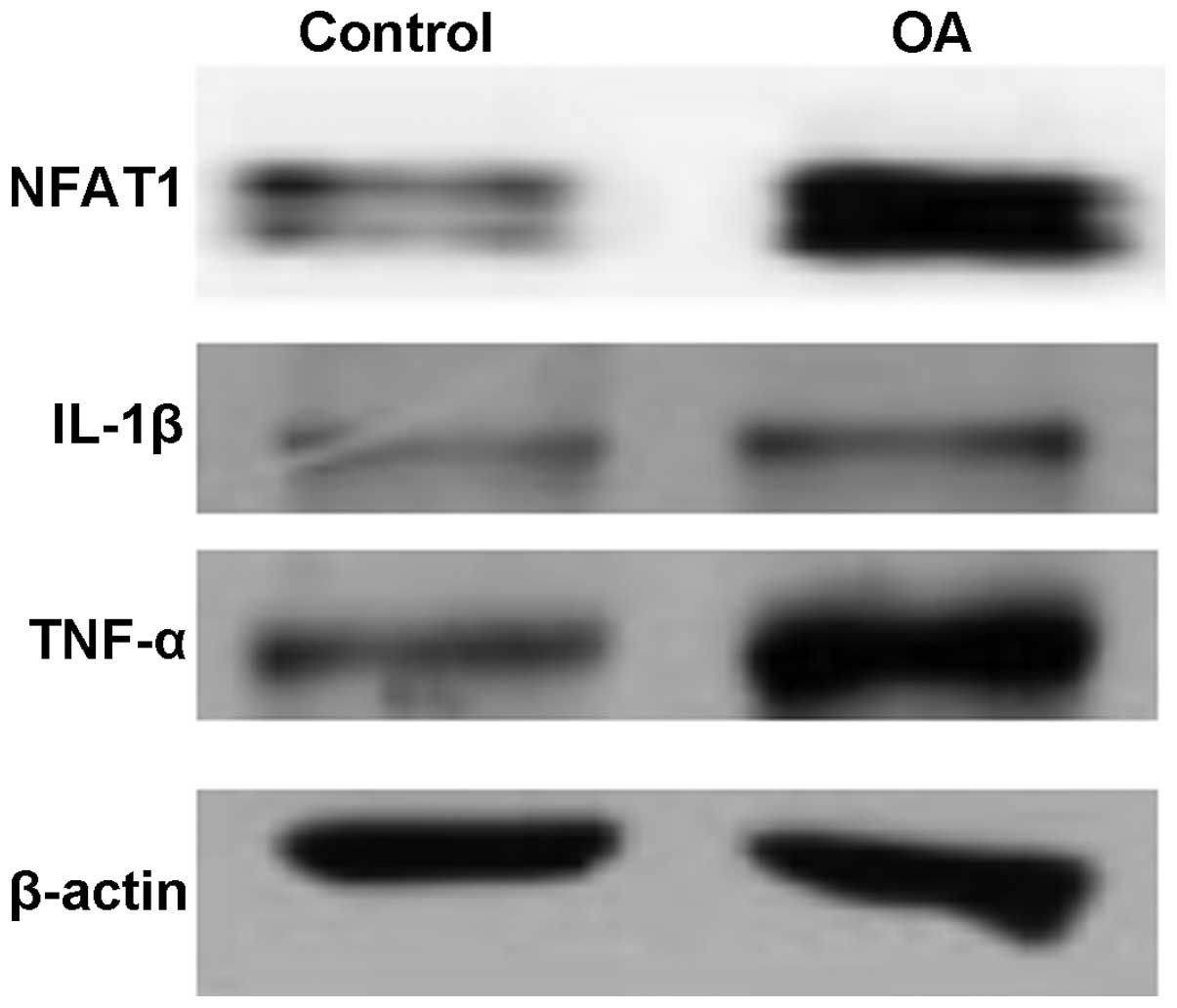
Expression levels of NFAT1, IL-1β and
TNF-α in OA
The expression levels of NFAT1, IL-1β and TNF-α in
the articular cartilage of patients with OA were higher than those
in the healthy individuals. As shown in Fig. 1, the levels of phosphorylated and
non-phosphorylated NFAT1 were observed to be higher in the patients
with OA than in the control group, implying that OA may be
associated with the transcription factor NFAT1. The expression
levels of pro-inflammatory cytokines, IL-1β and TNF-α, were also
increased in the patients with OA, and were positively correlated
with NFAT1.
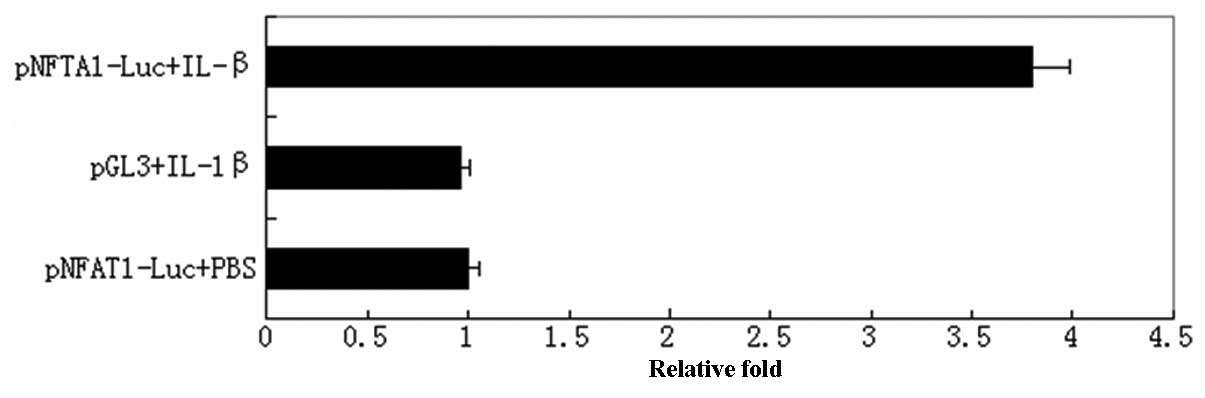
Activation of NFAT1 is mediated by
IL-1β
It is known that pro-inflammatory cytokines, such as
IL-1β and TNF-α, play a role in the pathogenesis of osteoarthritis;
therefore, the present study established a model of OA using normal
human articular cartilage cells stimulated by recombinant human
IL-1β. The model was used to evaluate the role of IL-1β in NFAT1
activation using the luciferase reporter assay method. As shown in
Fig. 2, IL-1β significantly
induced the activity of NFAT1 3-fold compared with that of the
control group (P<0.05).
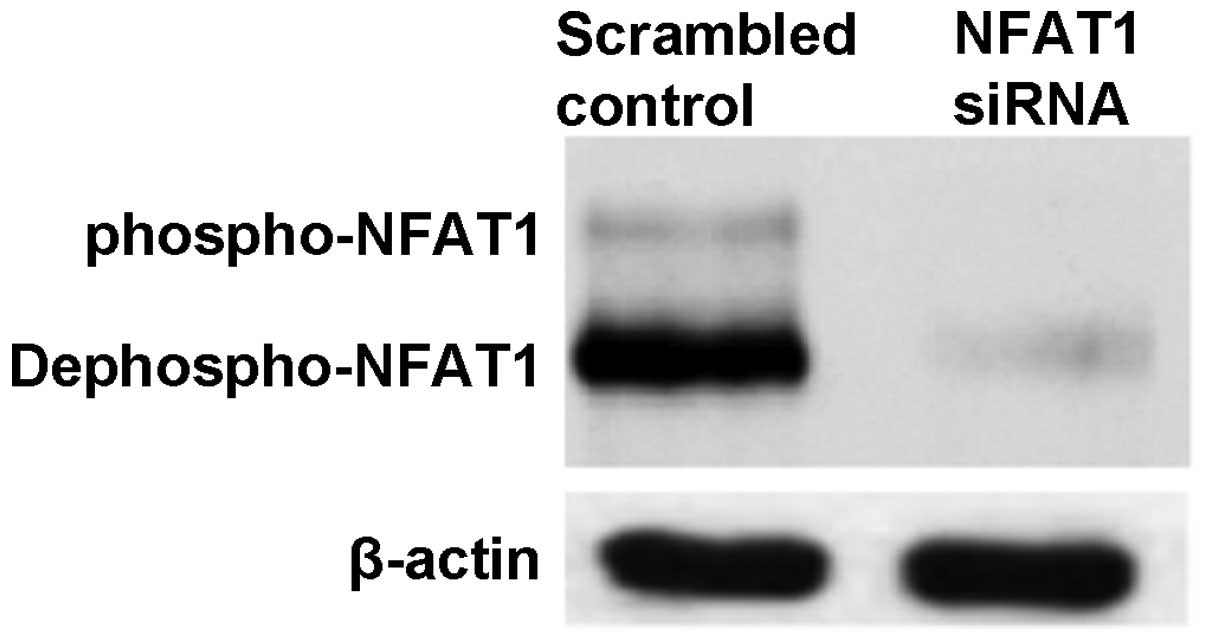
Silencing of NFAT1 reduced the expression
of TNF-α and MMPs
NFAT1 in cultured chondrocyte cells was silenced
using NFAT1-siRNA (Fig. 3). Cells
were then stimulated with IL-1β and the levels of TNF-α, MMP-1, -3
and -9 in the supernatant were detected by ELISA. As shown in
Table I, silencing of NFAT1
weakened the IL-1β-induced stimulation of TNF-α, MMP-1, -3 and -9
secretion (P<0.05).
 | Table ISilencing of NFAT1 in cultured
chondrocyte cells decreased the levels of TNF-α and MMPs stimulated
by IL-1β. |
Table I
Silencing of NFAT1 in cultured
chondrocyte cells decreased the levels of TNF-α and MMPs stimulated
by IL-1β.
| Group | TNF-α | MMP-1 | MMP-3 | MMP-9 |
|---|
| Scrambled
control | 0.93±0.10 | 0.95±0.06 | 0.92±0.07 | 1.04±0.12 |
| NFAT1-siRNA | 0.63±0.05a | 0.71±0.07a | 0.66±0.03a | 0.58±0.04b |
Discussion
The NFAT family, which consists of five members,
comprises the most important substrates of calcineurin (CaN). NFAT1
(NFATp), NFAT2 (NFATc) and NFAT4 (NFATx) predominantly exist in T
cells, while NFAT3 exists in other tissues, such as the heart.
NFAT5 is the original member of the NFAT family and is important
for permeability responses (11–13).
NFAT proteins consist of a trans-activation domain on the
N-terminal, and the NFAT homology region (NHR), also known as the
region of accommodation comprises nine conserved motifs, a highly
conserved Rel region (RSD) and a C terminal. NFAT proteins differ
from other phosphatase substrates in that they include ≥13
phosphorylation sites distributed in regions of accommodation and
spanning a range of nearly 400 amino acids (12). The special structure of NFAT allows
CaN to rapidly remove several phosphoric acids from the N terminal
of NFATc protein, subsequently causing conformation alteration and
exposure of the nuclear-localization sequence resulting in the
rapid transposition of NFATc to the nucleus (10,14).
The CaN-NFAT pathway may be blocked by inhibitors of CaN, such as
cyclosporine (CsA) and FK506 (15,16).
In the nucleus, NFATs participate in transcriptional regulation and
cooperate with other transcription factors, such as AP-1 family
members.
It is accepted that OA originates from the softening
of articular cartilage due to the actions of catabolic enzymes,
such as MMPs and dextranase. This results in slight inflammation
and increased levels of TNF-α and IL-1β, which further stimulates
the expression of catabolic enzymes and induces the transformation
of subchondral bone and articular peripheral cartilage (5). However, the transcription factors
involved in this transformation have not been identified. In the
present study, the expression levels of NFAT1, IL-1β and TNF-α were
evaluated in articular cartilage from patients with OA and healthy
individuals using western blot analysis. The results demonstrated
that the expression levels of NFAT1, IL-1β and TNF-α were
significantly increased in the cartilage tissues of patients with
OA (Fig. 1). The findings of this
study are in accordance with the results of previous studies
(16). Subsequently, a luciferase
reporter assay demonstrated that treatment with IL-1β significantly
induced the activity of NFAT1 in cultured human chondrocytes,
indicating that the CaN-NFAT pathway may be activated by IL-1β
(Fig. 3). The hypothesis is
further validated by the silencing of NFAT1. In chondrocyte cells
in which NFAT1 was silenced, the levels of TNF-α and MMPs induced
by IL-1β decreased significantly compared with those of the normal
chondrocyte cells, indicating that the CaN-NFAT pathway is closely
associated with OA. However, it remains unclear whether additional
pathways, such as nuclear factor-κ-light-chain-enhancer of
activated B cells (NF-κB), are important in this process. Notably,
there is a Rel domain that preserves a DNA-binding sequence with
homology to NF-κB in the regulatory region of NFATs (17). Further studies are required to
explore their roles of enhancing the proliferation and
differentiation of cartilage in OA.
Although the contribution of the CaN-NFAT pathway to
cartilage biology and OA remains unclear, a number of studies have
shown that the CaN-NFAT pathway may be important for the
proliferation and differentiation of cartilage and in reducing the
activity of catabolic enzymes (18). The results of present study also
support the hypothesis that inhibitors of CaN-NFAT may be effective
in the treatment of OA.
References
|
1
|
Mobasheri A: The future of osteoarthritis
therapeutics: targeted pharmacological therapy. Curr Rheumatol Rep.
15:3642013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lories RJ and Luyten FP: The
bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 7:43–49.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henry SP, Liang S, Akdemir KC and de
Crombrugghe B: The postnatal role of Sox9 in cartilage. J Bone
Miner Res. 27:2511–2525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fortier LA, Barker JU, Strauss EJ,
McCarrel TM and Cole BJ: The role of growth factors in cartilage
repair. Clin Orthop Relat Res. 469:2706–2715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim H and Kim HP: Matrix
metalloproteinase-13 expression in IL-1β-treated chondrocytes by
activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch
Pharm Res. 34:109–117. 2011.
|
|
6
|
Aoyama T, Nagayama S, Okamoto T, et al:
Mutation analyses of the NFAT1 gene in chondrosarcomas and
enchondromas. Cancer Lett. 186:49–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Gardner BM, Lu Q, et al:
Transcription factor Nfat1 deficiency causes osteoarthritis through
dysfunction of adult articular chondrocytes. J Pathol. 219:163–172.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Söder S and Aigner T: Osteoarthritis.
Etiology, typing, staging and histological grading. Pathologe.
32:183–192. 2011.(In German).
|
|
9
|
Samuels J, Krasnokutsky S and Abramson SB:
Osteoarthritis: a tale of three tissues. Bull NYU Hosp Jt Dis.
66:244–250. 2008.PubMed/NCBI
|
|
10
|
Li H, Rao A and Hogan PG: Interaction of
calcineurin with substrates and targeting proteins. Trends Cell
Biol. 21:91–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aramburu J, Heitman J and Crabtree GR:
Calcineurin: a central controller of signalling in eukaryotes. EMBO
Rep. 5:343–348. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fric J, Lim CX, Koh EG, et al:
Calcineurin/NFAT signalling inhibits myeloid haematopoiesis. EMBO
Mol Med. 4:269–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Müller MR and Rao A: NFAT, immunity and
cancer: a transcription factor comes of age. Nat Rev Immunol.
10:645–656. 2010.PubMed/NCBI
|
|
14
|
Oh-hora M and Rao A: Calcium signaling in
lymphocytes. Curr Opin Immunol. 20:250–258. 2008. View Article : Google Scholar
|
|
15
|
Müller MR, Sasaki Y, Stevanovic I, et al:
Requirement for balanced Ca/NFAT signaling in hematopoietic and
embryonic development. Proc Natl Acad Sci U S A. 106:7034–7039.
2009.PubMed/NCBI
|
|
16
|
Cippà PE, Kraus AK, Lindenmeyer MT, et al:
Resistance to ABT-737 in activated T lymphocytes: molecular
mechanisms and reversibility by inhibition of the calcineurin-NFAT
pathway. Cell Death Dis. 3:e2992012.PubMed/NCBI
|
|
17
|
Lee JH, Kim JW, Im YS, Seong GJ and Lee
HK: Cyclosporine A induces nerve growth factor expression via
activation of MAPK p38 and NFAT5. Cornea. 30:S19–24. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen QQ, Zhang W, Chen XF, Bao YJ, Wang J
and Zhu WZ: Electrical field stimulation induces cardiac fibroblast
proliferation through the calcineurin-NFAT pathway. Can J Physiol
Pharmacol. 90:1611–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|