Introduction
Acute liver failure is a significant clinical
syndrome in which a previously normal liver fails within days or
weeks. The prognosis of patients with acute liver failure remains
poor and the overall mortality rate is 90% (1). At present, there are no effective
treatment therapies for this disease and its complications result
in a high mortality rate and resource cost (2–4). In
the developing world, viral infections are predominant, with
hepatitis E infection recognized as a common cause of mortality in
many countries (5–8). The pathogenesis of acute liver
failure has not been fully elucidated and the apoptosis of liver
cells is an important event in the development of acute liver
failure (9,10). It has been demonstrated that the
serum from patients with liver failure may induce cytotoxicity and
oxidative stress of HHY41 cells, and reduce DNA synthesis, protein
synthesis and cytochrome P4501A activity (11). However, the effects of acute liver
failure serum on liver cell survival and apoptosis and the
underlying mechanisms have not been fully elucidated.
Endotoxin [lipopolysaccharide (LPS)] syndrome is a
particularly grave complication since bacterial infection is
confirmed in up to 80% of patients with fulminant hepatic failure
(12–14). The association of liver injury with
endotoxemia has been confirmed in a variety of experimental animals
(15,16). Endotoxin syndrome is a systemic
inflammatory response mediated by several of the cytokines produced
by lymphocytes and macrophages (17–19),
which exacerbates the disease condition of acute liver failure
(20). LPS is significant in the
development of liver failure (21).
The treatment of endotoxemia in liver failure is an
important research area. Antibodies against LPS are considered to
provide direct protective effects on endotoxemia, however, the
anti-endotoxin effects of antibodies against lipid A have not been
found to be satisfactory and the mechanisms of the protective
effects have not been elucidated (22). Therefore, the present study focused
on the effects of anti-LPS antibody recognizing core
polysaccharide.
In the present study, the LPS levels in the serum
from patients with hepatitis E virus (HEV)-related acute liver
failure were examined and the apoptotic effects of the serum on
human liver cells were investigated. In addition, the protective
effects of antibody on serum-induced apoptosis in human liver cells
were investigated.
Materials and methods
Reagents
The Quantitative Chromogenic Endpoint Tachypleus
Amebocyte Lysate Endotoxin Detection kit was purchased from Xiamen
Houshiji Ltd. (Xiamen, China). Anti-LPS antibody was purchased from
MyBiosource (San Diego, CA, USA), which could recognize core
polysaccharide. The Annexin V-FITC apoptosis detection kit was
obtained from Nanjing KeyGen Biotech. Co. Ltd (Nanjing, China).
RPMI-1640 medium, phosphate-buffered saline (PBS) and fetal bovine
serum (FBS) were purchased from Gibco-BRL (Carlsbad, CA, USA).
Serum collection and endotoxin
determination
This study was approved by the ethics committee of
Tongji Hospital (Wuhan, China). Whole blood samples from 13
patients with HEV-related acute liver failure and 13 normal
individuals were collected. All participants signed a consent form
approved by the ethics committee. Serum was separated from the
blood by centrifugation at room temperature (3,000 × g for 10 min)
and stored at −80°C. Serum endotoxin levels were measured using the
endotoxin detection kit following the manufacturer’s instructions.
Briefly, under aseptic conditions, 100 μl of the serum samples were
incubated with 100 μl of the limulus amebocyte lysate at 37°C for
10 min, followed by incubation with the provided chromogenic
substance at 37°C for 6 min. The absorbance was measured using a DU
730 spectrophotometer (Beckman Coulter Inc., Brea, CA, USA) at 545
nm following the addition of an azo reagent. The concentrations of
endotoxins were calculated on the basis of a standard curve. Two
serum samples with different concentrations of LPS were then used
to prepare the serums with the mean concentration of LPS, which
would be used in our subsequent experiment.
Cell culture with acute liver failure
serum
L02 human hepatic cell lines were preserved in a
laboratory at the Department of Infectious Disease (Wuhan, China).
The cells were maintained in RPMI-1640 medium supplemented with 10%
FBS and incubated at 37°C in a 5% CO2 atmosphere. For
experiments on cell apoptosis in different media, L02 cells were
prepared and plated at 2×105cells/well in 24-well plates
in 500 μl medium and allowed to settle for 24 h. The cultures were
later washed twice with warm PBS, and exposed to 20% (vol/vol) FBS,
20% (vol/vol) healthy serum or 20% (vol/vol) acute liver failure
serum in RPMI-1640 medium (500 μl total volume). Following
incubation for 20 h, cells were collected and prepared for the
detection of apoptosis.
Cell culture with antibody and acute
liver failure serum
To measure the protective effects of the antibody on
cells exposed to acute liver failure serum, L02 cells were plated
at 2×105cells/well in 24-well plates in 500 μl medium.
The cells were seeded in six wells, allowed to settle for 24 h and
the wells were divided into six groups: the FBS, healthy serum,
acute liver failure serum, FBS + antibody, healthy serum + antibody
and acute liver failure serum + antibody groups.
The cells were washed twice with warm PBS, and then
exposed to medium containing 20% (vol/vol) FBS, 20% (vol/vol)
healthy serum or 20% (vol/vol) acute liver failure serum.
Simultaneously, 15 μl antibody (diluted 1:300) was added to the
wells of the latter three groups.
Following incubation at 37°C in a 5% CO2
atmosphere for 20 h, the cells were collected and prepared for the
detection of apoptosis.
Measurement of apoptosis by flow
cytometry
After treatment of the cells as described above, the
cells were harvested, washed with PBS and stained using an Annexin
V-FITC apoptosis detection kit. The total sample solution contained
500 μl binding buffer, 5 μl Annexin V-FITC and 5 μl propidium
iodide. Following incubation at room temperature for 10 min, the
cells were examined using a FACS Calibur flow cytometer (Becton
Dickinson, Basel, Switzerland). Data were collected from
1×104 cells in the gated region of each sample.
Statistical analysis
Experiments were duplicated at least three times and
the results were expressed as the mean ± standard deviation.
One-way analysis of variance was used to analyze the statistical
differences. Statistical analysis was carried out using SPSS
software, version 13. 0 (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinical data of patients with
HEV-related acute liver failure
Serum was collected from the blood of 13 patients
with HEV-related acute liver failure and 13 normal individuals. The
basic characteristics of the patients are listed in Table I.
 | Table IBaseline characteristics of patients
with HEV-related acute liver failure and healthy individuals. |
Table I
Baseline characteristics of patients
with HEV-related acute liver failure and healthy individuals.
| Characteristics | Healthy (n=13) | Acute liver failure
(n=13) |
|---|
| Gender |
| Male | 8 | 10 |
| Female | 5 | 3 |
| Age (years) | 34.5±7.1 | 39.5±7.3 |
| ALT (U/l) | 30.6±5.5 | 694.9±331.7 |
| AST (U/l) | 29.0±4.6 | 455.8±217.7 |
| TBIL (μmol/l) | 11.2±3.2 | 258.8±66.1 |
| PTA (%) | 93.7±5.4 | 29.4±4.3 |
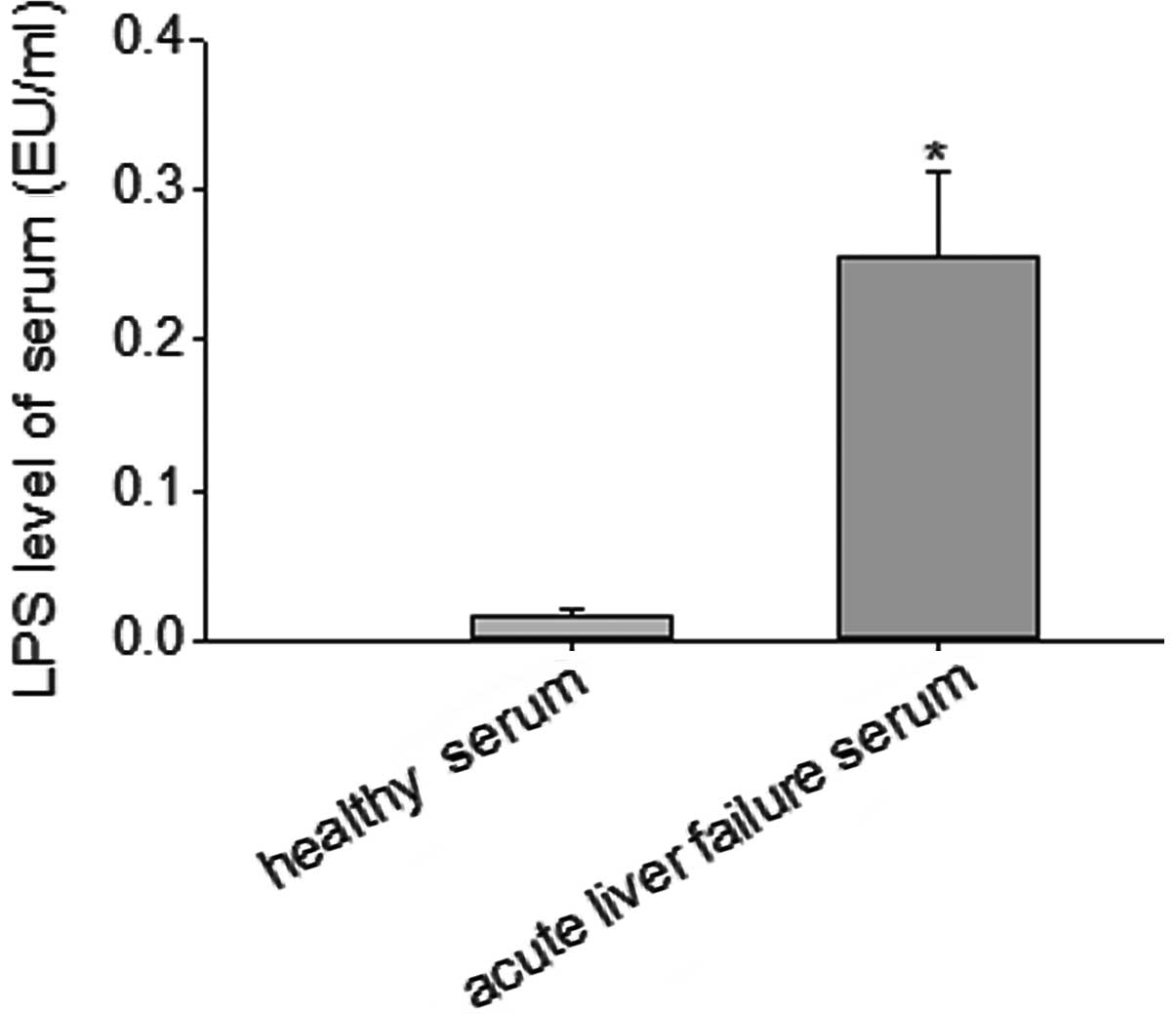
Serum LPS levels of patients with acute
liver failure
Serum was obtained from patients with HEV-related
acute liver failure and normal individuals. A quantitative
tachypleus amebocyte lysate-based endotoxin detection kit was used
to measure the serum levels of LPS. The results indicated that the
serum level of LPS in patients with acute liver failure was
0.26±0.02 EU/ml, which was significantly higher than that of the
healthy individuals (P<0.05; Fig.
1).
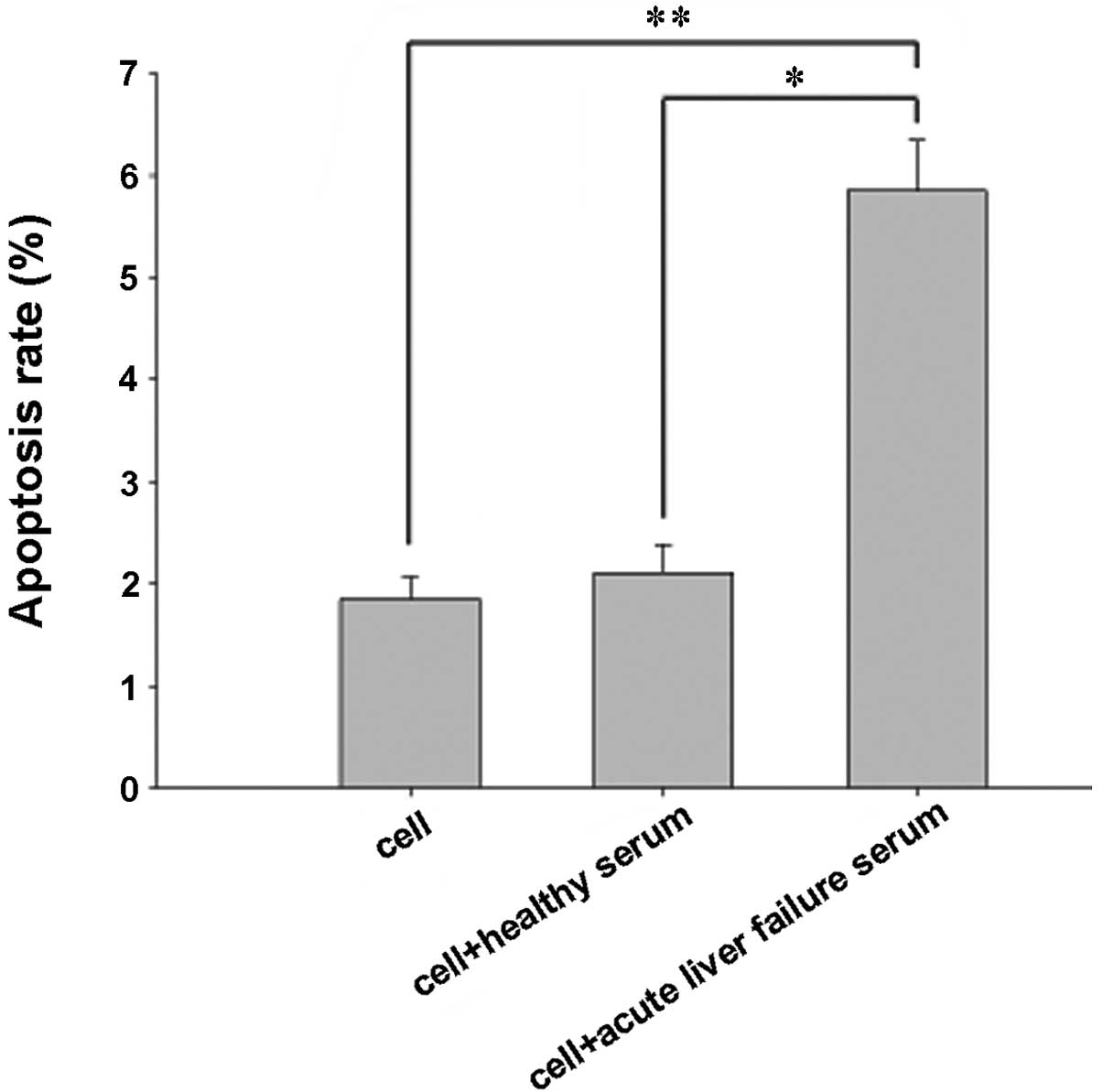
Proapoptotic effects of acute liver
failure serum on cells
To evaluate the proapoptotic effects of acute liver
failure serum on human liver cells, flow cytometry was used to
determine the apoptosis rate. The number of apoptotic cells at the
lower and upper right of the flow cytometric analysis chart
indicated the percentage of early and late apoptotic cells,
respectively. The results indicated that the apoptotic rate of the
cells incubated with acute liver failure serum was 5.83±0.42%, and
that the apoptosis rate was significantly increased by the acute
liver failure serum (P<0.05; Fig.
2).
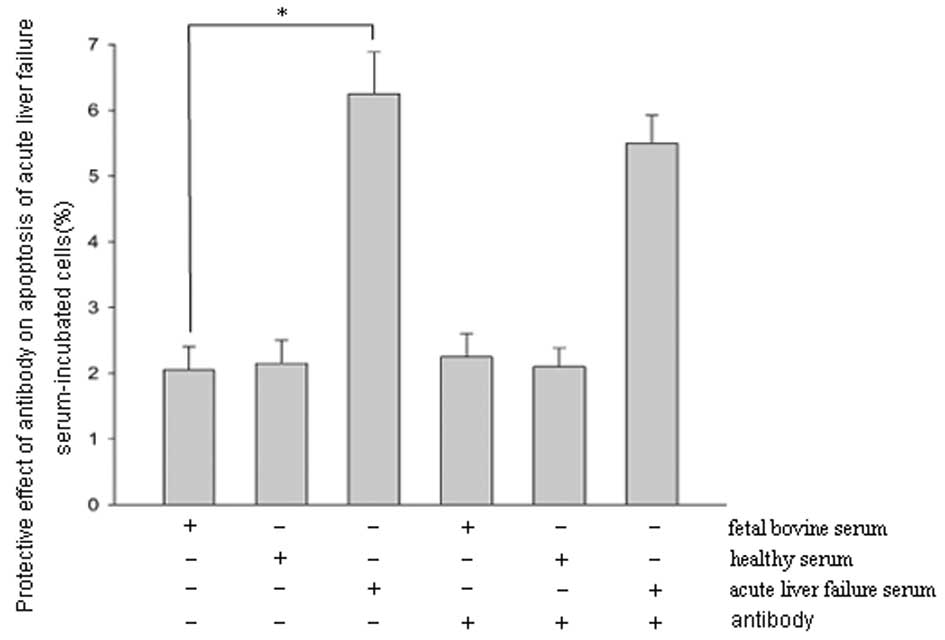
Protective effects of antibody against
acute liver failure serum-induced apoptosis
To evaluate the protective effects of the antibody,
cells were concurrently treated with the serum from patients with
acute liver failure and antibody and examined by flow cytometry.
The results showed that the rate of apoptosis in cells treated with
acute liver failure serum alone was 6.21±0.67%. The rate of
apoptosis decreased after 20 h of incubation with acute liver
failure serum and antibody; however, the reduction was not
statistically significant (Fig.
3).
Discussion
The results of the present study indicate that the
LPS levels in the serum of patients with HEV-related acute liver
failure were significantly increased compared with those of healthy
individuals, and that the acute liver failure serum was able to
induce the apoptosis of liver cells. Anti-LPS antibody recognizing
core polysaccharide demonstrated no significant protective effects
on the apoptosis of cells treated with acute liver failure serum.
The composition of the serum from patients with HEV-related acute
liver failure is complicated and, the present study investigated
only the serum levels of LPS and intervention against LPS using
antibody.
Endotoxin syndrome occurs during the course of acute
liver failure (2). In the present
study, the acute liver failure in the patients was also associated
with endotoxemia. It has been identified that the serum from
patients with fulminant hepatic failure due to paracetamol overdose
has the ability to induce apoptosis in primary hepatocytes
(23). However, the mechanisms
resulting in the apoptosis of liver cells are not fully understood.
In a previous study, the plasma from patients with acute chronic
liver failure did not significantly reduce cytochrome P450 mRNA
expression levels in immortalized human hepatocytes (HepLi-2
cells); however, the ammonia removal and drug metabolism properties
of the cells remained stable following incubation with plasma
(24). Therefore, it may be
speculated that the serum from patients with acute chronic liver
failure does not result in apoptosis of HepLi-2 cells. In the
present study, serum was collected from patients with HEV-related
acute liver failure and the serum levels of LPS were evaluated. LPS
has the ability to induce the apoptosis of liver cells without
macrophages in vitro(25).
Hence, it may be speculated that LPS in the serum may be the direct
cause of the liver cell apoptosis observed in the present study;
however, other substances that cause apoptosis of liver cells must
not be excluded. The discrepancies in the findings concerning the
effects of serum on liver cell survival and apoptosis may be
attributed to several factors, such as differences in the cell
lines, cell density and the sera from patients with liver failure
due to a variety of causes.
The composition of acute liver failure serum is
complex, containing various cytokines, bile salts and a number of
other components. Each ingredient of the serum has different
effects on liver cells, and the mechanisms of action have not been
fully elucidated (26,27). Following the loss of liver
synthesis and excretory functions, the levels of bile salts
significantly increase in patients with liver failure. In initial
studies, bile salts were viewed as intervention targets (28,29),
however, the results were not satisfactory. LPS is the main
component of the gram-negative bacterial cell wall and is
associated with various biological effects (30,31).
LPS consists of an outer membrane macromolecular complex of
polysaccharides, lipids and proteins (32). Lipid A is the most conservative
component of the LPS structure. The antibody against lipid A is
considered to possess the most direct protective effects. In a
study in which freeze-dried human plasma rich in anti-LPS IgG was
used to treat septic shock, anti-LPS appeared to significantly
reduce mortality and morbidity in patients with septicemia
(33). However, the components of
freeze-dried human plasma are complex. Human monoclonal IgM
antibody that binds specifically to the lipid A domain of
endotoxins has been shown to be safe and effective for the
treatment of patients with sepsis and gram-negative bacteremia
(34). However, an additional
study found that anti-lipid A mAbs are not able to attenuate the
toxic effects of LPS (22). The
studies of the protective effects of lipid A antibody have provided
greatly different results. Lipid A may have various epitopes and
different antibodies against the epitopes may have correspondingly
varied biological effects.
Core polysaccharide is a core component of LPS and
following to the in-depth analysis of the lipid A antibody,
scientists gradually shifted their attention to the core
polysaccharide. In the present study, the core polysaccharide serum
antibodies did not demonstrate significant protective effects
against the actions of serum collected from patients with acute
liver failure due to HEV infection. It was possible that the serum
contained other harmful substances, which resisted the protective
effects of the antibody.
In the present study, serum collected from patients
with HEV-related acute liver failure induced apoptosis of human
liver cells. This experiment was an in-depth study concerning the
toxicity of the serum obtained from patients with acute liver
failure. The effects of serum from patients with HEV-related acute
liver failure on the survival and apoptosis rate of human liver
cells were directly explored. However, the mechanisms by which
acute liver failure serum affects liver cell survival and apoptosis
have not been fully elucidated.
Bacterial LPS is an unavoidable aspect of liver
failure development, which directly mediates the apoptosis of liver
cells and aggravates the disease condition (25,35).
However, in the present study, the antibody did not show protective
effects and further study is required. The current study did not
consider the complexity of the serum components and failed to take
appropriate intervention measures. The aim of future studies is to
analyze the different components of acute liver failure serum, as
well as the mechanisms of cell survival and apoptosis affected by
these components. This is likely to assist in clarifying the
pathogenesis of acute liver failure and the therapeutic effects of
LPS antibody on acute liver failure.
References
|
1
|
Lee WM: Acute liver failure. Semin Respir
Crit Care Med. 33:36–45. 2012. View Article : Google Scholar
|
|
2
|
Mas A and Rodés J: Fulminant hepatic
failure. Lancet. 349:1081–1085. 1997. View Article : Google Scholar
|
|
3
|
Macnaughtan J and Thomas H: Liver failure
at the front door. Clin Med. 10:73–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neuberger J: Prediction of survival for
patients with fulminant hepatic failure. Hepatology. 41:19–22.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernal W, Auzinger G, Dhawan A and Wendon
J: Acute liver failure. Lancet. 376:190–201. 2010. View Article : Google Scholar
|
|
6
|
Tanaka Y, Takahashi K, Orito E, et al:
Molecular tracing of Japan-indigenous hepatitis E viruses. J Gen
Virol. 87:949–954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li TC, Chijiwa K, Sera N, et al: Hepatitis
E virus transmission from wild boar meat. Emerg Infect Dis.
11:1958–1960. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chau TN, Lai ST, Tse C, et al:
Epidemiology and clinical features of sporadic hepatitis E as
compared with hepatitis A. Am J Gastroenterol. 101:292–296. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Togo S, Kubota T, Matsuo K, et al:
Mechanism of liver failure after hepatectomy. Nihon Geka Gakkai
Zasshi. 105:658–663. 2004.(In Japanese).
|
|
10
|
Pathil A, Warth A, Chamulitrat W and
Stremmel W: The synthetic bile acid-phospholipid conjugate
ursodeoxycholyl lysophosphatidylethanolamide suppresses
TNFα-induced liver injury. J Hepatol. 54:674–684. 2011.PubMed/NCBI
|
|
11
|
McCloskey P, Tootle R, Selden C, Larsen F,
Roberts E and Hodgson HJ: Modulation of hepatocyte function in an
immortalized human hepatocyte cell line following exposure to
liver-failure plasma. Artif Organs. 26:340–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rolando N, Wade J, Davalos M, Wendon J,
Philpott-Howard J and Williams R: The systemic inflammatory
response syndrome in acute liver failure. Hepatology. 32:734–739.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rolando N, Harvey F, Brahm J, et al:
Prospective study of bacterial infection in acute liver failure: an
analysis of fifty patients. Hepatology. 11:49–53. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaquero J, Polson J, Chung C, et al:
Infection and the progression of hepatic encephalopathy in acute
liver failure. Gastroenterology. 125:755–764. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamimoto M, Mizuno S and Nakamura T:
Reciprocal regulation of IL-6 and IL-10 balance by HGF via
recruitment of heme oxygenase-1 in macrophages for attenuation of
liver injury in a mouse model of endotoxemia. Int J Mol Med.
24:161–170. 2009.PubMed/NCBI
|
|
16
|
McAvoy EF, McDonald B, Parsons SA, Wong
CH, Landmann R and Kubes P: The role of CD14 in neutrophil
recruitment within the liver microcirculation during endotoxemia. J
Immunol. 186:2592–2601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raulf-Heimsoth M, Liebers V and Brüning T:
Mechanism of endotoxin action and pattern of diseases. Gefahrstoffe
Reinhaltung Der Luft. 67:351–353. 2007.(In German).
|
|
18
|
Li T, Hu J, Thomas JA and Li L:
Differential induction of apoptosis by LPS and taxol in monocytic
cells. Mol Immunol. 42:1049–1055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muto Y, Nouri-Aria KT, Meager A, Alexander
GJ, Eddleston AL and Williams R: Enhanced tumour necrosis factor
and interleukin-1 in fulminant hepatic failure. Lancet. 2:72–74.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jalan R, Olde Damink S, Hayes PC, Deutz
NEP and Lee A: Pathogenesis of intracranial hypertension in acute
liver failure: inflammation, ammonia and cerebral blood flow. J
Hepatol. 41:613–620. 2004. View Article : Google Scholar
|
|
21
|
Han DW: Intestinal endotoxemia as a
pathogenetic mechanism in liver failure. World J Gastroenterol.
8:961–965. 2002.PubMed/NCBI
|
|
22
|
Warren HS, Amato SF, Fitting C, et al:
Assessment of ability of murine and human anti-lipid A monoclonal
antibodies to bind and neutralize lipopolysaccharide. J Exp Med.
177:89–97. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Newsome PN, Tsiaoussis J, Masson S, et al:
Serum from patients with fulminant hepatic failure causes
hepatocyte detachment and apoptosis by a beta(1)-integrin pathway.
Hepatology. 40:636–645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du WB, Pan XP, Yu XP, et al: Effects of
plasma from patients with acute on chronic liver failure on
function of cytochrome P450 in immortalized human hepatocytes.
Hepatobiliary Pancreat Dis Int. 9:611–614. 2010.PubMed/NCBI
|
|
25
|
Chen M, Zhou J, Li H, Chen A, Zhang Z and
Tian D: Effects of endotoxin on liver Smac apoptosis channel. J
Huazhong Univ Sci Technolog Med Sci. 28:660–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nie B, Park HM, Kazantzis M, et al:
Specific bile acids inhibit hepatic fatty acid uptake in mice.
Hepatology. 56:1300–1310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li T, Jahan A and Chiang JY: Bile acids
and cytokines inhibit the human cholesterol 7 alpha-hydroxylase
gene via the JNK/c-Jun pathway in human liver cells. Hepatology.
43:1202–1210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reinehr R and Häussinger D: Inhibition of
bile salt-induced apoptosis by cyclic AMP involves serine/threonine
phosphorylation of CD95. Gastroenterology. 126:249–262. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karimian G, Buist-Homan M, Mikus B,
Henning RH, Faber KN and Moshage H: Angiotensin II protects primary
rat hepatocytes against bile salt-induced apoptosis. PLoS One.
7:e526472012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mayorga M, Iborra A, Estany S and Martínez
P: Protective effect of vitamin E in an animal model of LPS-induced
inflammation. Am J Reprod Immunol. 52:356–361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McGhee JR, Kiyono H, Michalek SM, Babb JL,
Rosenstreich DL and Mergenhagen SE: Lipopolysaccharide (LPS)
regulation of the immune response: T lymphocytes from normal mice
suppress mitogenic and immunogenic responses to LPS. J Immunol.
124:1603–1611. 1980.PubMed/NCBI
|
|
32
|
Hitchcock PJ, Leive L, Mäkelä PH,
Rietschel ET, Strittmatter W and Morrison DC: Lipopolysaccharide
nomenclature - past, present, and future. J Bacteriol. 166:699–705.
1986.PubMed/NCBI
|
|
33
|
Lachman E, Pitsoe SB and Gaffin SL:
Anti-lipopolysaccharide immunotherapy in management of septic shock
of obstetric and gynaecological origin. Lancet. 1:981–983. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ziegler EJ, Fisher CJ Jr, Sprung CL, et
al; The HA-1A Sepsis Study Group. Treatment of gram-negative
bacteremia and septic shock with HA-1A human monoclonal antibody
against endotoxin. A randomized, double-blind, placebo-controlled
trial. N Engl J Med. 324:429–436. 1991. View Article : Google Scholar
|
|
35
|
Recknagel P, Gonnert FA, Halilbasic E, et
al: Mechanisms and functional consequences of liver failure
substantially differ between endotoxaemia and faecal peritonitis in
rats. Liver Int. 33:283–293. 2013. View Article : Google Scholar : PubMed/NCBI
|