Introduction
Hepatic veno-occlusive disease (VOD) is a clinical
syndrome characterized by hepatomegaly, ascites, weight gain and
jaundice (1–3). VOD has been described in a patient
who drank an infusion of a traditional herbal medicine that
contained pyrrolizidine alkaloids (4).
Small venous occlusion of the liver was first
described in infants with cirrhosis by McFarlane and Branday
(5) in 1945, and the underlying
liver lesion was recognized as VOD for the first time by Bras et
al(6) in 1954. Classified
small venous occlusion as a VOD was generally accepted from that
date. VOD is frequently observed in individuals who have consumed
wild plants or herbs containing pyrrolizidine alkaloids, and
patients who have undergone hematopoietic stem cell transplantation
(HSCT) (7), chemotherapy or
radiotherapy; however, VOD following liver transplantation is rare.
A patient with VOD following liver transplantation was diagnosed
and treated at The Institute of Organ Transplantation (Beijing,
China) in 2011. Combining the clinical data and relevant
literature, this study aimed to consider the relevant clinical
diagnosis, auxiliary examination features, pathological changes and
treatment of this case, and the possible causes and pathogenesis of
VOD following liver transplantation.
Case report
Patient medical history
This study was approved and registered by The Ethics
Committee of The General Hospital of Chinese People’s Armed Police
Forces in January 2011, the Ethics committee approved relating
screening, treatment and data collection. The patient signed a
written informed consent form. All works were undertaken following
the provisions of the Declaration of Helsinki. A 42-year-old male
who had a long history of taking traditional Chinese medicine
(essential components unknown) underwent an orthotropic liver
transplantation in a local hospital on January 14, 2011, having
been diagnosed with small venous occlusion disease of the liver
subsequent to splenectomy, combined with incurable ascites. The
patient was treated with tacrolimus as an antirejection therapy
following the surgery, and developed right upper quadrant pain and
fatigue. Routine liver function examinations were conducted which
showed that the patient’s alanine transaminase (ALT), aspartate
transaminase (AST) and γ-glutamyl transpeptidase (GGT) levels were
normal and the alkaline phosphatase (ALP) level was 150–250 IU/l.
The total bilirubin (TBIL) level gradually increased to 70 mmol/l
and the direct bilirubin (DBIL) level gradually increased to 50
mmol/l, both of which were outside the normal range. The patient’s
serum albumin (ALB) level ranged from 25 to 30 g/l and
cholinesterase (CHE) level ranged from 500 to 1300 IU/l. The
patient’s renal function test indices were: Urea, 20 mmol/l; uric
acid (UA), 800–1,000 μmol/l; and creatinine (Cr), 130–140 μmol/l.
An ultrasonic B scan of the abdomen showed that there was a low to
moderate level of pleural effusion and a large amount of peritoneal
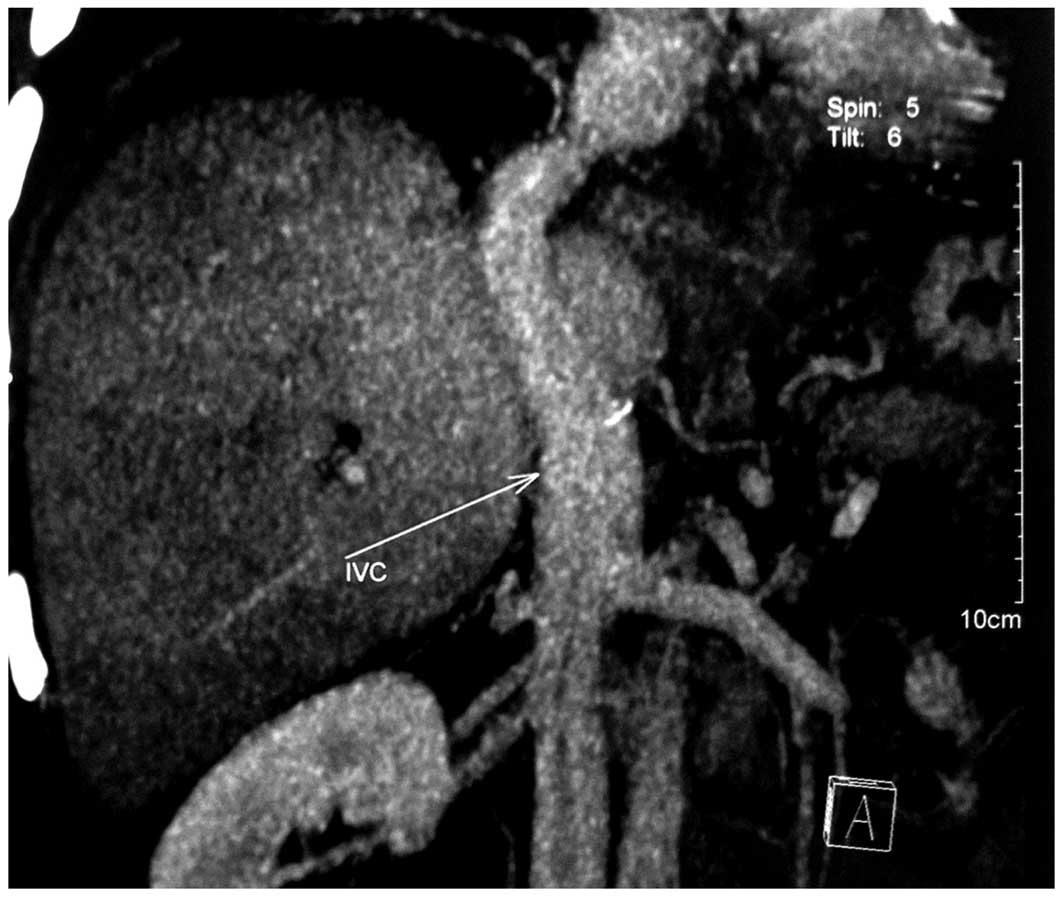
effusion; the abdominal computed tomography (CT) and CT angiography
(CTA) results showed that anastomotic stenosis was present in the
liver artery and inferior vena cava (Fig. 1). The patient received balloon
dilatation in the inferior vena cava three times in the local
hospital. Following an abdominal CT review, it was revealed that
there was no significant stenosis in the inferior vena cava
anastomosis; however, the effusion in the patient’s abdomen did not
decrease. The patient was then prescribed a maintenance treatment
comprising a continual protein supply, diuretic therapy,
intermittent hydrothorax fluid extraction (with the maximum volume
of 1,000 ml yellow-clear fluid) and catheter drainage of ascites
(with a volume of 1,000–1,500 ml/day clear-yellow ascites).
Admission examination
The patient was admitted to The Institute of Organ
Transplantation on April 24, 2011. The examination results on
admission showed that the patient was weak and in a poor general
physical condition. The patient cooperated well, but was only able
to lie, not sit. A moderate yellowish discoloration of the
patient’s skin and sclera was observed. The right inferior
pulmonary percussion result was dull. Auscultation of the
bronchovesicular breath sound was weak, and moist and dry rales, as
well as pleural friction rub sounds, were not audible in both
lungs. The patient’s abdomen was flat and gastroscopy showed no
varicose veins in the gastrointestinal tract. The longitudinal
splenectomy incision scar of the liver transplant procedure was
present in the center of the upper abdomen in an ‘L’ shape, and had
healed well. The patient’s abdomen was soft without tenderness,
rebound pain, muscle tension or masses. The liver tissue 6 cm under
the patient’s xiphoid process and 4 cm under the ribs was harder,
with blunt edges, and auscultation showed a shifting dullness
exited. There was no percussion pain in the region of the liver and
kidneys. The patient’s gurgling sound in the belly had a frequency
of four times per minute and there was no edema in the lower
extremities. The left abdominal drainage tube was not blocked and
the volume of pale yellow ascites drainage was 1,000–1,600 ml per
day, and the urine volume was 500–600 ml per day. The patient’s
routine blood examination, liver and kidney function and T/B
lymphocyte subset test results are shown in Table I. The tests for tests for hepatitis
and liver cancer were normal, the autoimmune hepatitis antibody
spectrum for antinuclear antibody (ANA) was particle type (1:80)
and the immunoglobulin G level and liver fibrosis tests were
normal. Furthermore, the erythrocyte sedimentation rate (ESR),
adenosine deaminase (ADA) and C-reactive protein (CRP) levels,
tuberculosis anti-body (TBAB) and γ-interferon antibody release
test for Mycobacterium tuberculosis in the ascites were all
normal. The patient’s ascitic fluid was yellow, with a total cell
number of 1.12×109/l, and the Rivalta test was negative.
The ascites biochemical test results are shown in Table I. An abdominal ultrasound B-mode
scan showed that there were diffuse lesions in the graft liver,
with no evident blood flow abnormalities, and that the liver was
enlarged. The liver was located 4.5 cm under the ribs, and the
maximum thickness of the right, left and caudate lobes was 13.60,
8.41 and 2.07 cm, respectively. There was moderate peritoneal
effusion and a small amount of right-sided pleural effusion. The
lung CT revealed encapsulated effusion in the right side of the
thorax, and there was right lower pulmonary atelectasis. The
abdominal CT and CTA scan results showed that the liver volume had
increased and that stenosis was present in the hepatic artery
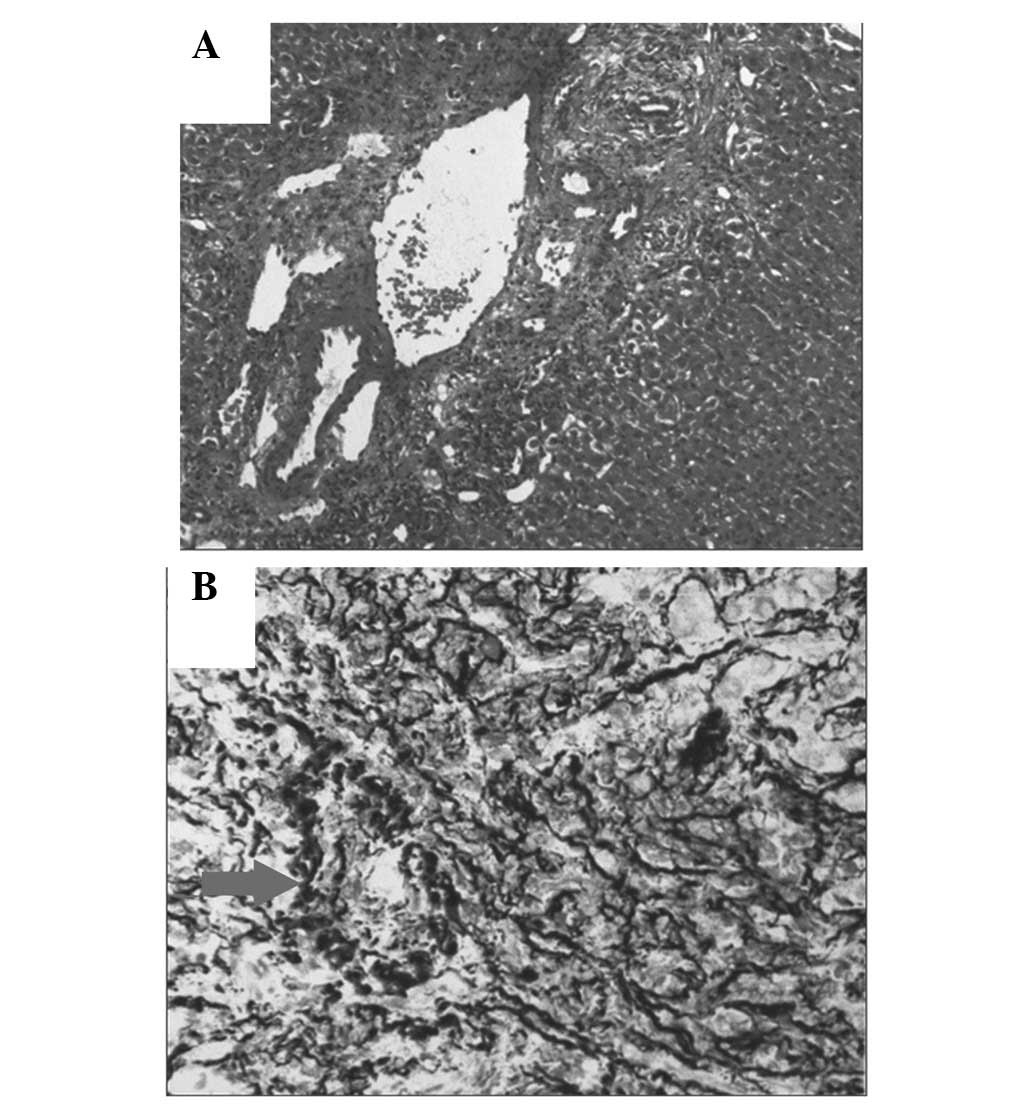
anastomosis. The test results of the liver biopsy (Fig. 2) showed that the hepatic vein was
occluded, and that there was necrosis with blood stasis in the
center of the liver, combined with chronic cholangitis.
 | Table IPatient examination results. |
Table I
Patient examination results.
| Level or
concentration |
|---|
|
|
|---|
| Examination | Admission | 40th day of hospital
treatment |
|---|
| Routine blood
examination |
| WBC (per liter) |
12.67×109 | - |
| NEUT (%) | 41.2 | - |
| HGB (g/l) | 110 | - |
| PLT (per liter) |
270×109 | - |
| Liver and kidney
function |
| ALT (IU/l) | 56 | 29 |
| AST (IU/l) | 170 | 31 |
| GGT (IU/l) | - | 184 |
| ALP (IU/l) | - | 91 |
| TBIL (μmol/l) | 235.1 | 23.3 |
| DBIL (μmol/l) | 169.6 |
141.0×104 |
| ALB (g/l) | 30.1 | 31.6 |
| CHE (IU/l) | 1132 | 2676 |
| B macroglobulin
(mg/l) | 17.42 | 4.26 |
| Urea (mmol/l) | 31.68 | 19.59 |
| UA (μmol/l) | 828 | 529 |
| Cr (μmol/l) | 178 | 81 |
| T/B lymphocyte
subsets |
| CD4 (cells/μl) | 1485 | - |
| FK (ng/ml) | 5068.5 | - |
| Ascites biochemical
tests |
| Glu (mmol/l) | 7.04 | - |
| LDH (U/l) | 68 | - |
| TP (g/l) | 29.8 | - |
| ADA (IU/l) | 6 | - |
| ALB (g/l) | 11.9 | - |
Treatment process
The patient was treated with methylprednisolone (500
mg/day) as a pulse treatment for two days, with adjuvant
antisecretory, gastric mucosa-protective, antibiotic and antiviral
(ganciclovir) therapies. When the patient began vomiting on the
third day, the pulse treatment was stopped and changed to oral
Medrol (Methylprednisolone; 28 mg/day), while the tacrolimus
(Prograf) antirejection therapy was stopped and changed to
sirolimus tablets (2 mg/day). However, the routine blood
examination results for hemoglobin (HGB) decreased significantly
(ranging from 80 to 85 g/l), which resulted in the antirejection
treatment being adapted with enteric-coated mycophenolate sodium
tablets (540 mg/12 h, 3 weeks). In addition, the patient received
fluid infusion and treatments to protect the liver, lower
transaminase levels, remove jaundice and dieresis, increase ALB and
improve the microcirculation of the liver [2 ml/day Prostaglandin
E1 injection and 50 ml/day Danhong injection]. The liver gradually
reduced in size and was located 2 cm under the xiphoid process and
2 cm under the ribs. The peritoneal drainage volume decreased
gradually to 800–1,000 ml/day, the urine volume increased and liver
and renal function improved gradually. The liver and kidney
function examination results on the patient’s 40th day of hospital
treatment (Table I) showed that
the levels of ALT, AST, TBIL, B macroglobulin, DBIL, urea, UA and
Cr had decreased markedly, while the level of CHE had increased.
The results of the abdominal ultrasound B-mode scan showed that no
significant ultrasonographical or blood abnormalities existed in
the graft liver (Fig. 2). The
maximum thickness of right, left and caudate lobes was 11.25, 7.84
and 1.69 cm, respectively.
The general condition of the patient improved
markedly and an increase in appetite, weight gain and the ability
to walk 2–3 km were observed. The patient underwent a transjugular
intrahepatic portosystemic shunt (TIPS) procedure on June 18, 2011
(day 66 of hospital treatment), from which he recovered well. One
week later, the patient’s ascites had disappeared and his liver and
renal function had returned to normal.
Discussion
Medication may lead to the development of VOD
following liver transplantation. Izaki et al(8) described a number of cases of VOD
following liver transplantation; however, the specific mechanism
has not been elucidated. This, in combination with other studies
(9,10), suggests that liver transplantation
may induce postoperative VOD. Previous studies have revealed that
the use of the immunosuppressant azathioprine following
transplantation may damage the end of the small hepatic vein, the
hepatic sinusoidal endothelial cells and the liver cells of the
three zones of the hepatic lobules, causing multi-factorial
abnormal pathophysiological processes, such as immune and
inflammatory reactions and coagulation (11,12),
leading to VOD. In the present case, the patient was treated with
long-term tacrolimus following the liver transplantation, without
any other drugs; we hypothesized that tacrolimus may have been the
cause of the VOD. Since there have been no relevant reports, the
mechanism remains to be elucidated.
The typical symptoms of VOD following liver
transplantation include hepatomegaly, tenderness, weight gain,
peripheral edema, ascites and jaundice. Prior to the onset of these
symptoms, the patient may have had a history of HSCT, chemotherapy,
radiotherapy or liver transplantation, and a long history of
drinking or eating substances containing monocrotaline toxin,
including tea beverages, health products, food and herbal or
certain special medicines. There are two standard diagnostic
references for VOD prior to HSCT and 20 days subsequent to
transplantation. One is the Baltimore VOD diagnostic criteria
(13), which comprises TBIL ≥34
μmol/l, combined with at least two of the following clinical
manifestations: hepatomegaly accompanied by right upper abdominal
pain, ascites and weight gain ≥5%. The other is the Seattle VOD
diagnostic criteria (14), which
includes the presence of at least two of the following
manifestation and symptoms: TBIL ≥34 μmol/l, hepatomegaly, pain in
the right upper quadrant or over the liver, and weight gain >20%
compared with the baseline weight. In the present case, the main
clinical manifestations of the patient were jaundice, hepatomegaly
and pain over the liver and ascites, which were consistent with the
previously mentioned diagnostic standards for VOD.
With regard to the auxiliary examinations for VOD,
liver and kidney function examinations have revealed that, in cases
of mild VOD, patients exhibit a mildly elevated TBIL level, while
in patients with moderate and serious VOD, the TBIL level increases
to 143 and 615 μmol/l, respectively; ALT and ALP levels increase
concurrently (15). In addition,
N-terminal domain type III procollagen peptide levels have been
used in the diagnosis of VOD (16), with the majority of patients with
VOD exhibiting levels of >100 μg/l. Following HSCT, patients’
CRP levels often decrease prior to the development of VOD, showing
a predictive value for VOD of 91%, a specificity of 87% and a
sensitivity of 69% (15). In
addition, plasminogen activator agent inhibitor-1 (PAI-1) levels
have been indicated to be a diagnostic marker of VOD following
HSCT, with levels increasing in the early stages of VOD (17). With regard to imaging examinations,
color Doppler ultrasound is of no use in the diagnosis of VOD.
However, the measurement of wedged hepatic vein pressure and
hepatic venous pressure gradient (HVPG) using an intravenous
cannula may identify whether portal hypertension is a result of
VOD, with HVPG >10 mmHg prompting a diagnosis of VOD (18). Furthermore, irregular small hepatic
vein waves and patchy contrast medium filling the liver parenchyma
in hepatic venography are imaging results that are indicative of
VOD. In addition to the previously mentioned diagnostic methods, an
abdominal CT of patients with VOD typically shows an increased
liver volume and congestion of the liver; however, liver vascular
remodeling does not support the stenosis of the hepatic vein and
inferior vena cava, which is one differentiating factor from
Budd-Chiari syndrome.
In conclusion, the pathophysiology of VOD has not
been fully elucidated. As an increasing number of cases of patients
with VOD following liver transplantation are investigated, it may
be possible to obtain an enhanced understanding of the mechanism
underlying the disease.
References
|
1
|
Richardson P and Guinan E: The pathology,
diagnosis, and treatment of hepatic veno-occlusive disease: current
status and novel approaches. Br J Haematol. 107:485–493. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bearman SI: The syndrome of hepatic
veno-occlusive disease after marrow transplantation. Blood.
85:3005–3020. 1995.PubMed/NCBI
|
|
3
|
McDonald GB, Sharma P, Matthews DE,
Shulman HM and Thomas ED: Venocclusive disease of the liver after
bone marrow transplantation: diagnosis, incidence, and predisposing
factors. Hepatology. 4:116–122. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuckerman M, Steenkamp V and Stewart MJ:
Hepatic veno-occlusive disease as a result of a traditional remedy:
confirmation of toxic pyrrolizidine alkaloids as the cause, using
an in vitro technique. J Clin Pathol. 55:676–679. 2002. View Article : Google Scholar
|
|
5
|
McFarlane AL and Branday WJ: Enlarged
liver with ascites in children. Br Med J. 1:838–840. 1945.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bras G, Jelliffe DB and Stuart KL:
Veno-occlusive disease of liver with nonportal type of cirrhosis,
occurring in Jamaica. AMA Arch Pathol. 57:285–300. 1954.PubMed/NCBI
|
|
7
|
Tascilar NF, Akman-Demir G, Demiryurek BE,
Tokgoz O, Akgun N and Ozen Barut B: An unusual case of
neuro-Behçet’s disease presenting with co-occurence of cerebral
venous sinus thrombosis with basilar artery occlusion. Neurol Sci.
34:785–788. 2013.
|
|
8
|
Izaki T, Inomata Y, Asonuma K, Okajima H,
Ohshiro H, et al: Early graft failure due to a veno-occlusive
disease after a pediatric living donor liver transplantation.
Pediatr Transplant. 8:301–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamada N, Urahashi T, Ihara Y, Sanada Y,
Wakiya T, et al: Veno-occlusive disease/sinusoidal obstruction
syndrome associated with potential antibody-mediated rejection
after pediatric living donor liver transplantation: a case report.
Transplant Proc. 44:810–813. 2012. View Article : Google Scholar
|
|
10
|
Wozniak LJ, Yang HM, Lassman CR, Federman
N and Wu SS: Extramedullary acute myelocytic leukemia following
liver transplantation for VOD with immunodeficiency. J Pediatr
Gastroenterol Nutr. 53:346–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurzawski M, Dziewanowski K, Safranow K
and Drozdzik M: Polymorphism of genes involved in purine metabolism
(XDH, AOX1, MOCOS) in kidney transplant recipients receiving
azathioprine. Ther Drug Monit. 34:266–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dumortier J, Guillaud O, Pittau G,
Salandre J, Adham M, et al: Introduction of mycophenolate mofetil
in maintenance liver transplant recipients: what can we expect?
Results of a 10-year experience. Transplant Proc. 42:2602–2606.
2010.PubMed/NCBI
|
|
13
|
Giles FJ, Kantarjian HM, Kornblau SM,
Thomas DA, Garcia-Manero G, et al: Mylotarg (gemtuzumab ozogamicin)
therapy is associated with hepatic venoocclusive disease in
patients who have not received stem cell transplantation. Cancer.
92:406–413. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dignan FL, Wynn RF, Hadzic N, et al:
BCSH/BSBMT guideline: diagnosis and management of veno-occlusive
disease (sinusoidal obstruction syndrome) following haematopoietic
stem cell transplantation. Br J Haematol. September 17–2013.(Epub
ahead of print).
|
|
15
|
Liang KH: Hepatology. Hepatic Vascular
Disease. People’s Medical Publ Corp; Beijing: pp. 576–638. 2008
|
|
16
|
Rio B, Bauduer F, Arrago JP and Zittoun R:
N-terminal peptide of type III procollagen: a marker for the
development of hepatic veno-occlusive disease after BMT and a basis
for determining the timing of prophylactic heparin. Bone Marrow
Transplant. 11:471–472. 1993.PubMed/NCBI
|
|
17
|
Kaleelrahman M, Eaton JD, Leeming D,
Bowyer K, et al: Role of plasminogen activator inhibitor-1 (PAI-1)
levels in the diagnosis of BMT-associated hepatic veno-occlusive
disease and monitoring of subsequent therapy with defibrotide (DF).
Hematology. 8:91–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Senzolo M, Germani G, Cholongitas E, Burra
P and Burroughs AK: Veno occlusive disease: update on clinical
management. World J Gastroenterol. 13:3918–3924. 2007. View Article : Google Scholar : PubMed/NCBI
|