Introduction
15-hydroxyprostaglandin dehydrogenase (15-PGDH) is a
major rate-limiting enzyme in the biodegradation of PG. 15-PGDH was
first identified as a novel colorectal cancer gene. Previous
studies have indicated that 15-PGDH is closely associated with the
occurrence and development of numerous tumors in vivo and a
reduction in its expression level promotes the occurrence,
development, infiltration and metastasis of tumors, as well as the
formation of tumor blood vessels (1,2).
Gastric cancer is a commonly observed malignant tumor worldwide.
Although its incidence rate has decreased in developed countries,
its mortality rate has not markedly declined. Among all malignant
tumors, its mortality rate ranks second in the world and first in
China. Studies show that 15-PGDH expression levels decrease
significantly in gastric cancer cell strains, while the restoration
of its expression induces the apoptosis of gastric cancer cells and
blocks the cell cycle (3). These
results further indicate that 15-PGDH may be important in
inhibiting the occurrence, development, infiltration and metastasis
of gastric cancer, and may become a new target for treatment of
gastric cancer.
The aim of the present study was to restore 15-PGDH
expression in cultured gastric cancer cells in vitro using
gene transfection technology and to observe the inhibitory effect
of 15-PGDH on the proliferation of gastric cancer cells. These
results are likely to provide a theoretical basis for further in
vivo studies and new indications for developing therapeutic
drugs targeting gastric cancer.
Materials and methods
Reagents, cell strains and plasmid
15-PGDH primers were synthesized by Sangon Biotech
Co., Ltd. (Shanghai, China). pMD18-T vectors and T4 ligase were
purchased from Takara Bio, Inc. (Dalian, China). pcDNA3.1
eukaryotic expression vectors and Lipofectamine 2000 were purchased
from Invitrogen Life Technologies (Carlsbad, CA, USA). BALB/c
female mice (weighing 17–20 g) were purchased from the Experimental
Animal Center of Yunnan (Kunming, China). The study was approved by
the ethics committee of The First Hospital of Yunnan Province,
Kunming, China.
Construction of recombinant plasmids
Total RNA was extracted from the gastric tissues of
each BALB/c mouse using TRIzol reagent. The RT-PCR amplification
mixture contained the following primers: 15-PGDH,
5′-AAGCTTCTGCACCATGCACGTGA-3′ (upstream) and
5′-GCGGATCCTTCAGCTATGGCTAAC-3′ (downstream). AAGCTT and GGATCC are
recognition sequences of the restriction endonucleases,
HindIII and BamHI. PCR products were combined with
the pMD-18T simple vector to construct the cloning vector
pMD18-T/15-PGDH, the double-enzyme cleavage cloning vector
pMD18-T/15-PGDH with two restrictive endonucleases HindIII
and XbaI and the empty vector pcDNA3.1. T4 DNA ligase
connected with the target gene fragments of 15-PGDH and the linear
plasmid of pcDNA3.1. The connected product (pcDNA3.1/15-PGDH)
transforms Escherichia coli DH5α. Following this, positive
clones were screened and plasmids were extracted for enzyme
cleavage identification and sequencing identification.
Cell transfection and screening of stable
transfected cell strains
During culture of the gastric cancer cell murine
foregastric carcinoma (MFC), 24-mesh cell culture plates were used
to inoculate 1 ml culture solution containing 0.6×105
cells, using Lipofectamine 2000 as a transfection reagent. MFC
cells were transfected separately with pcDNA 3.1 and pcDNA
3.1/15-PGDH for 4–6 h. Next, complete culture solutions were
changed, and following subculture, G418 was added to screen the
stable transfected cell strains, which were frozen until use.
Drawing of cell growth curves
The MTT assay was used to cultivate
15-PGDH-transfected, empty vector-transfected and parent cells. The
culture plates were collected on days 1–6. Subsequently, 20 μl MTT
(5 mg/ml) and 100 μl dimethyl sulfoxide were added to each mesh for
detection of absorbance at 490 nm using an ELISA. This absorbance
is also called optical density, which reflects the cell count and
may be used to construct cell growth curves.
Detection of 15-PGDH expression by
reverse transcription polymerase chain reaction (RT-PCR)
The 15-PGDH-transfected, empty vector-transfected
and untransfected cells were separately cultured to extract total
RNA and to synthesize cDNA through reverse transcription. 15-PGDH
genes were amplified by PCR. The amplified products were identified
and analyzed via agarose gel electrophoresis.
Clone formation
The 15-PGDH-transfected, empty vector-transfected
and untransfected cells were separately cultured in a 5%
CO2 incubator at 37°C and saturated humidity for 10–14
days until cloning formation was observed. Subsequently, the
culture solutions were discarded. The cells were fixed by methanol
at room temperature for 10 min and stained by 0.4% crystal violet
for 10 min, followed by 3 washings with sterile water. The cells
were observed under a light microscope (Guiguang Company, Guilin,
China) to determine cell number, and images were captured of the
colonies in each dish. Each experiment was repeated three times.
Plating efficiency (PE) was calculated as follows: PE = (average
colony count/inoculation count) × 100.
Statistical methods
All data were analyzed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA) The means were compared between two and between
multiple using t-tests and Student Newman Keuls, respectively.
P<0.05 and P<0.01 were considered to indicate an extremely
significant difference.
Results
Identification of recombinant plasmids by
RT-PCR
Competent DH5α was transformed by recombinant
plasmids and 15-PGDH genes in colonies were amplified by PCR. The
products were observed using 1% agarose gel electrophoresis.
Observation of a specific band at ~845 bp represented the size of
the target fragment (Fig. 1).
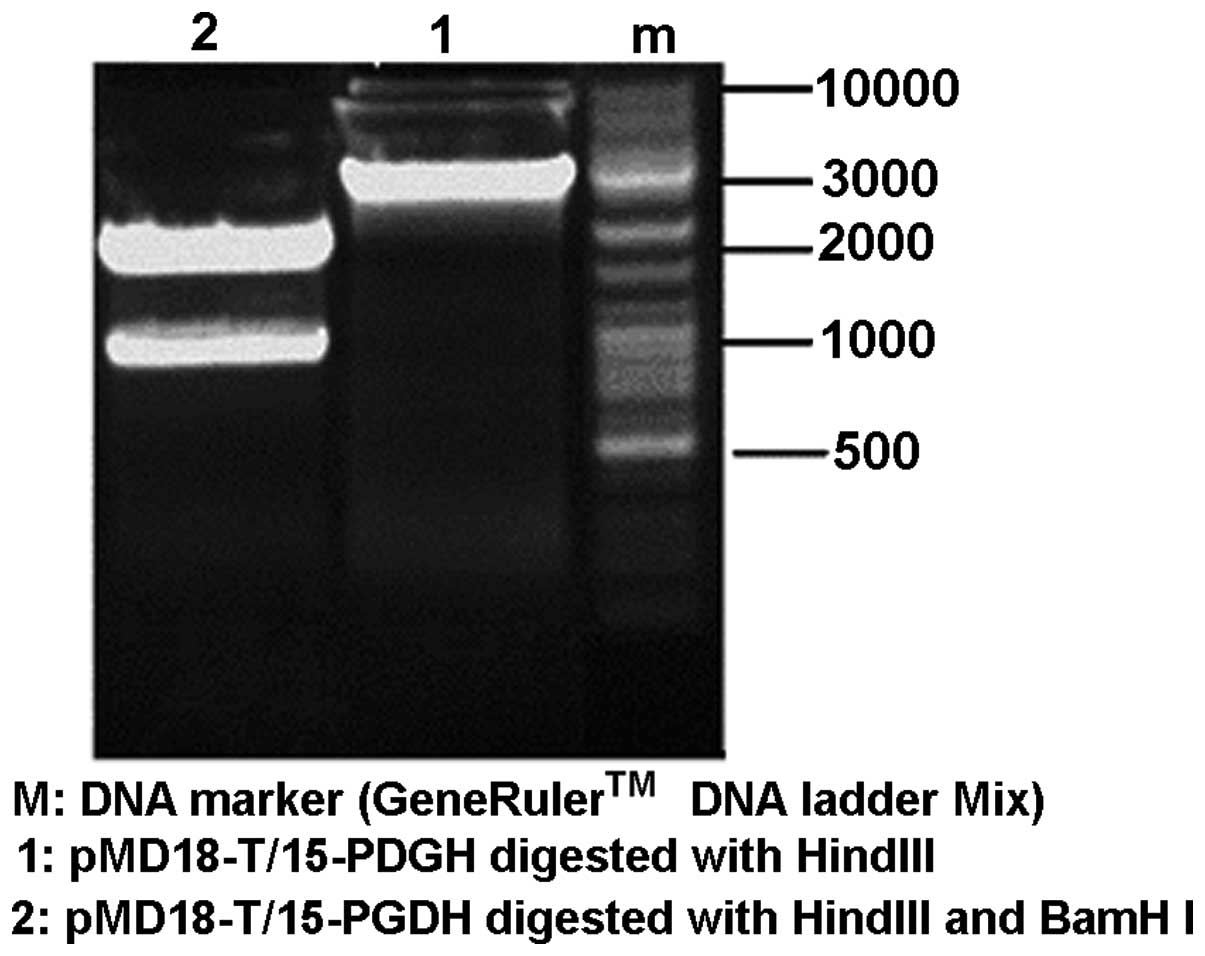
Identification of the cloning vector
pMD18-T/15-PGDH using enzyme cleavage
After the pMD18-T/15-PGDH vector was cleaved by
restriction endonucleases, HindIII and BamHI, two DNA
bands of ~2,700 and 845 bp in length were obtained (Fig. 2). This indicated that the gene
sequence of 15-PGDH was successfully constructed into the
EcoRV multiple clone site on the cloning vector pMD18-T.
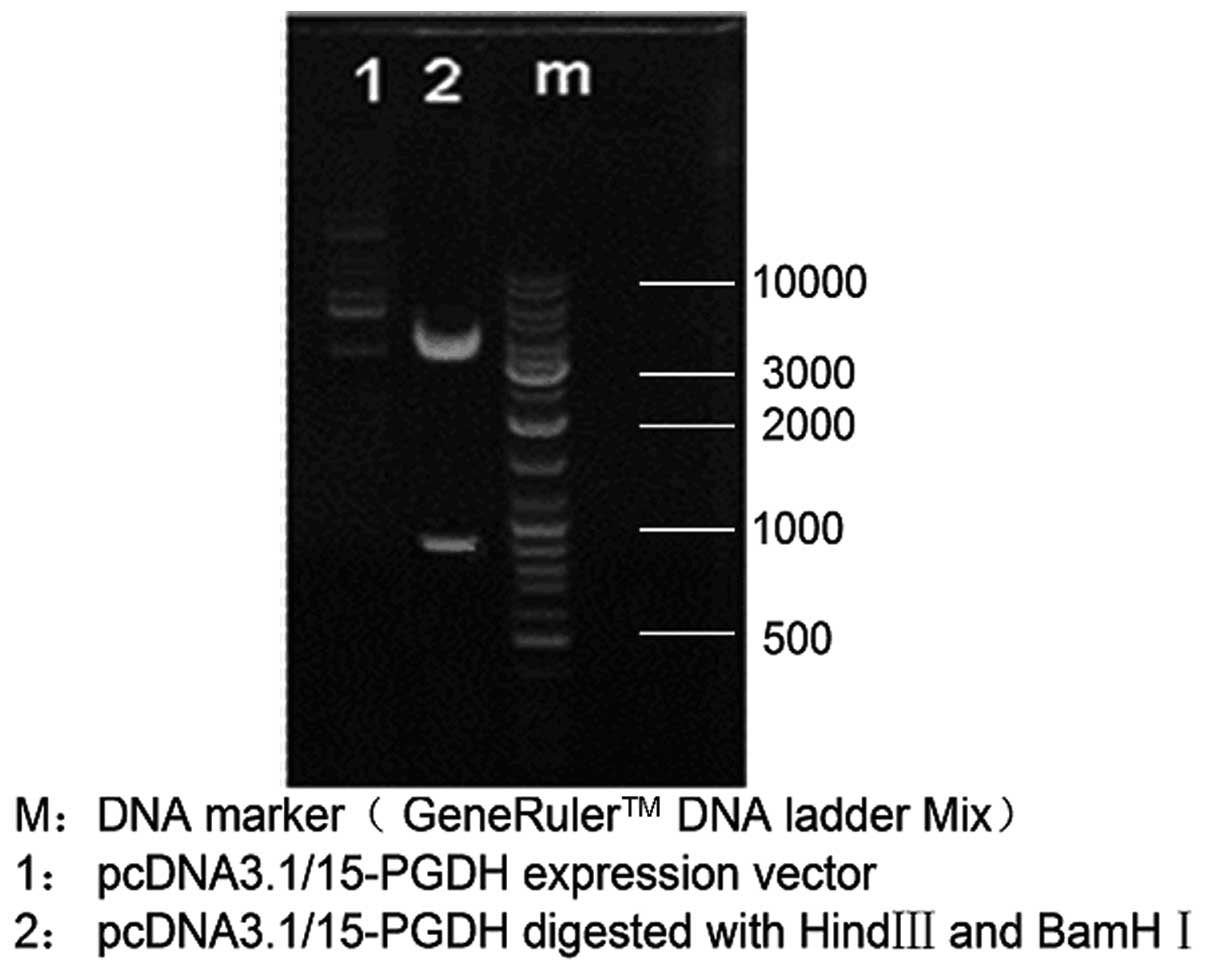
Identification of eukaryotic expression
vector pcDNA3.1/15-PGDH using enzyme cleavage
HindIII and BamHI were used in double
enzyme cleavage of the constructed vector pcDNA3.1/15-PGDH and the
results (Fig. 3) indicated that
the sequence of 15-PGDH was successfully constructed into the
eukaryotic expression vector, pcDNA 3.1.
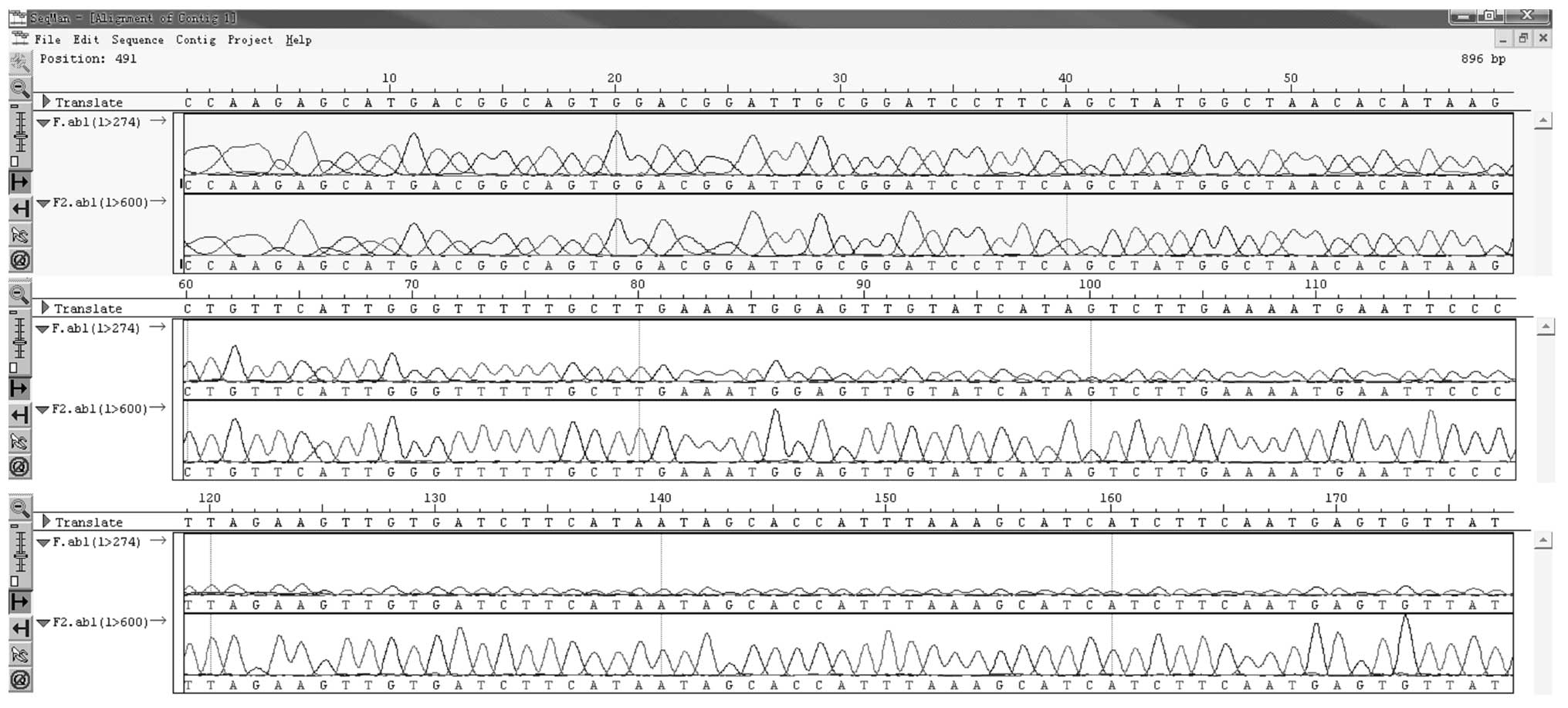
Sequencing identification of recombinant
plasmid
The recombinant plasmid pcDNA3.1/15-PGDH was
sequenced. The PCR-obtained 15-PGDH gene fragment was fully
consistent with the sequence of the 15-PGDH open reading frame in
the NCBI nr database, showing 100% consistency. A section of the
sequence is showed in Fig. 4.
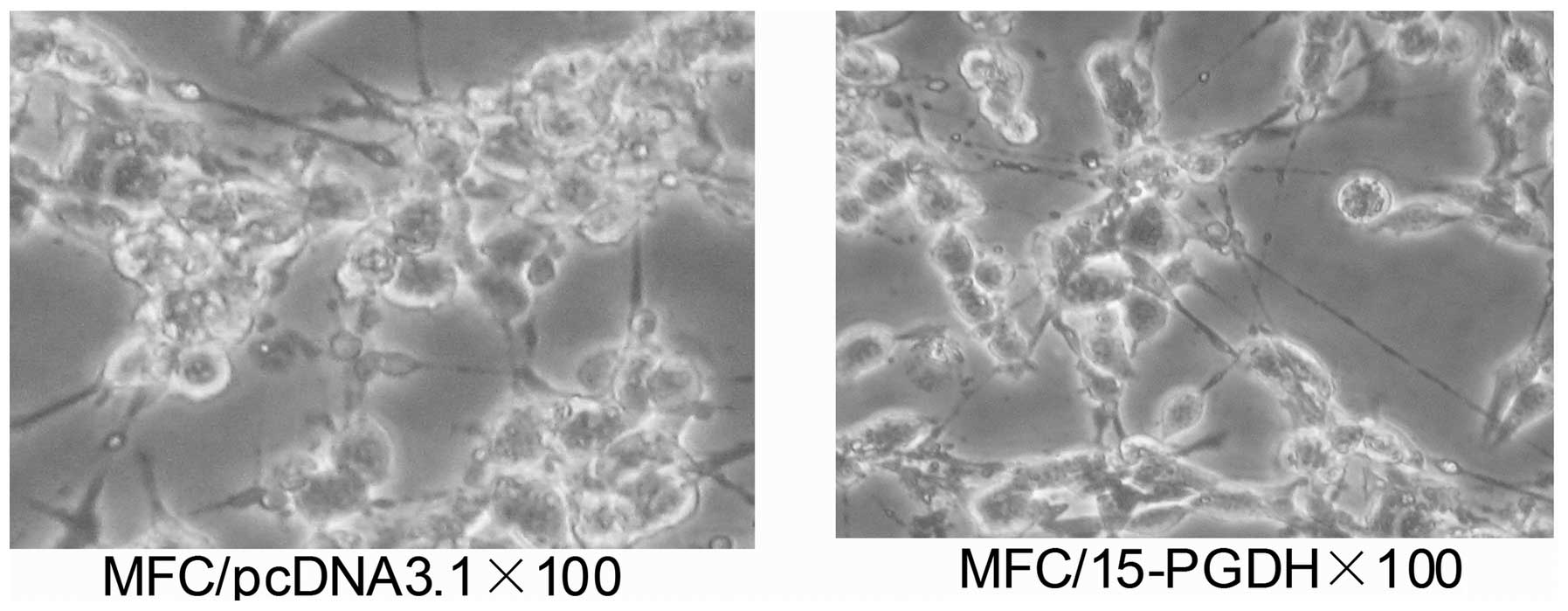
Morphologic changes following
transfection of MFC cells with the recombinant plasmids
Following transfection with 15-PGDH, significantly
less cell stacking or multilayers were observed. The length of the
cell body increased, cytoplasm transparency decreased and granular
secretion increased (Fig. 5).
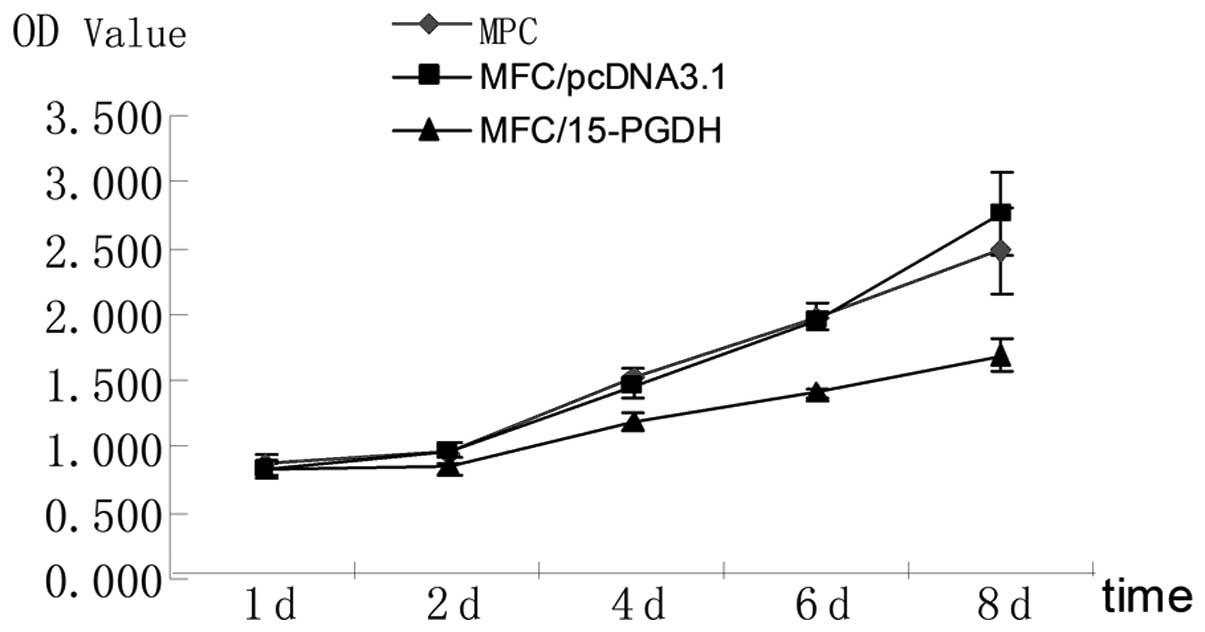
Determination of cell growth curves
The MTT results indicated that on days 4, 6 and 8,
the growth of the 15-PGDH-transfected cells slowed down
significantly (P<0.05) compared with the parent and empty
vector-transfected cells (Table
I). These results demonstrated that 15-PGDH exerts specific
inhibitory effects on the growth of gastric cancer cells (Fig. 6).
 | Table IMTT results of cells in each group
(mean ± standard deviation). |
Table I
MTT results of cells in each group
(mean ± standard deviation).
| Time (days) | MFC | MFC/pcDNA3.1 | MFC/15-PGDH |
|---|
| 1 | 0.865±0.062 | 0.822±0.067 | 0.832±0.064 |
| 2 | 0.953±0.066 | 0.975±0.091 | 0.846±0.074 |
| 4 | 1.534±0.082 | 1.452±0.062 | 1.173±0.072a |
| 6 | 1.972±0.073 | 1.955±0.098 | 1.405 ±0.040a |
| 8 | 2.477±0.319 | 2.755±0.324 | 1.689±0.116a |
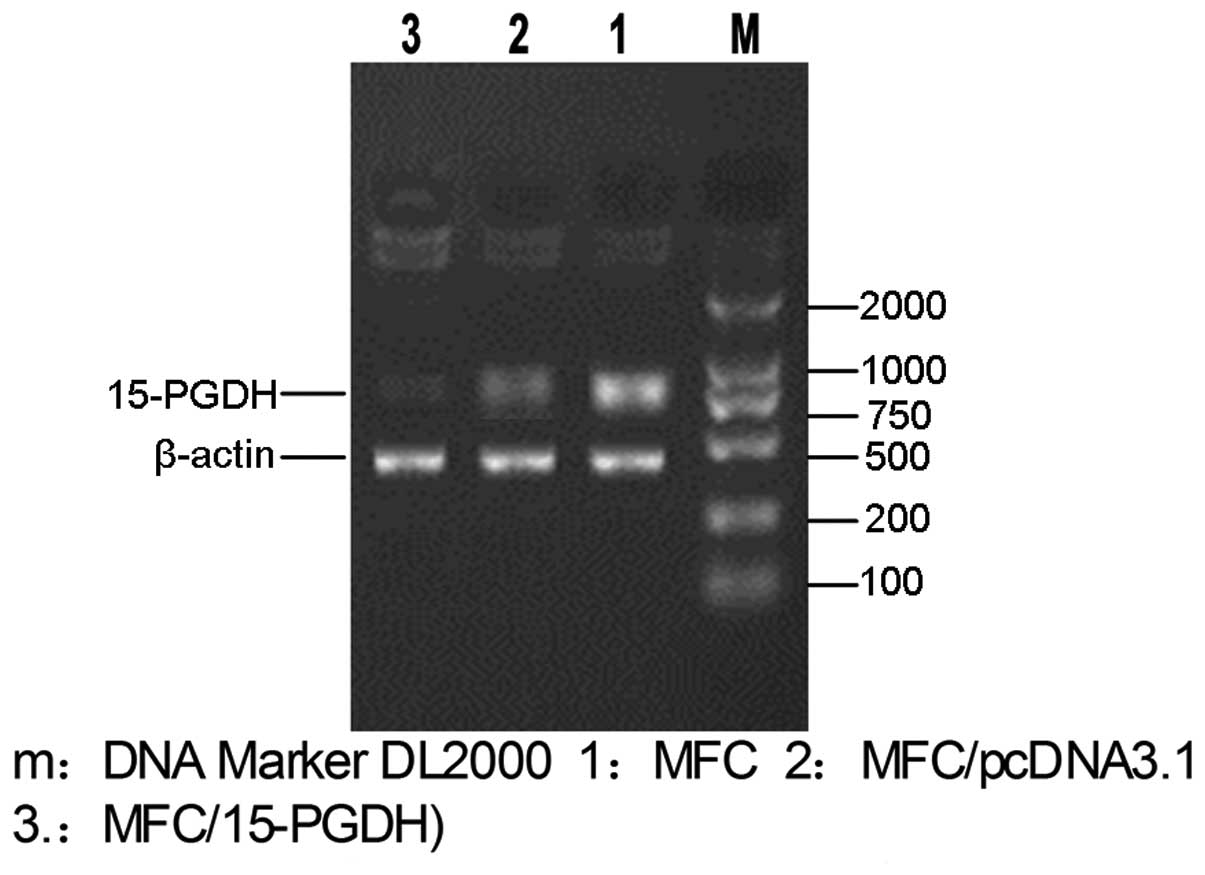
15-PGDH expression following transfection
of MFC cells with recombinant plasmids
MFC cells are a type of gastric cancer cells and
have low expression levels of 15-PGDH. Following extraction of
total RNA from cDNA of the empty plasmid-transfected and the
recombinant plasmid-transfected cells (MFC/15-PGDH), the 15-PGDH
genes were amplified. The results showed that 15-PGDH expression
levels were low in MFC/pcDNA3.1 cells but high in MFC/15-PGDH cells
with clear bands (Fig. 7).
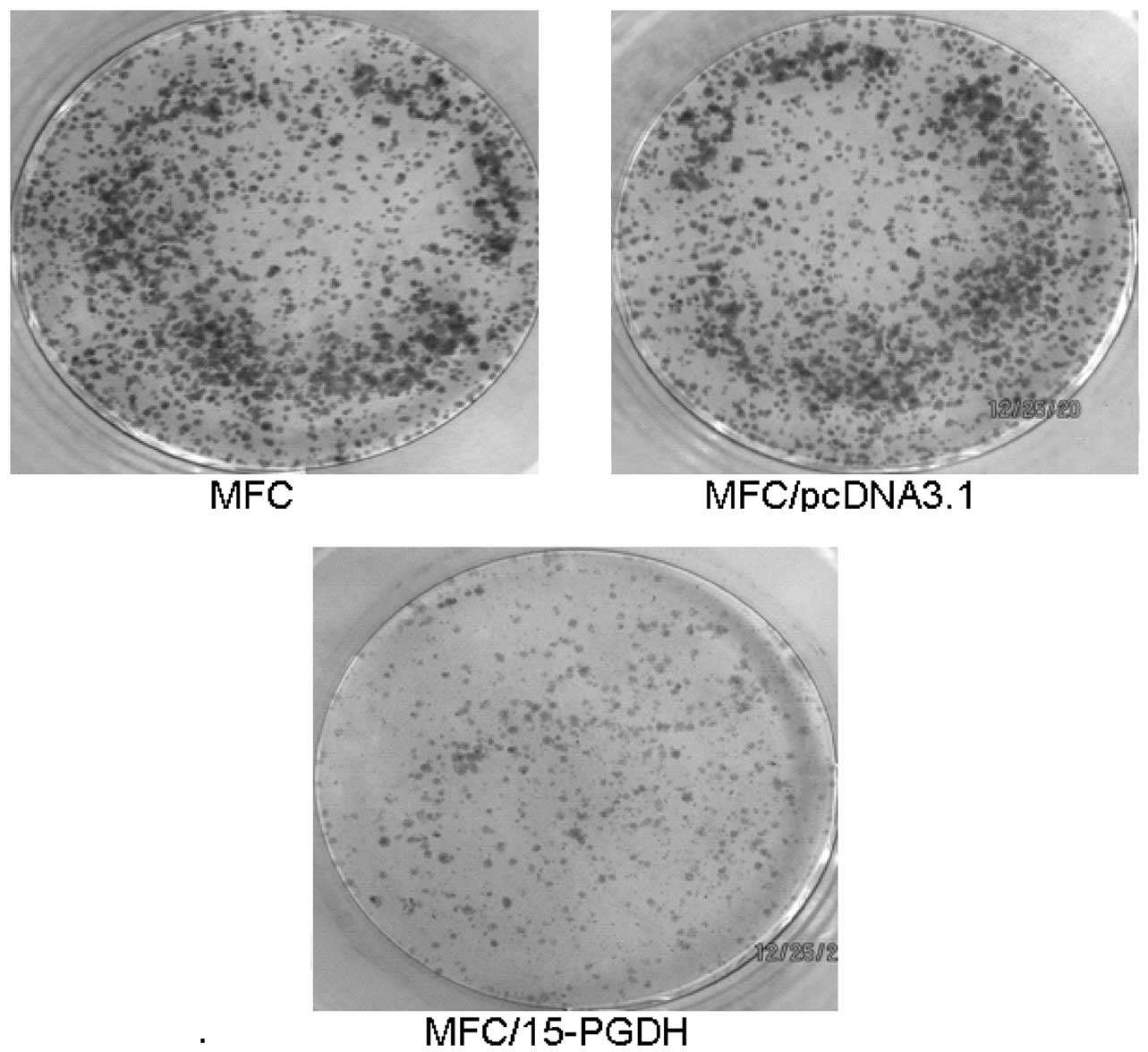
15-PGDH transfection inhibits clone
formation in MFC proliferation
Cells in the control groups showed visible colonies
after only 10 days of inoculation (Fig. 8). The experimental group
(recombinant plasmid-transfected cells) had a PE of 18%, which is
significantly lower (P<0.01) than the untransfected cells (63%)
and the empty vector-transfected cells (59%). These results
indicated that the expression of 15-PGDH may inhibit the
proliferation of gastric cancer cells.
Discussion
15-PGDH is a tumor suppressor gene, however, the
mechanism by which it inhibits tumor proliferation is yet to be
elucidated. Three theories have been put forward. Firstly, 15-PGDH
inhibits tumor proliferation through antagonism of COX-2; COX-2 and
the PGE2 it synthesizes may irritate the development of tumors by
regulating their growth, proliferation and infiltration, the
formation of blood vessels and the apoptosis of tumor cells
(4). 15-PGDH may degrade PGE2 and
thus exhibit natural antagonism against COX-2 (5–8). The
second theory relates to the regulation of apoptosis. Previously,
Li et al(3) reported that
following transfection by 15-PGDH, the expression of proapoptosis
genes, BAK, BAX and p53, increase, while the expression of
anti-apoptosis genes, BCL-2 and BCL-XL, decrease. 15-PGDH may
induce the apoptosis of SGC-7901 gastric cancer cells and inhibit
the cell cycle. Thirdly, the irregular methylation in the promoter
zone of 15-PGDH gene may cause its expression loss and after its
methylation is reversed and 15-PGDH proteins may be reexpressed
(9).
Expression of 15-PGDH is reduced, lost or its
bioactivity is decreased in a number of malignant tumors (for
example, colon cancer, gastric cancer, non-small cell lung cancer
and prostate cancer). These may be the early events upon the
occurrence of tumors (10,11). The occurrence and development of
tumors may be inhibited if the expression of 15-PGDH is restored.
This theory has been preliminarily proven in colon cancer and
non-small cell lung cancer. Firstly, 15-PGDH genes were transferred
into Vaco-400 colon cancer cells and injected into the forelimb of
a nude mouse transplantation tumor experiment through injection
into the forelimb. Although 15-PGDH expression was still lower than
in normal cells, tumor growth slowed significantly compared with
the control group (12). Secondly,
in a A549 nude mice transplantation tumor model of non-small cell
lung cancer, the restoration of 15-PGDH expression was also
demonstrated to significantly inhibit the growth of tumors
(13).
The existence of the correlation between 15-PGDH and
gastric cancer is rarely reported. Specific studies have shown that
15-PGDH expression is reduced or lost in gastric cancer tissues and
is significantly associated with the pathological type, degree of
differentiation, the occurrence of distant metastasis and TNM
staging (14,15). Whether restoration of 15-PGDH
expression inhibits the growth and metastasis of gastric cancer
cells has not been reported. To the best of our knowledge, the
present study is the first discussion of this inhibition.
In the present study, a eukaryotic expression vector
pcDNA3.1-PGDH was constructed. Subsequently, this recombinant
plasmid was used to transfect gastric cancer MFC cells. The
relative expression levels of 15-PGDH increased significantly
compared with the empty vector-transfected group (control group).
The effects of 15-PGDH transfection on the proliferation of MFC
were observed. The growth curves show that on days 4,6 and 8, the
growth of the 15-PGDH-transfected cells was markedly reduced,
indicating that 15-PGDH has specific inhibitory effects on the
growth of gastric cancer cells. Clone formation experiments
revealed that PE was 18% in the recombinant plasmid-transfected
cells and 63% in the untransfected cells, indicating that 15-PGDH
expression may inhibit the proliferation of gastric cancer
cells.
In general, the restoration of 15-PGDH expression
inhibits the proliferation of gastric cancer cells. The reduction
or loss of 15-PGDH expression is closely correlated with the
occurrence of gastric cancer. Results of the present study indicate
that 15-PGDH may play an important role in inhibiting the
occurrence, development, infiltration and metastasis of gastric
cancer cells. In addition, 15-PGDH may represent a novel target for
the prevention and treatment of gastric cancer (16).
References
|
1
|
Myung SJ, Rerko RM, Yan M, et al:
15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of
colon tumorigenesis. Proc Natl Acad Sci USA. 103:12098–12102. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rao CV, Wang CX, Simi B, et al:
Enhancement of experimental colon cancer by genistein. Cancer Res.
57:3717–3722. 1997.PubMed/NCBI
|
|
3
|
Li HL, Jing DD, Lai YX, et al: 15-PGDH is
reduced and induces apoptosis and cell cycle arrest in gastric
carcinoma. World J Gastroenterol. 18:1028–1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jang TJ, Jeon KH and Jung KH:
Cyclooxygenase-2 expression is related to the
epithelial-to-mesenchymal transition in human colon cancers. Yonsei
Med J. 50:818–824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts HR, Smartt HJ, Greenhough A, et
al: Colon tumour cells increase PGE(2) by regulating COX-2 and
15-PGDH to promote survival during the microenvironmental stress of
glucose deprivation. Carcinogenesis. 32:1741–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun WH, Zhu F, Chen GS, et al: Blockade of
cholecystokinin-2 receptor and cyclooxygenase-2 synergistically
induces cell apoptosis and inhibits the proliferation of human
gastric cancer cells in vitro. Cancer Lett. 263:302–311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Z, Wang X, Lu Y, et al: Expression of
15-PGDH is downregulated by COX-2 in gastric cancer.
Carcinogenesis. 29:1219–1227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moore AE, Greenhough A, Roberts HR, et al:
HGF/Met signalling promotes PGE(2) biogenesis via regulation of
COX-2 and 15-PGDH expression in colorectal cancer cells.
Carcinogenesis. 30:1796–1804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaliberova LN, Kusmartsev SA,
Krendelchtchikova V, et al: Experimental cancer therapy using
restoration of NAD+-linked 15-hydroxyprostaglandin
dehydrogenase expression. Mol Cancer Ther. 8:3130–3139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tseng-Rogenski S, Gee J, Ignatoski KW, et
al: Loss of 15-hydroxyprostaglandin dehydrogenase expression
contributes to bladder cancer progression. Am J Pathol.
176:1462–1468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiel A, Ganesan A, Mrena J, et al:
15-hydroxyprostaglandin dehydrogenase is down-regulated in gastric
cancer. Clin Cancer Res. 15:4572–4580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan M, Rerko RM, Platzer P, et al:
15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist,
is a TGF-beta-induced suppressor of human gastrointestinal cancers.
Proc Natl Acad Sci USA. 101:17468–17473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding Y, Tong M, Liu S, et al:
NAD+-linked 15-hydroxyprostaglandin dehydrogenase
(15-PGDH) behaves as a tumor suppressor in lung cancer.
Carcinogenesis. 26:65–72. 2005.PubMed/NCBI
|
|
14
|
Song HJ, Myung SJ, Kim IW, et al:
15-hydroxyprostaglandin dehydrogenase is downregulated and exhibits
tumor suppressor activity in gastric cancer. Cancer Invest.
29:257–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lou LH, Jing DD, Lai YX, et al: 15-PGDH is
reduced and induces apoptosis and cell cycle arrest in gastric
carcinoma. World J Gastroenterol. 18:1028–1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Na HK, Park JM, Lee HG, et al:
15-Hydroxyprostaglandin dehydrogenase as a novel molecular target
for cancer chemoprevention and therapy. Biochem Pharmacol.
82:1352–1360. 2011. View Article : Google Scholar : PubMed/NCBI
|