Introduction
Herpes stromal keratitis (HSK), caused by herpes
simplex virus type 1 (HSV-1) infection of the eye, is an eye
disease that severely affects visual function, with a high
blindness and relapse rate. Matrix metalloproteinases (MMPs) are a
family of protein-cleaving enzymes that degrade the extracellular
matrix (ECM) and basement membrane components. They are involved in
a number of physiological processes, including damage repair, and
in the pathological processes of specific diseases, for example
tumor metastasis and keratopathy. A previous study (1) showed that, following HSV-1 infection
of the cornea, MMP-2 (produced by corneal cells and corneal
epithelial cells) plays an important role in the development of
HSK-induced corneal ulceration. However, these observations only
confirmed a change in MMP-2 expression levels and activity in the
HSK model and the mechanisms by which HSK affects MMP-2 expression,
secretion and activity, remain unclear.
Previous studies have shown that the secretion of
MMPs may be regulated by activating multiple intracellular
signaling pathways, including the focal adhesion kinase (FAK)
signaling pathway (2). FAK is an
important non-receptor protein tyrosine kinase that is important in
intercellular adhesion and adhesion between cells and the ECM.
Simultaneously, it is closely associated with embryonic
development, cell cycle regulation and angiogenesis. Following
activation, FAK undergoes a conformational change which results in
the exposure of the catalytic domain, simultaneous to
autophosphorylation and formation of phosphorylated-FAK (p-FAK)
(3). After activation, FAK is
widely involved in a variety of biological processes through
multiple pathways, including the integrin pathway (4). Previous studies (5–8) have
demonstrated that activation of FAK may result in the formation of
the FAK-Src-p130Cas-Dock180 signaling complex and elevated
activation of the c-Jun NH2-terminal kinase (JNK). Thereby,
expression levels and activity of MMP is increased. We hypothesize
that HSV-1 infection of corneal epithelial cells may activate FAK
through the virus or via the release of cytokines by inflammatory
cells, resulting in increased MMP levels in the corneal tissue and
accelerated formation of corneal ulcers and necrotic lesions. The
present study investigated the secretion of MMP-2, which is
directly induced by HSV-1 infection, and its association with FAK
and p-FAK expression, using immunohistochemical staining and
molecular biology techniques to further explore the mechanisms of
corneal ulceration in HSK.
Materials and methods
Construction of an experimental animal
model of HSK
The Institutional Animal Care and Use Committee of
Wuhan University (Wuhan, China) approved the study protocol. Animal
care guidelines comparable to those published by the Institute for
Laboratory Animal Research (Guide for the Care and Use of
Laboratory Animals, 8th edition) were followed. Thirty female
BALB/c mice (weight, 60–80 g; age, 6–8 weeks), with normal
bilateral anterior segment examination results, were provided by
the Zhongnan Hospital Experimental Animal Center, Wuhan University.
The animals were randomly divided into five groups (n=6 per group),
the negative control group and four experimental groups. Mice in
the experimental groups were anesthetized by intraperitoneal
injection of sodium pentobarbital (50 mg/kg). Under a slit lamp
microscope (SL 115 Slit Lamp; Carl Zeiss, Oberkochen, Germany),
cross-shaped corneal epithelial scratches were made with a 5-ml
syringe needle in the right eyes of the animals in the experimental
groups to the depth of the Bowman's layer within the limbus. One
drop of 5 μl HSV-1 suspension (Institute of Virology, College of
Medicine, Wuhan University; 105 PFU) was placed onto the
cornea and allowed to remain for 20 sec. Commercially-available
chloramphenicol eye drops were applied to the eyes daily, and one
experimental group was sacrificed each day at 1, 7, 14 and 28 days
following infection. The eyes were collected and fixed in 4%
formalin solution for 6 h. The controls were sacrificed 28 days
following infection by cervical vertebra dislocation and the eyes
were collected and fixed as described.
Immunohistochemical staining
Following fixation, each eye was embedded in
paraffin with the cornea facing to the side to facilitate
sectioning. The cornea was sectioned into 5 μm-thick slices. The
slices were dewaxed and washed with phosphate-buffered saline (PBS;
pH 7.4) three times for 5 min each. Slices were placed in boiling
water containing EDTA (pH 8.0) under a high pressure for 10 min to
retrieve the antigen. The sections were then placed in cold water
and cooled to room temperature. Slices were incubated in 3%
hydrogen peroxide solution at room temperature for 10 min to block
endogenous peroxidase and then washed with PBS (pH 7.4) and
incubated overnight at 4°C with anti-FAK antibody (1:100; Wuhan
Boster Biological Technology, Ltd., Wuhan, China). The two-step
anti-rabbit/mouse universal immunohistochemistry kit (EnVision
Detection Systems Peroxidase/DAB, Rabbit/Mouse; DakoCytomation,
Glostrup, Denmark) was used for color reaction. The slices were
observed and images were captured under a microscope (UB102i;
Chongqing UOP Photoelectric Technology, Chongqing, China).
Cell count
FAK-positive cells were counted at the periphery and
center of the cornea using 10×10 grid under a high-powered field
(magnification, ×450) at 1, 7, 14 and 28 days following infection
in the experimental group and the results were compared with the
corneal slices of the negative control group. We selected 6 slices
from each group (total 30 slices) to conduct the cell count.
Primary culture of human corneal
epithelial (HCE) cells
The Institutional Review Board of Wuhan University
approved the use of HCE cells. The tissue block culturing method
was used. Tissue blocks were obtained from the remaining donor
corneas following penetrating keratoplasty. Under a surgical
microscope (OPMI Lumera 700; Carl Zeiss), the endothelial cell
layer and matrix layer were peeled off and inoculated in a Petri
dish with the epithelial cell layer at the top. Dulbecco's modified
Eagle's medium (DMEM; HyClone, Waltham, MA, USA) solution was used.
The samples were cultured in an incubator with 5% CO2 at
37°C for 72 h. The second-generation cells were collected for
immunohistochemistry with mouse anti-human cytokeratin 3 monoclonal
antibody to confirm the identity of the cell line (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Cells of passages 2–4
were collected for further experiments.
Infection of HCE cells with HSV-1
When HCE cells reached 70–90% confluence the medium
was discarded. The HSV-1 (F strain) suspension was inoculated using
an optimal multiplicity of infection (MOI) of 5. Samples were
placed in an incubator for 1 h and the flask was shaken once every
15 min to ensure the cells evenly absorbed the virus suspension.
UltraCULTURE Serum-free Medium (12–725F; LONZA Inc., Basel,
Switzerland) was added to the negative control group and the cells
used were non-infected cells. The suspension was discarded after 1
h, serum-free medium was added and the samples were cultured in an
incubator with 5% CO2 at 37°C. Cells positive for
enhanced green fluorescent protein (EGFP) represented cells
successfully infected with HSV-1. The number of infected cells and
the total number of cells in the same field were counted. The
infection efficiency (%) was calculated using the following
formula: Number of EGFP-positive cells/total number of cells ×
100.
Detection of MMP-2 and FAK mRNA
expression using reverse transcription-polymerase chain reaction
(RT-PCR)
HCE cells were collected at 2, 20 and 40 h following
HSV-1 infection and total RNA was extracted. The cDNA was obtained
through RT of random primers and was used for PCR amplification.
According to the mRNA sequences of β-actin, FAK and MMP-2 in
GenBank, specific amplification primers were designed using Primer
5 software (Primer Premier 5.0; Premier Biosoft Inc., Canada) as
follows: β-actin (accession no. NM_001101) upstream,
5′-GTCCACCGCAAATGCTTCTA-3′ and downstream
5′-TGCTGTCACCTTCACCGTTC-3′ (length, 190 bp); FAK (accession no.
NM_005607.3) upstream, 5′-CCCTATGGTGAAGGAAGTCG-3′ and downstream,
5′-TGCCATCTCAATCTCTCGGT-3′ (length 106 bp); and MMP-2 (accession
no. NM_004530.4) upstream, 5′-AGTGACGGAAAGATGTGGTGTG-3′ and
downstream, 5′-CTTGGTGTAGGTGTAAATGGGTG-3′ (length 182 bp).
Following electrophoresis of the RT-PCR products, gels were scanned
using the computer image analyzer (AlphaEaseFC software; Alpha
Innotech Corporation, San Leandro, CA, USA) and the gray values of
the bands were analyzed using the electrophoresis gel image
analysis system (AlphaEaseFC, Genetic Technologies, Inc., Miami,
FL, USA). The mean gray values of the target fragment and the
internal reference were compared in order to calculate the
ratio.
Detection of MMP-2, FAK and p-FAK protein
expression using western blot analysis
Cell lysis buffer (100 μl) was added to a Petri dish
at 2, 20 and 40 h after HSV-1 infection and the cells were
collected following 30 min of lysis on ice. Cells were sonicated
and centrifuged at 12,000 rpm for 5 min at 4°C (Labofuge 400R;
Thermo Fisher Scientific, Rockford, IL, USA). The supernatant was
collected, the protein content of each sample was measured with the
spectrophotometer using the BAC method and the concentration of the
sample for loading was determined. SDS-PAGE was performed for
protein detection and the proteins were transferred to
nitrocellulose membranes (LC2000; Invitrogen, Carlsbad, CA, USA).
Nonspecific immunoglobulin binding was blocked with skimmed milk
and bovine serum albumin for 1 h. The filter membrane was incubated
with rabbit anti-human MMP-2, FAK and p-FAK polyclonal antibodies
(Wuhan Boster Biological Technology, Ltd.) at 4°C overnight.
Following rinsing with PBS buffer, the membrane was incubated with
the corresponding horseradish peroxidase-conjugated secondary
antibody (Wuhan Boster Biological Technology, Ltd.) at room
temperature for 1 h. The enhanced chemiluminescence method was used
to detect protein expression [BeyoECL Plus kit (Beyotime, P0018);
Beyotime Institute of Biotechnology, Shanghai, China] and images
were captured of the membranes. The gel image processing system was
used to analyze the molecular weight and net optical density values
of the target bands.
Detection of MMP-2, FAK and p-FAK protein
expression using immunohistochemical staining
Cells were seeded in 24-well plates at 2, 20 and 40
h following infection and fixed with 4% paraformaldehyde for 30
min. The samples were treated with Triton X-100 for 15 min and
blocked with 3% H2O2-methanol for 15 min.
Rabbit anti-human MMP-2, FAK and p-FAK polyclonal antibodies were
then added. The two-step anti-rabbit/mouse universal
immunohistochemistry kit was used for staining development. The
samples were observed under a microscope and images were
captured.
Statistical analysis
The experimental data are expressed as the mean ±
standard deviation. Repeated measures analysis of variance was
performed. P<0.05 was considered to indicate a statistically
significant difference. Data was analyzed using SPSS software,
version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
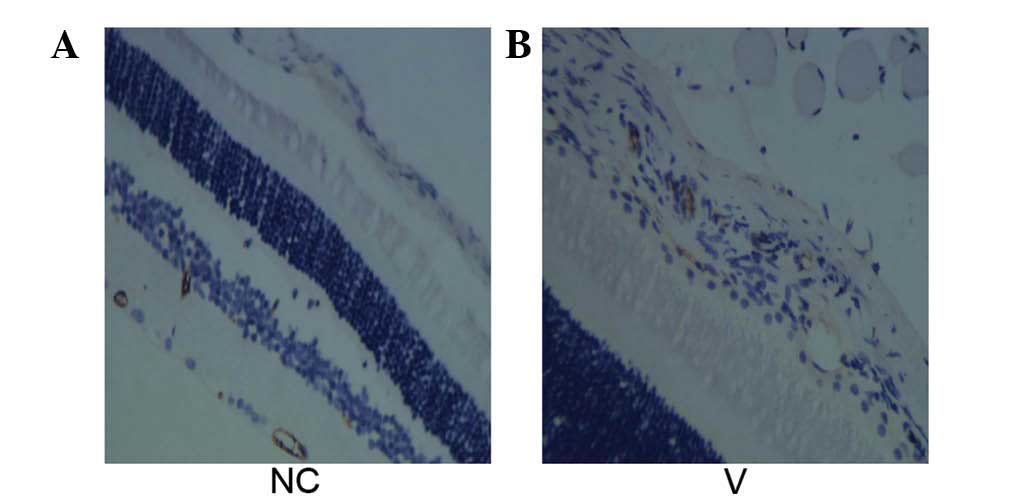
Morphological changes following HSV-1
infection
The corneal epithelium of the negative control group
was orderly and tightly arranged. No neutrophils were observed in
the epithelium or stroma. Epithelial edema and neutrophil
infiltration were observed on the first day following HSV-1
infection. Over time, vacuolar degeneration and necrosis gradually
occurred in epithelial cells. Cell arrangement was disordered,
matrix edema was present and the number of neutrophils increased
(Fig. 1).
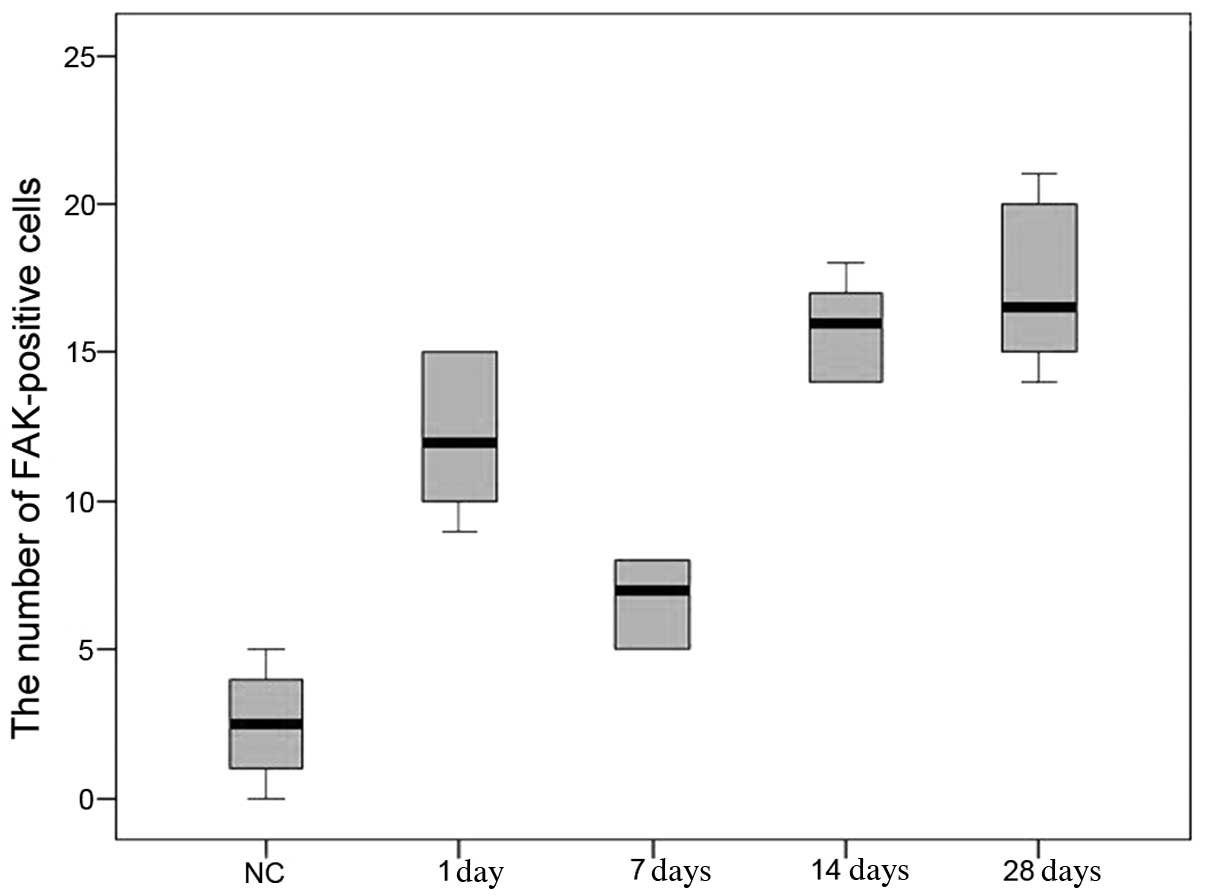
Tissue localization of FAK and cell
count
In the cornea of the negative control group,
FAK-positive cells were located mainly near the basement membrane
in the corneal epithelial cells. In the corneal epithelium, the
number of FAK-positive cells increased significantly 1 day
following HSV-1 infection compared with that of the negative
control group (P<0.05); and the number of FAK-positive cells
declined on day 7 compared with that on day 1 (P<0.05). The
number increased again on days 14 and 28, differing significantly
from that of the negative control group and the four groups with
HSV-1 infection (P<0.05). However, the number of FAK-positive
cells on days 14 and 28 were not significantly different
(P>0.05) (Fig. 2).
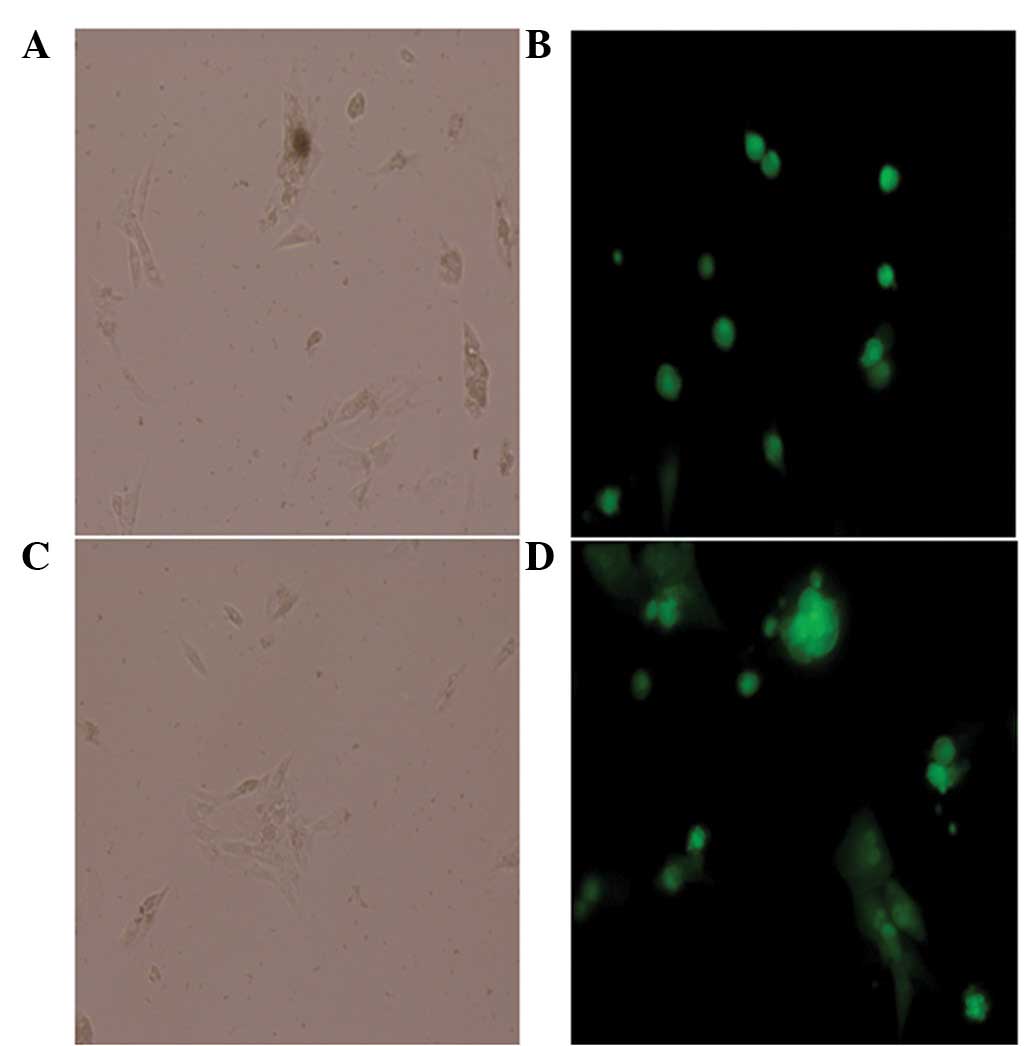
Cell number
The number of cells marked with green fluorescence
and the total number of cells in the same field were counted under
a phase contrast fluorescence inverted microscope (Fig. 3). The virus infection efficiency
was 92% when the MOI was 5, which met the experimental
requirement.
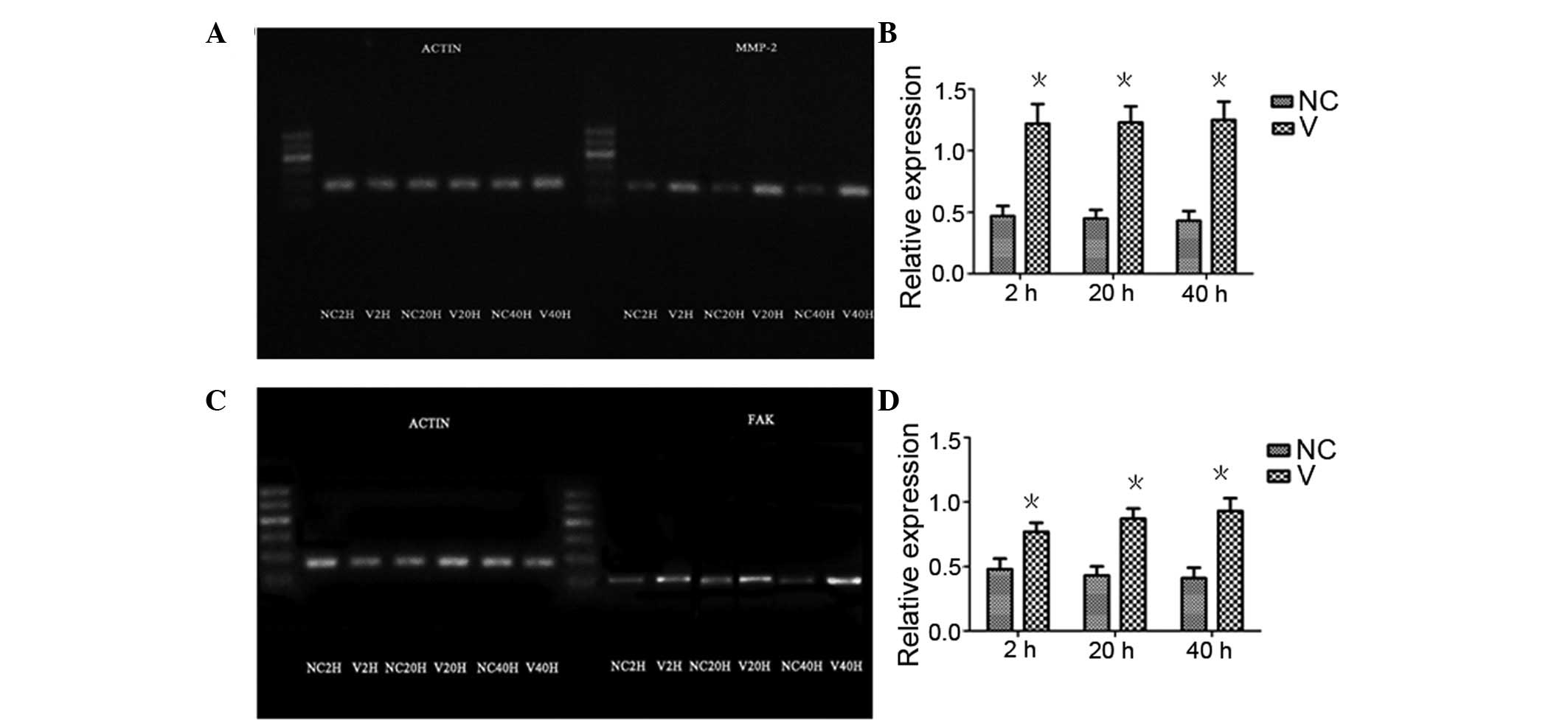
MMP-2 and FAK mRNA expression levels in
HCE cells detected by RT-PCR
The MMP-2 and FAK mRNA expression levels in the HCE
cells detected by RT-PCR at 2, 20 and 40 h following HSV-1
infection, are shown in Fig. 4 and
Tables I and II. The repeated measures analysis of
variance revealed no significant differences in MMP-2 and FAK mRNA
expression levels at different time points (F=0.968, P=0.436). Both
MMP-2 and FAK mRNA expression levels differed significantly between
infected cells and non-infected cells (F=47.649, P=0.000), with no
interaction between groups and time points (F=0.757, P=0.536).
Pairwise comparisons showed a greater mRNA expression in the
virus-infected cells than in non-infected cells at each time point
(P<0.01). Over time, virus-infected and non-infected cells
showed no significant differences in mRNA expression levels of
MMP-2 and FAK.
 | Table IRelative gray values of matrix
metalloproteinase-2 mRNA electrophoretic bands in infected and
non-infected cells at various time points, obtained from
RT-PCR. |
Table I
Relative gray values of matrix
metalloproteinase-2 mRNA electrophoretic bands in infected and
non-infected cells at various time points, obtained from
RT-PCR.
| Parameters | 2 h | 20 h | 40 h | F statistic | P-value |
|---|
| Non-infected
cells | 0.47±0.05 | 0.45±0.02 | 0.43±0.07 | 0.771 | 0.485 |
| Infected cells | 1.22±0.02 | 1.23±0.09 | 1.25±0.10 | 0.190 | 0.830 |
| t-test | 31.142 | 18.918 | 15.021 | - | - |
| P-value | 0.000 | 0.000 | 0.000 | - | - |
 | Table IIRelative gray values of the focal
adhesion kinase mRNA electrophoretic bands in infected and
non-infected cells at various time points, obtained from
RT-PCR. |
Table II
Relative gray values of the focal
adhesion kinase mRNA electrophoretic bands in infected and
non-infected cells at various time points, obtained from
RT-PCR.
| Parameters | 2 h | 20 h | 40 h | F statistic | P-value |
|---|
| Non-infected
cells | 0.40±0.03 | 0.43±0.06 | 0.41±0.08 | 0.321 | 0.731 |
| Infected cells | 0.87±0.05 | 0.87±0.04 | 0.93±0.04 | 3.610 | 0.079 |
| t-test | 18.024 | 13.644 | 13.000 | - | - |
| P-value | 0.000 | 0.000 | 0.000 | - | - |
p-FAK expression in HCE cells detected by
western blot analysis
p-FAK expression in HCE cells detected by western
blot analysis at 2, 20 and 40 h following HSV-1 infection, are
shown in Fig. 5D. No statistically
significant differences were observed between infected and
non-infected cells 2 h after infection (P>0.05). At 20 and 40 h
following infection, p-FAK expression differed significantly
between infected and non-infected cells (P<0.05). p-FAK
expression levels in the infected cells 2 h after infection was
significantly different than that at 20 and 40 h (P<0.05). Over
time, p-FAK expression of non-infected cells did not change
significantly (P>0.05). FAK expression levels in HCE cells at 2,
20 and 40 h following HSV-1 infection (Fig. 5C) was in line with the
aforementioned p-FAK expression. MMP-2 expression levels (Fig. 5B) in HCE cells at 2, 20 and 40 h
following HSV-1 infection showed no statistically significant
difference between infected cells and non-infected cells 2 h after
infection (P>0.05). At 20 and 40 h, MMP-2 expression levels were
significantly greater in infected cells (P<0.05 and P<0.01,
respectively) than the non-infected cells. At 2 h, MMP-2 expression
levels in infected cells was significantly lower than that at 20
and 40 h (P<0.05). At 40 h, MMP-2 expression levels in
non-infected cells was significantly different from that in the
same cells at 2 and 20 h after infection (P<0.05).
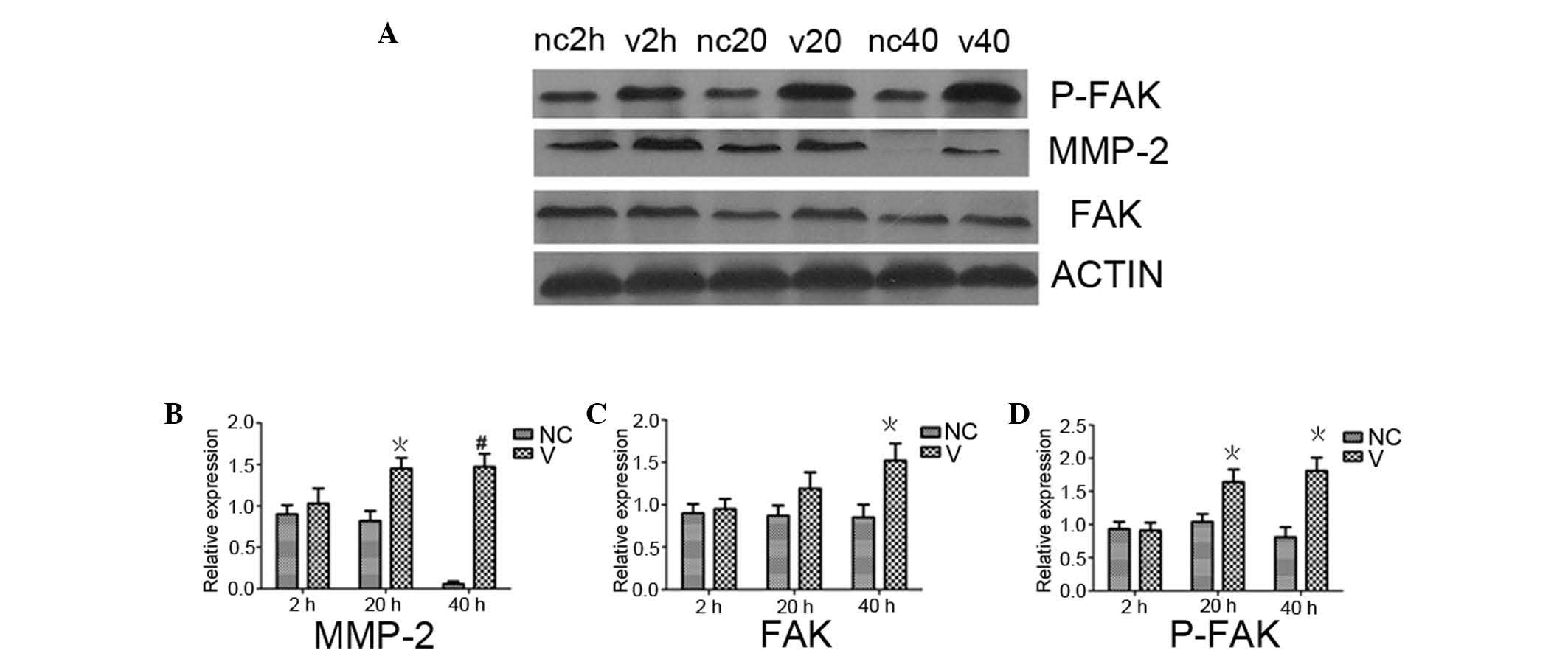 | Figure 5Western blot analysis of MMP-2, p-FAK
and FAK protein expression levels in HCE cells following HSV-1
infection. (A) MMP-2, p-FAK and FAK protein expression levels in
HCE cells following HSV-1 infection. Histogram of the relative
expression of (B) MMP-2, (C) FAK, and (D) p-FAK in V and NC cells
in (A). V cells showed an increasing trend in protein expression of
MMP-2, p-FAK and FAK with increased infection time.
*P<0.05 and #P<0.01, vs. NC group.
MMP-2, matrix metalloproteinase-2; phosphorylated-FAK, focal
adhesion kinase; HCE, human corneal epithelial; HSV-1, type 1
herpes simplex virus; V, infected with HSV-1; CN normal
control. |
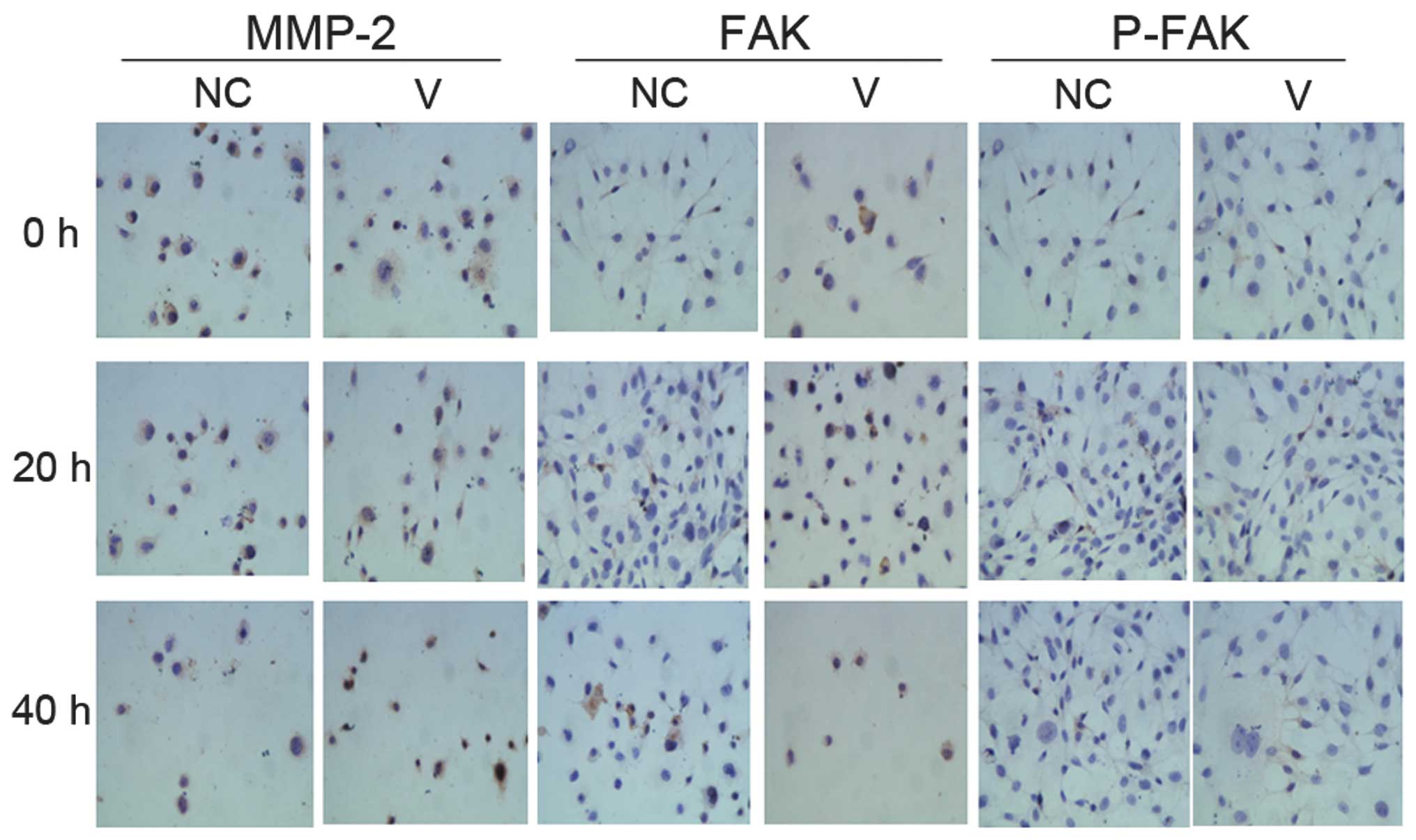
Cells with positive immunohistochemical
staining for MMP-2, FAK and p-FAK
Cells with positive immunohistochemical staining for
MMP-2, FAK and p-FAK showed blue nuclei. The cytoplasm was stained
a specific brownish yellow (Fig.
6). As infection time increased, the number of positively
stained cells increased.
Discussion
The present study demonstrated the model
construction of HSK in rats. We observed morphological changes in
cells of the epithelial layer during viral infection and increased
neutrophil infiltration in the corneal stroma, confirming that
HSV-1 infection induced leukocyte migration into the limbal blood
vessels and activated the inflammatory response.
In the negative control group, several FAK-positive
cells were identified in the corneal epithelium of mice suggesting
that FAK is important in maintaining the normal physiological
function of cells. On day 1 following HSV-1 infection, FAK-positive
cells increased in the corneal epithelium, but the number of
FAK-positive cells decreased 7 days after infection. The number of
FAK-positive cells increased again on day 14 and 28 after infection
in an upward step-wise fashion. The pattern of FAK expression
levels was similar to that of MMP-2 in the cornea of an
experimental animal model of HSK by Yang et al(1). These results indicate that MMP-2 is
important in the development of corneal stromal ulcers.
A previous study (9) also showed that p-FAK is important in
the transfer of viral capsids to the host nuclear pore complex.
Therefore, we hypothesized that in the initial stage of HSV-1
infection, the main purpose of activating a large amount of FAK
protein is to respond to viral replication and invasion of the host
cells. When the host immune function is activated, viral
replication and invasion are decreased, therefore, FAK activation
is also reduced. In this case, the viruses that have invaded the
host cell destroyed these cells, and the large amount of FAK
protein may activate the expression of cytokines, for example
MMP-2, which further assists in the formation of corneal stromal
ulcers.
The present study successfully infected HCE cells
with HSV-1 in vitro and detected MMP-2 mRNA and protein
expression levels at the early stages of infection. The results
showed that mRNA expression levels 2 h following infection were
significantly greater than that of normal cells, and continued to
increase up to 40 h after infection. Increases in MMP-2 protein
expression were slower and were not significantly different from
that of the normal cells 2 h after infection, but increased with
time from 20 to 40 h. These results are consistent with the
findings of previous studies. Using in vivo animal models of
HSK, Yang et al(10)
indicated that MMP-2 protein expression peaked between days 2 and
14 following infection, and the expression was mainly located at
the base of the epithelial cells and in the superficial stroma.
After 2 days, the epithelium healed and protein expression levels
declined until inflammatory cells synthesized a large number of
MMP-2 proteins again following formation of stromal ulcers. The
present study validated the change in MMP-2 protein expression in
corneal epithelial cells within 2 days of infection, indicating
that MMP-2 synthesized by epithelial cells at the early stages of
infection is important in the formation of corneal stromal
ulcers.
Cheshenko et al(9) found that at the early stages of HSV-1
infection of cervical epithelial cells, p-FAK is important in the
transfer of viral capsids to the host cell nuclear pore complex.
Therefore, silencing the FAK gene can reduce the viral infection
rate by 90%. In the present study, the authors observed that FAK
mRNA expression 2 h after infection was significantly higher than
that in the normal cells and expression continued to increase up to
40 h. The levels of protein expression of FAK and p-FAK did not
differ significantly from those of normal cells 2 h after
infection. The expression of these proteins increased significantly
with time from 20 to 40 h and the change in expression was similar
to that of MMP-2 protein. Hsia et al(8) identified that FAK activation may
result in the formation of the FAK-src-p130Cas-Dock180 signaling
complex and an increased level of Rac and JNK activation, thereby
stimulating the expression and enhancing the activity of MMP.
Heiligenhaus et al(11)
previously performed gelatin zymography and identified that
following inoculation the corneas of mice with HSV-1, and the
activity levels of MMP-2, −8 and −9 in the corneal tissue were
significantly elevated on day 2. The results of present study
indicated that at the early stages of HSV-1 infection of cultured
HCE cells in vitro, p-FAK not only participates in the
transfer of viral capsids into the nucleus but also activates MMP-2
expression, resulting in the formation of corneal stromal ulcers.
Following HSV-1 infection of the corneal epithelium, the FAK
signaling pathway was activated, resulting in increased secretion
of MMP-2 in the corneal tissue and accelerated formation of corneal
ulcers and necrotic lesions. The present study investigated the
secretion of MMP-2, which is directly induced by HSV-1 infection,
and its association with FAK and P-FAK expression. P-FAK plays an
important role in MMP-2 activation when the HSV-1 infects cells.
Further study is required on the mechanisms of corneal ulceration
in HSK.
References
|
1
|
Yang YN, Bauer D, Wasmuth S, Steuhl KP and
Heiligenhaus A: Matrix metalloproteinases (MMP-2 and 9) and tissue
inhibitors of matrix metalloproteinases (TIMP-1 and 2) during the
course of experimental necrotizing herpetic keratitis. Exp Eye Res.
77:227–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Budagian V, Bulanova E, Orinska Z, Pohl T,
Borden EC, Silverman R and Bulfone-Paus S: Reverse signaling
through membrane-bound interleukin-15. J Biol Chem.
279:42192–42201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: in command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen LA and Guan JL: Mechanisms of focal
adhesion kinase regulation. Curr Cancer Drug Targets. 5:629–643.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu B, Jarzynka MJ, Guo P, Imanishi Y,
Schlaepfer DD and Cheng SY: Angiopoietin 2 induces glioma cell
invasion by stimulating matrix metalloprotease 2 expression through
the alphavbeta1 integrin and focal adhesion kinase signaling
pathway. Cancer Res. 66:775–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Segarra M, Vilardell C, Matsumoto K,
Esparza J, Lozano E, Serra-Pages C, Urbano-Márquez A, Yamada KM and
Cid MC: Dual function of focal adhesion kinase in regulating
integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells.
FASEB J. 19:1875–1877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akahane T, Akahane M, Shah A, Connor CM
and Thorgeirsson UP: TIMP-1 inhibits microvascular endothelial cell
migration by MMP-dependent and MMP-independent mechanisms. Exp Cell
Res. 301:158–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsia DA, Mitra SK, Hauck CR, et al:
Differential regulation of cell motility and invasion by FAK. J
Cell Biol. 160:753–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheshenko N, Liu W, Satlin LM and Herold
BC: Focal adhesion kinase plays a pivotal role in herpes simplex
virus entry. J Biol Chem. 280:31116–31125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Xing YQ, Dirk B and Arnd H:
Localization of matrix metalloproteinase-2 in experimental herpes
simple virus keratitis. Med J Wuhan Univ. 23:316–318. 2002.(In
Chinese).
|
|
11
|
Heiligenhaus A, Li HF, Yang Y, Wasmuth S,
Steuhl KP and Bauer D: Transplantation of amniotic membrane in
murine herpes stromal keratitis modulates matrix metalloproteinases
in the cornea. Invest Ophthalmol Vis Sci. 46:4079–4085. 2005.
View Article : Google Scholar : PubMed/NCBI
|