Introduction
Neuronal cell apoptosis is associated with various
neurological disturbances, including radiation (1). Research concerning the molecular
mechanisms of neuronal cell apoptosis following radiation has
enriched the number of therapeutic strategies for protection
against neuronal cell death caused by radiation (2). Mitochondria are important for the
initiation and/or reinforcement of cell apoptotic pathways
(3,4). During apoptosis, a key event is the
release of second mitochondria-derived activator of caspase
(Smac)/direct IAP binding protein with low pI (DIABLO) and
cytochrome c (cyto c) from mitochondria to the
cytosol (5). When the discovery of
Smac by Du et al was first published (6), Verhagen et al concurrently
reported on the same protein, which they named DIABLO (7). Hence, the name Smac/DIABLO is
typically assigned to the molecule to credit the work of both
groups. For simplicity, in the present study this molecule is
referred to as Smac.
Radiation and other agents induce caspase activation
fundamentally via the mitochondrial pathway, which includes the
mitochondrial integration of apoptotic signals and the subsequent
release of cyto c, Smac, Omi/HtrA2 and apoptosis-inducing
factor into the cytosol (6,8).
This release allows the assembly of the apoptosome. The apoptosome
activates caspase-9, which subsequently induces the activation of
caspase-3, -6 and -7. The effector caspases cleave their cellular
specific substrates and generate the typical morphology of
apoptosis (8).
The inhibitors of apoptosis protein (IAP) family
prevent apoptosis by interacting with and then controlling the
activities of caspase-8, -9, -3 and -7 (8). Cellular IAP-1 (c-IAP1), c-IAP2 and
X-linked IAP (XIAP) are three significant members of the IAP
family; XIAP is particularly significant as it has numerous domains
that interact with different caspases, such as caspase-3, -7 and -9
(9,10) and its BIR2 domain inhibits
caspase-7 in a non-competitive manner (11). As XIAP blocks apoptosis at the
effector phase, a point where multiple signaling pathways converge,
treatments targeting XIAP may prove to be effective in overcoming
resistance. Smac was identified as a protein that may antagonize
the inhibition of apoptosis by IAPs following its release from the
mitochondria in response to apoptotic stimuli (6,7). It
has been demonstrated that the domain of Smac, which interacts with
IAPs is a particular NH2-terminal residue consisting of
four amino acids, Ala-Val-Pro-Ile (12–14).
Studies have shown that apoptosis-associated cyto c and Smac
release from mitochondria occur via different mechanisms and that
the release of Smac may be a key event linking the mitochondrial
and death receptor pathways (15,16).
Previous studies of XIAP and Smac have mainly
concentrated on tumors and cerebral ischemia reperfusion injury
with less focus on radiation brain injury (17,18).
Whether the interaction of XIAP and Smac affects neuronal apoptosis
following brain injury induced by radiation remain unclear. It is
also not known whether the expression levels of XIAP induced by
radiation injury change markedly or, following irradiation, whether
caspase family members are activated sequentially. Changes in the
hypoglossal nucleus were investigated in rats following radiation
injury, with and without caspase inhibition, to explore these
unknown factors.
Materials and methods
Radiation model
All animal procedures were performed in a facility
accredited by the Radiation Hazard Evaluation Laboratory of the
Institute of Radiation Medicine of Chinese Academy of Medical
Science and Peking Union Medical College (Nankai, China). All
experimental procedures were performed according to the Guide for
the Care and Use of Laboratory Animals published by the US National
Institutes of Health (publication no. 85–23, revised 1996). Adult
male Sprague-Dawley rats (weight, 250–300 g) were randomly divided
into the irradiation group (IR group, n=12), the irradiation with
z-VAD-fmk group (IRVAD group, n=12) or control group (con group,
n=12). The irradiation of the rats in the former group was
performed at room temperature using a Cs-137 γ-ray instrument
(Atomic Energy of Canadian Inc., Mississauga, Canada) to administer
a 4-Gy dose of radiation at a dose rate of 0.71 116 Gy/min. The
animals in the control group did not receive any radiation. The
study was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of Institute of Radiation Medicine of
Chinese Academy of Medical Science and Peking union Medical College
(Tianjin, China).
Intracerebroventricular administration of
z-VAD-fmk
With a rat brain stereotaxic apparatus (Stoelting
Co., Wood Dale, IL, USA), rats were implanted
intracerebroventricularly (i.c.v.) with a cannula [anteroposterior
(AP)=−2.4 mm, length, −1.4 mm; height, −3.0 mm] and osmotic
micropump (Alzet® micro-osmotic pump Model 1007D, Durect
Corporation, CA, USA). Infusion of 2 μg z-VAD-fmk (BioVision,
Mountain View, CA, USA) in 10 μl vehicle was conducted at a rate of
0.2 μg/h for 1 h. The drug vehicle was 0.5% dimethyl sulfoxide in
phosphate-buffered saline (PBS). Infusions were performed at the
onset of radiation administration, as previously described
(19). The rats in the IRVAD group
were infused with z-VAD-fmk, the other two groups were infused with
vehicle. Non-irradiated controls received vehicle i.c.v. and
radiation controls received z-VAD-fmk. Twenty-four hours subsequent
to irradiation, the rats from each group were anesthetized with 10%
chloral hydrate (30 mg/kg body weight) by intraperitoneal
anesthesia.
Immunohistology and terminal
deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL)
staining
Rat brains were harvested and immediately frozen in
2-methylbutane at −30°C. The brainstem was cut into 12-μm thick
sections with a cryostat (CM 3000; Leica, Manheim, Germany) at the
level of the hypoglossal nucleus (20) and then stored at −80°C until
required for further experiments. Coronal sections were air dried
for 15 min, post-fixed in 10% formalin for 15 min, washed twice in
PBS and then processed for immunohistology with rabbit anti-XIAP
(1:1,500 dilution; Abcam, Cambridge, MA, USA). The
avidin-biotin-peroxidase complex method was conducted as previously
described (21). For detection of
DNA fragmentation, the fluorescein-based TUNEL assay (Roche
Molecular Biochemicals, Indianapolis, IN, USA) was used. TUNEL
staining was conducted according to the manufacturer’s
instructions. Briefly, sections were incubated for 90 min at 37°C
with TUNEL reaction mixture. Positive control sections were
incubated with 200 U/ml DNase I (Gibco-BRL, Carlsbad, CA, USA) for
5 min prior to fixation. Negative control sections underwent the
same procedure but terminal deoxynucleotidyl transferase was
omitted from the reaction buffer to evaluate nonspecific labeling.
TUNEL cell counts were performed on brain sections (n=3) from the
hypoglossal nuclei. TUNEL-positive cells were averaged from counts
on three adjacent brain sections of a rat. Images were visualized
using a Leica microscope under an excitation/emission wavelength of
500/550 nm (green), captured using an Optronics DEI-750 3-chip
camera equipped with a BQ 8000 sVGA frame grabber and analyzed with
Bioquant software (Bioquant Image Analysis Corporation, Nashville,
TN, USA).
Generation of cytosolic fractions
Brainstems containing the hypoglossal nucleus were
collected from the rats, and cytosolic fractionation was performed
as previously described (20).
Briefly, the brainstem samples (6 samples per group) were
homogenized in radioimmunoprecipitation assay buffer (Sigma-Aldrich
Inc., St. Louis, MO, USA) containing protease inhibitors. The
protein concentration of the supernatant homogenate was determined
using a Bio-Rad kit (Bio-Rad, Hercules, CA, USA). Samples were then
centrifuged at 2,500 × g for 15 min at 4°C to precipitate the
nuclei and cellular debris. The supernatant was then centrifuged at
15,000 × g for 20 min at 4°C to remove the mitochondria. The
supernatant was subsequently centrifuged at 100,000 × g for 60 min
to at 4°C obtain the cytosol (supernatant).
Western blot analysis
The protein concentration from the cytosol
(supernatant) was determined spectrophotometrically from the
absorbance at 595 nm (A595 nm) using the Bradford method (22). Samples (80 μg) were denatured in
gel-loading buffer and separated on 15% SDS-PAGE gels, then
transferred to polyvinylidene difluoride membranes and incubated
with the following primary antibodies: Rabbit polyclonal anti-XIAP
(1:500 dilution; Abcam), rabbit polyclonal antibody raised against
Smac (1:500 dilution; Chemicon, Temecula, CA, USA), rabbit
polyclonal antibody raised against cyto c (1:200 dilution;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit
anti-β-actin (1:1,500 dilution; Sangon Biotech, Shanghai, China)
and goat anti-rabbit IgG conjugated to horseradish peroxidase
(1:800 dilution; ZSGB-BIO, Beijing, China).
RNA extraction, cDNA synthesis and
quantitative polymerase chain reaction (qPCR)
Total RNA was purified and extracted as conducted
previously by Chen et al(23). Equal concentrations of total RNA
were reverse-transcribed using Prime Script RT reagent kit (Takara
Bio, Inc., Shiga, Japan) according to the manufacturer’s
instructions. cDNA samples were blended with primers and SYBR
Master Mix (Invitrogen Life Technologies, Carlsbad, CA, USA) in a
total volume of 25 μl. All samples were assayed in triplicate using
an ABI PRISM 7500 Sequence Detection system (Applied
Biosystems®-Life Technologies, Foster City, CA, USA).
The cycle threshold (CT) values for each reaction were determined
and the mean was calculated using TaqMan SDS analysis software
(Applied Biosystems-Life Technologies). The expression levels of
target genes were calculated by the comparative CT method [fold
changes=2(−ΔΔCT)]. PCR primers for caspase-3, -8 and -9
and the housekeeping gene, GAPDH, were obtained from Sangon
Biotech. The primer pairs used were as follows: CASP3,
5′-ATCACAGCAAAAGGAGCAGTTT-3′ (forward) and 5′-ACACCACT
GTCTGTCTCAATGC-3′ (reverse); CAPS8, 5′-TAGGGACAGGAATGGAACACA-3′
(forward) and 5′-TGGGAGAGGATACAGCAGATG-3′ (reverse); CASP9,
5′-TCTGGAGGATTTGGTGATGTC-3′ (forward) and
5′-CATTTTCTTGGCAGTCAGGTC-3′ (reverse); and GAPDH,
5′-ATGACATCAAGAAGGTGGTG-3′ (forward) and 5′-CATACCAGGAAA
TGAGCTTG-3′ (reverse).
Caspase activation assay
The activities of caspase-3, -8 and -9 were analyzed
using a fluorogenic caspase assay with Ac-DEVD-AFC, Ac-IETD-AFC and
Ac-LEHD-AFC (BD Pharmingen, San Diego, CA, USA), respectively, as
substrates (23). The results are
expressed as the fold change compared with that of the control
according to the previously described technique by Chen et
al(23).
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed using one-way analysis of variance with a post
hoc test (multiple comparison test), which determined the
significant differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of XIAP and TUNEL-positive
cells within the hypoglossal nuclei
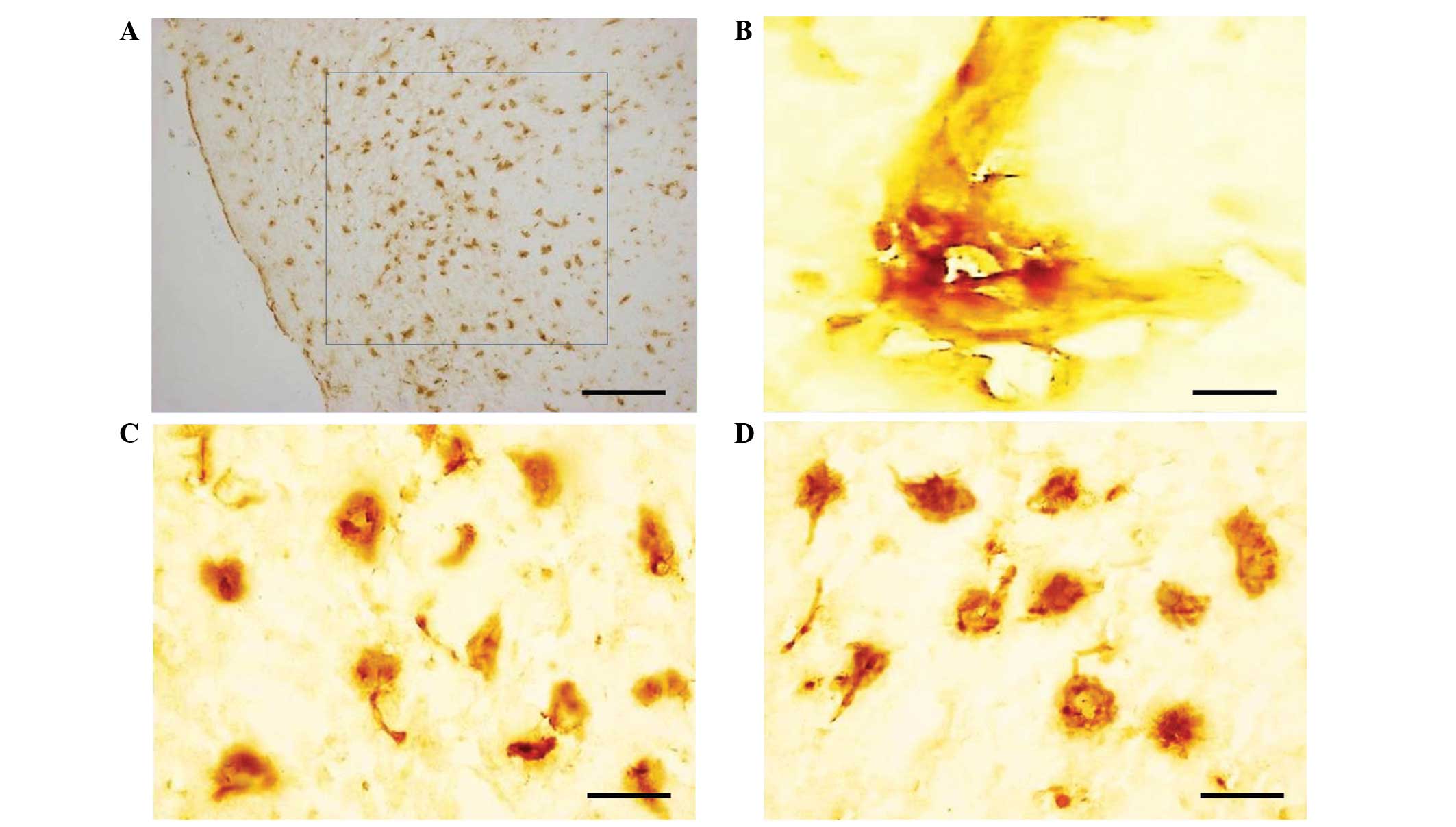
XIAP was mainly expressed in the cytoplasm with
positive yellow-brown staining and a high concentration of brown
granules (Fig. 1A and B). In the
brain tissue of the normal control group, XIAP was predominantly
expressed in the perinuclear region of neurons (Fig. 1C). The levels of XIAP present in
the brainstems following irradiation (Fig. 1D) were similar to those in the
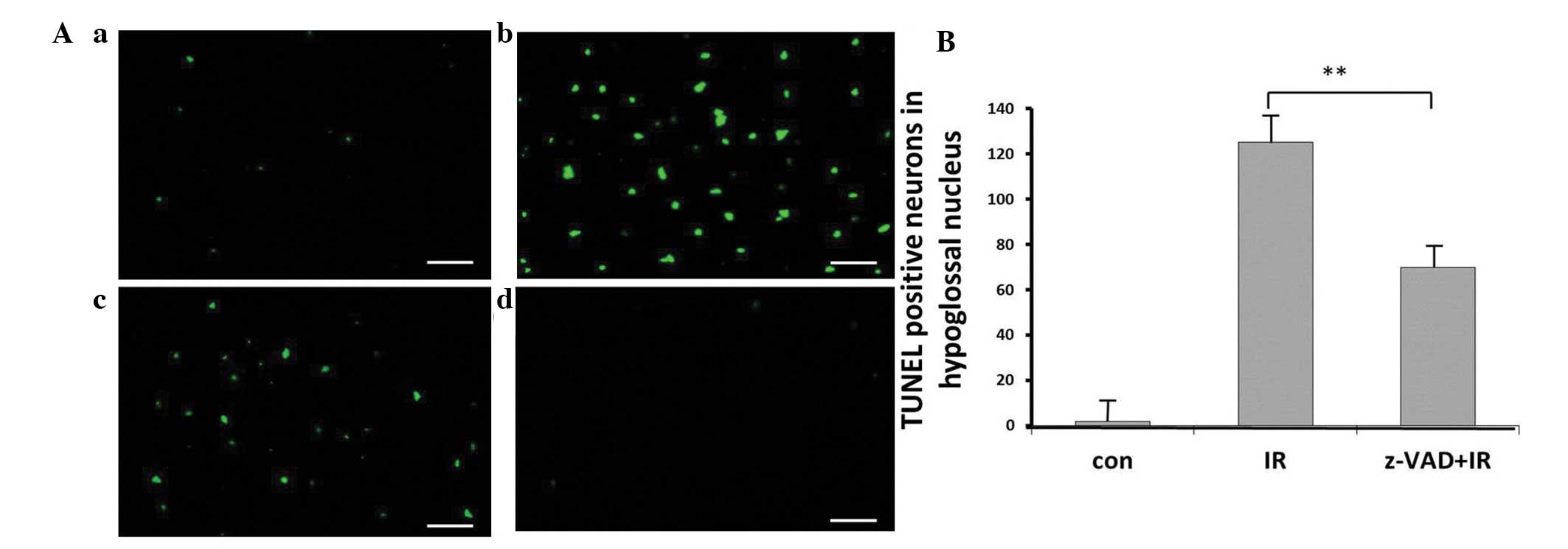
normal control. TUNEL-positive cells were visible mainly in the
hypoglossal nuclei of the group treated with radiation alone
(Fig. 2A). The number of
TUNEL-positive cells detected in the control rats was low (Fig. 2B).
Neuroprotective effects of the
pan-caspase inhibitor, z-VAD-fmk, in vivo
The quantification of TUNEL-positive neurons 24 h
after exposure to radiation in the hypoglossal nuclei indicated the
neuroprotective effects of the pan-caspase inhibitor, z-VAD-fmk
(Fig. 2). The number of
TUNEL-positive neurons observed in the hypoglossal nuclei in the
irradiation plus z-VAD-fmk group following irradiation was reduced
compared with that of the radiation alone group (P<0.01;
Fig. 2).
Western blot analysis of XIAP, Smac and
cyto c following irradiation
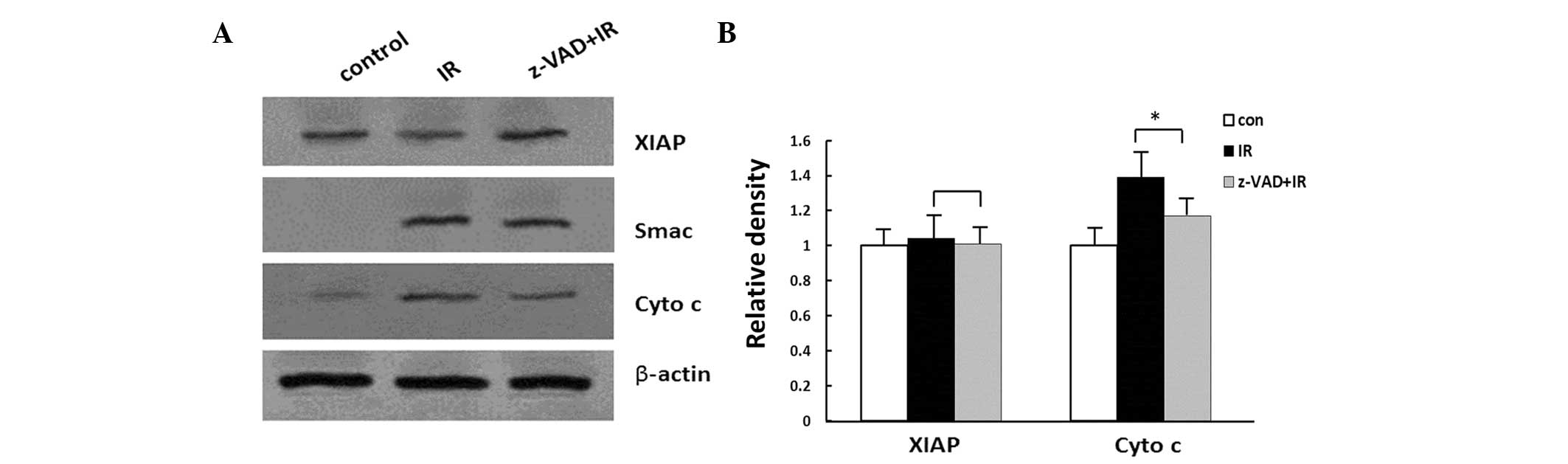
The results of the western blot analysis of XIAP,
Smac and cyto c following irradiation are shown in Fig. 3. The presence of Smac and cyto
c in the cytosol was observed following radiation
(P<0.05). No significant changes were identified in the
expression levels of XIAP following radiation (P>0.05; Fig. 3).
Effects of z-VAD-fmk on cyto c and XIAP
following irradiation
The rats were injected with z-VAD-fmk i.c.v. to
investigate the effects of caspase inhibition on the expression of
XIAP, as well as the release of cyto c and Smac. Western
blot analysis demonstrated that the inhibition of caspase induced
by z-VAD-fmk following radiation decreased the expression levels of
cyto c in animals treated with z-VAD-fmk for 24 h
(P<0.01; Fig. 3). By contrast,
no differences in the cytoplasmic expression levels of XIAP or Smac
between the z-VAD-fmk-treated animals and the vehicle-treated
animals were identified following irradiation (P>0.05; Fig. 3).
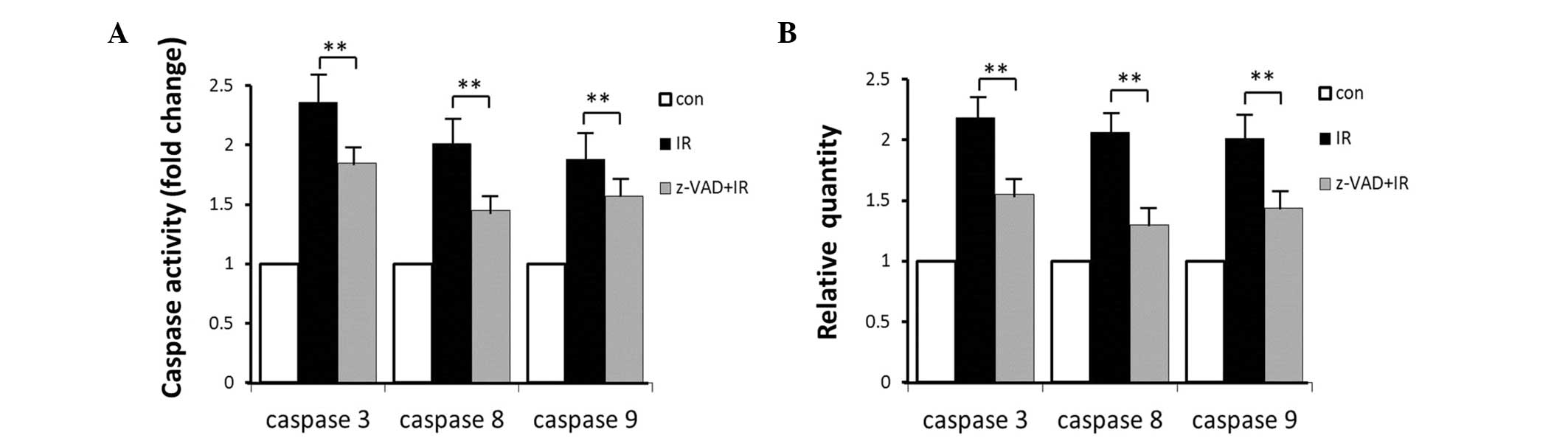
Caspase expression and activity
The effects of exposure to radiation were dependent
on caspase expression and activity. The mRNA expression levels of
caspase-3, -8 and -9 were measured. In the brainstem, treatment
with radiation induced 2.16-, 2.06- and 2.01-fold increases in the
RNA expression levels of caspase-3, -8 and -9, respectively, and
increased the activities of these caspases by 2.36-, 2.01- and
1.88-fold, respectively. Combined treatment with radiation and
z-VAD-fmk resulted in significant reductions in the caspase RNA
expression levels of 1.55-, 1.30- and 1.44-fold and activity of
1.85-, 1.45- and 1.57-fold for caspase-3, -8 and -9, respectively
(Fig. 4).
Discussion
The IAP proteins are signal transducers that perform
a diverse array of functions, which affect numerous signaling
pathways and elicit multiple responses in the cell (24,25).
IAPs are a family of proteins that are defined by the presence of
70 amino acids and a baculovirus IAP repeat (BIR), which was
originally described as a domain utilized by viruses to compromise
cell death machinery in its host (26); homologs have been identified in
species ranging from baculovirus to man (25). The BIR domain is responsible for
mediating protein-protein interactions, which allow certain IAPs to
directly bind to and suppress caspase function to inhibit cell
death (25). In addition to BIR
domains, the mammalian IAPs, XIAP, c-IAP1 and c-IAP2 contain a
carboxy-terminal RING domain that provides them with E3 ubiquitin
ligase activity (27). In addition
to their ability to conjugate ubiquitin onto target substrates,
XIAP, c-IAP1 and c-IAP2 all contain a ubiquitin-associated domain
(25). This motif interacts with
ubiquitin chains and allows IAP proteins to participate in
ubiquitin-dependent signaling pathways. c-IAP1 and c-IAP2 each
contain an additional caspase activation and recruitment domain,
which is thought to mediate protein-protein interactions (28). However, its function within the
context of the c-IAPs remains unclear.
Smac resides within the mitochondrial intermembrane
space and is subsequently released into the cytosol upon the
induction of apoptosis (6). Smac
and additional mitochondrial proteins, such as Omi, adenylate
kinase-2, cyto c and apoptosis-inducing factors are released
into the cytosol through permeability of the mitochondrial
membrane. Proteins of the Bcl-2 family are pivotal as they may
facilitate this process (29).
Although mitochondrial membrane changes may result in the
coincidental release of Smac and cyto c, the interaction of
these mitochondrial proteins is also significantly interrelated, as
cells deficient in cyto c are unable to release Smac from
the mitochondria even in the presence of Bax (30). Conversely, Smac release may be
necessary for the efficient release of cyto c(31). Furthermore, although Smac and cyto
c release from the mitochondria occurs in the intrinsic
pathway of apoptosis, complete functioning of the extrinsic pathway
may require Bax-stimulated release of Smac as well. This is due to
the ability of Smac to attenuate the inhibitory effects of IAPs on
caspases, which may result in alterations of upstream caspases and
the upregulation of extrinsic pathway receptors. Thus, although
IAPs inhibit apoptosis, mitochondrial release of Smac serves to
promote apoptosis by blocking the effects of IAPs on caspases.
To completely understand the role of caspases in the
hypoglossal nucleus model, a cell permeable pan-caspase inhibitor,
z-VAD-fmk, was used to investigate the effects of caspase blockade
in vivo. TUNEL staining demonstrated that z-VAD-fmk reduced
the numbers of TUNEL-positive cells within the hypoglossal nucleus,
suggesting that intervention in the caspase cascade following
radiation may have therapeutic applications. The results of the
present study confirmed that inhibition of caspase by z-VAD-fmk
reduced the expression levels and activation of caspase -3, -8 and
-9, indicating that caspase may be a potential therapeutic target
for the treatment of brain radiation injury. z-VAD-fmk reduced the
appearance of cyto c within the cytosolic fraction following
radiation. A previous study demonstrated that Smac and IAP family
members are involved in regulating caspase activation (18). In the present study, western blot
analysis indicated that z-VAD-fmk decreased the mitochondrial
release of cyto c, indicating that removal of caspase
activity reduced mitochondrial events in addition to caspase-3
activation. In contrast to cyto c, the expression of Smac
was not altered by z-VAD-fmk. A previous study found that Smac is
not released from the mitochondria during apoptotic signaling in
cells deficient in cyto c, indicating that Smac release is
dependent on cyto c(32).
The hypoglossal nucleus in the brainstem has a
relatively large size and the distribution of neurons is relatively
balanced, so locating the nucleus is relatively easy (Fig. 1A). Since z-VAD-fmk does not
penetrate the blood-brain barrier (19), it was applied
intracerebroventricularly as a bolus injection to overcome this
limitation. Via the cerebrospinal fluid circulating through the
fourth ventricle, the agent reaches the neurons through the process
of osmosis. The hypoglossal nucleus may be used a model of
radiation-induced injury in the central nervous system, providing
visual information and apoptotic nuclear morphology. The
hypoglossal nucleus has a large number of mitochondria (33), and therefore was an effective model
for this study. In the present study, changes of the hypoglossal
nucleus in an animal model established by exposure to radiation
were examined. Notably, the cytosol was extracted from cells of the
brainstem corresponding to the hypoglossal nucleus. In conclusion,
the inhibition of caspase induced by z-VAD-fmk reduced the
expression and activation of the caspase-3, -8 and -9. The present
results may provide a potential theoretical basis for the therapy
of brain radiation injury.
Acknowledgements
This study was supported by the Special Foundation
of the Ministry of Health (grant no. 201002009), the National
Natural Science Foundation of China (grant nos. 31170804, 31240052
and 31200634), the Natural Science Foundation of Tianjin (grant
nos. 10JCZDJC16900, 11ZCGYSY02400, 12JCYBJC15300 and
12JCYBJC32900), the Science Research Foundation for Doctor-Subject
of High School of the National Education Department (grant nos.
20101106110046, 20121106120044 and 20121106120043), the PUMC Youth
Fund and Fundamental Research Funds for the Central Universities
(grant nos. 2012G01 and 2012J05), and the PUMC graduate student
innovation fund.
References
|
1
|
Kim JS, Yang M, Kim SH, Shin T and Moon C:
Neurobiological toxicity of radiation in hippocampal cells. Histol
Histopathol. 28:301–310. 2013.PubMed/NCBI
|
|
2
|
Bladen CL, Kozlowski DJ and Dynan WS:
Effects of low-dose ionizing radiation and menadione, an inducer of
oxidative stress, alone and in combination in a vertebrate embryo
model. Radiat Res. 178:499–503. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reed JC and Kroemer G: Mechanisms of
mitochondrial membrane permeabilization. Cell Death Differ.
12:11452000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou LL, Zhou LY, Luo KQ and Chang DC:
Smac/DIABLO and cytochrome c are released from mitochondria through
a similar mechanism during UV-induced apoptosis. Apoptosis.
10:289–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belikova NA, Jiang J, Tyurina YY, Zhao Q,
Epperly MW, Greenberger J and Kagan VE: Cardiolipin-specific
peroxidase reactions of cytochrome c in mitochondria during
irradiation-induced apoptosis. Int J Radiat Oncol Biol Phys.
69:176–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verhagen AM, Ekert PG, Pakusch M, Silke J,
Connolly LM, Reid GE, et al: Identification of DIABLO, a mammalian
protein that promotes apoptosis by binding to and antagonizing IAP
proteins. Cell. 102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Srinivasula SM, Hegde R, Saleh A, Datta P,
Shiozaki E, Chai J, et al: A conserved XIAP-interaction motif in
caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis.
Nature. 410:112–116. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chai J, Shiozaki E, Srinivasula SM, Wu Q,
Datta P, Alnemri ES and Shi Y: Structural basis of caspase-7
inhibition by XIAP. Cell. 104:769–780. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riedl SJ, Renatus M, Schwarzenbacher R,
Zhou Q, Sun C, Fesik SW, Liddington RC and Salvesen GS: Structural
basis for the inhibition of caspase-3 by XIAP. Cell. 104:791–800.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC
and Takahashi R: X-linked inhibitor of apoptosis protein (XIAP)
inhibits caspase-3 and -7 in distinct modes. J Biol Chem.
276:27058–27063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chai J, Du C, Wu JW, Kyin S, Wang X and
Shi Y: Structural and biochemical basis of apoptotic activation by
Smac/DIABLO. Nature. 406:855–862. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Green DR: Apoptotic pathways: paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu G, Chai J, Suber TL, Wu JW, Du C, Wang
X and Shi Y: Structural basis of IAP recognition by Smac/DIABLO.
Nature. 408:1008–1012. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng Y, Lin Y and Wu X: TRAIL-induced
apoptosis requires Bax-dependent mitochondrial release of
Smac/DIABLO. Genes Dev. 16:33–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Y, Srinivasula SM, Druilhe A,
Fernandes-Alnemri T and Alnemri ES: Caspase-2 induces apoptosis by
releasing proapoptotic proteins from mitochondria. J Biol Chem.
277:13430–13437. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dean EJ, Ranson M, Blackhall F, Holt SV
and Dive C: Novel therapeutic targets in lung cancer: Inhibitor of
apoptosis proteins from laboratory to clinic. Cancer Treat Rev.
33:203–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saito A, Hayashi T, Okuno S, Ferrand-Drake
M and Chan PH: Interaction between XIAP and Smac/DIABLO in the
mouse brain after transient focal cerebral ischemia. J Cereb Blood
Flow Metab. 23:1010–1019. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wiessner C, Sauer D, Alaimo D and
Allegrini PR: Protective effect of a caspase inhibitor in models
for cerebral ischemia in vitro and in vivo. Cell Mol Biol
(Noisy-le-grand). 46:53–62. 2000.PubMed/NCBI
|
|
20
|
Graeber MB, López-Redondo F, Ikoma E,
Ishikawa M, Imai Y, Nakajima K, Kreutzberg GW and Kohsaka S: The
microglia/macrophage response in the neonatal rat facial nucleus
following axotomy. Brain Res. 813:241–253. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lotocki G, Alonso OF, Frydel B, Dietrich
WD and Keane RW: Monoubiquitination and cellular distribution of
XIAP in neurons after traumatic brain injury. J Cereb Blood Flow
Metab. 23:1129–1136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luan G, Zhao Y, Zhai F, Chen Y and Li T:
Ketogenic diet reduces Smac/Diablo and cytochrome c release and
attenuates neuronal death in a mouse model of limbic epilepsy.
Brain Res Bull. 89:79–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen F, Xu C, Du L, Wang Y, Cao J, Fu Y,
Guo Y, Liu Q and Fan F: Tat-SmacN7 induces radiosensitization in
cancer cells through the activation of caspases and induction of
apoptosis. Int J Oncol. 42:985–992. 2013.PubMed/NCBI
|
|
24
|
Li T, Lu C, Xia Z, Xiao B and Luo Y:
Inhibition of caspase-8 attenuates neuronal death induced by limbic
seizures in a cytochrome c-dependent and Smac/DIABLO-independent
way. Brain Res. 1098:204–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srinivasula SM and Ashwell JD: IAPs:
what’s in a name? Mol Cell. 30:123–135. 2008.
|
|
26
|
Crook NE, Clem RJ and Miller LK: An
apoptosis-inhibiting baculovirus gene with a zinc finger-like
motif. J Virol. 67:2168–2174. 1993.PubMed/NCBI
|
|
27
|
Wilson R, Goyal L, Ditzel M, Zachariou A,
Baker DA, Agapite J, Steller H and Meier P: The DIAP1 RING finger
mediates ubiquitination of Dronc and is indispensable for
regulating apoptosis. Nat Cell Biol. 4:445–450. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hofmann K, Bucher P and Tschopp J: The
CARD domain: a new apoptotic signalling motif. Trends Biochem Sci.
22:155–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kandasamy K, Srinivasula SM, Alnemri ES,
Thompson CB, Korsmeyer SJ, Bryant JL and Srivastava RK: Involvement
of proapoptotic molecules Bax and Bak in tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced
mitochondrial disruption and apoptosis: differential regulation of
cytochrome c and Smac/DIABLO release. Cancer Res. 63:1712–1721.
2003.
|
|
30
|
Hansen TM, Smith DJ and Nagley P:
Smac/DIABLO is not released from mitochondria during apoptotic
signalling in cells deficient in cytochrome c. Cell Death Differ.
13:1181–1190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu J, Wang P, Ming L, Wood MA and Zhang L:
SMAC/Diablo mediates the proapoptotic function of PUMA by
regulating PUMA-induced mitochondrial events. Oncogene.
26:4189–4198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Masoumi KC, Cornmark L, Lønne GK, Hellman
U and Larsson C: Identification of a novel protein kinase Cδ-Smac
complex that dissociates during paclitaxel-induced cell death. FEBS
Lett. 586:1166–1172. 2012.
|
|
33
|
van Loo G, Saelens X, van Gurp M,
MacFarlane M, Martin SJ and Vandenabeele P: The role of
mitochondrial factors in apoptosis: a Russian roulette with more
than one bullet. Cell Death Differ. 9:1031–1042. 2002.PubMed/NCBI
|