Introduction
Bispectral index (BIS) has been suggested as a new
method for electroencephalogram (EEG) signal analysis which is able
to transfer the mixed information from analysis of the power and
frequency of EEG into a numerical output. BIS is expressed as a
score between 0 and 100 which reflects the sedation depth and
clear-mindedness grade. A score of 100 is entirely clear-minded,
whereas decreased scores indicate increased inhibition of the
cerebral cortex (1). BIS combines
the frequency, power, phase and harmonic wave of EEG and includes a
greater amount of original EEG information which rapidly reflects
the functional status of the cerebral cortex. Therefore, BIS has
been extensively used for judging the depth of anesthesia and
consciousness state and has been considered as an index with high
sensitivity and accuracy for evaluating the consciousness state of
patients (2–6).
BIS has also been used for the diagnosis of brain
death and the evaluation of nervous system disease, which expands
its clinical applications (7). In
1996, the use of a BIS monitoring instrument in operating rooms was
approved by the United States Food and Drug Administration (FDA).
Studies have shown that monitoring has been successfully used to
assess the depth of sedation and awareness levels of patients in
intensive care units (ICUs). Therefore, BIS monitoring may be used
for a wide range of clinical applications. Since BIS monitoring has
differences between individuals, methods for evaluating the level
of the patients’ awareness, predicting the prognosis of severe
brain injury patients and diagnosing brain death remain
controversial (6,8–10).
Blood from the brain flows mainly through the
internal jugular vein and therefore, the jugular bulb venous oxygen
saturation (SjO2) index represents the cerebral oxygen
metabolism level and reflects cerebral circulation and brain
function recovery. Certain studies have noted that the difference
between the arterial oxygen saturation (SaO2) and
SjO2 (SaO2-SjO2) may directly
reflect the cerebral tissue oxygen consumption situation. Factors
which increase cerebral oxygen consumption cause increases in
SaO2-SjO2, while the
SaO2-SjO2 value is decreased by reductions in
cerebral oxygen consumption. Therefore, after cardiopulmonary
resuscitation, the correlation between SaO2 and
SjO2 may objectively reflect the cerebral oxygen
metabolic status of patients. There is a correlation between BIS
values and ischemic brain injury and brain edema. The BIS value in
patients is consistent with cerebral cortex cell oxygen consumption
(11). The present study evaluated
the prognosisof patients following acute cardiopulmonary
resuscitation by dynamically measuring BIS and SjO2
values.
Materials and methods
Clinical data
A total of 33 patients were selected, including 20
males and 13 females with an average age of 51.82±18.65 years. Of
the 33 patients, there were 16 with coronary atherosclerotic heart
disease, 6 with acute exacerbation chronic obstructive pulmonary
disease (AECOPD), 4 with uremia, 2 with hypertrophic obstructive
cardiomyopathy, 2 with pulmonary infection and 3 with
cardiopulmonary arrest of unknown cause. The resuscitation from
cardiopulmonary arrest occurred at the hospital for 24 patients and
away from hospital for 9 patients. Clinical mortality or brain
death after 1 week of therapy were used as the end-point. The
criteria of brain death included, i) irreversible coma with clear
cause; ii) persisting disappearance of brainstem reflex (complete
disappearance of brain function including brainstem function); and
iii) unrecoverable respiratory arrest. The exclusion criteria were
based on the following: children <1 year old, patients using
sedation and muscle relaxants and patients with a previous history
of epilepsy. The present study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of the Affiliated Nanjing Hospital of Nanjing Medical University.
Written informed consent was obtained from the immediate families
of all participants.
BIS monitoring methods
A multi-functional monitor (Intelli Vue MP40;
Philips, Hamburg, Germany) equipped with monitor modules for blood
pressure, heart rate, SpO2 and BIS was used. The monitor
module for BIS was obtained from Philips and the BIS sensor with an
Aspect 4-electrode from Aspect Medical Systems, Inc. (Newton, MA,
USA). All patients were treated by mechanical ventilation and
circulatory support immediately after admission or
post-cardiopulmonary resuscitation. The BIS values were monitored
as soon as the patients circulation and respiration stabilized. The
process of BIS monitoring included, i) wiping away grease from the
patient’s forehead skin using an alcohol tampon until evaporation;
ii) placing the sensor on the level of the lateral canthus; iii)
immobilizing BIS electrode patches and tightly pressing for 5 sec
to ensure good connections; iv) connecting the BIS electrode,
sensor and BIS module; and v) opening the BIS window in the monitor
and selecting the sensor display from the installed menu. After an
impedance test, the monitor showed a numerical value and graph.
When the signal quality index (SQI) was >80% and electromyogram
(EMG) was <40, the data was recorded. If the patients were
shivering or had other autonomic nerve reactions, the BIS values
were unsuitable for the requirements of the study and were not
accurate. At this point, the patients were injected intramuscularly
with 1.5 mg/kg tramadol and 5 mg droperidole during shivering or
hypothermic autonomic nerve reactions until the SQI and EMG values
improved. BIS monitoring was avoided during sputum suction,
turnover and management of nursing.
Other indices
The GCS grading of all patients was routinely
recorded following admission or post-cardiopulmonary resuscitation.
The acute physiology and chronic health evaluation (APACHE) II
scores were acquired following admission or cardiopulmonary
resuscitation within 24 h. The SaO2, SjO2 and
BIS values were determined simultaneously. In 23 patients, blood
was obtained from the internal jugular vein (basilar vein),
measured for SjO2 and the difference between
SaO2 and SjO2 was calculated.
Therapy methods
All patients underwent routine cerebral
resuscitation in the ICU, including i) mechanical ventilation with
a pattern of synchronized intermittent mandatory ventilation and
pressure support ventilation (SIMV+PSV), FiO2 40–60% and
maintenance of SpO2 ≥95%; ii) circulatory support to
maintain a mean arterial pressure >80 mmHg and ensure cerebral
perfusion; and iii) application of mannitol, albumin for
dehydration and cattle encephalon glycoside and ignotin or
Xingnaojing (a traditional Chinese medicine) injection for the
activation of cerebral metabolism and function. Ice packs or ice
blankets were used to lower temperatures to ensure that the
patients axillary temperatures did not exceed 35°C. The use of
sedative drugs which interfere with BIS was avoided.
Statistical analysis
Data were presented as the mean ± SD and statistical
analysis was performed using the SPSS 13.0 statistical analysis
software (SPSS, Inc., Chicago, IL, USA). The Student’s t-test was
used for the comparison of the mean of two samples. Pearson
correlation analysis was used for rank correlation analysis between
BIS and GCS or BIS and APACHE II scores. P<0.05 was considered
to indicate a statistically significant difference.
Results
Comparison of BIS, SaO2,
SjO2 and SaO2-SjO2
The data for these parameters are shown in Table I. Blood from 23 of the patients was
obtained from the internal jugular vein to measure SjO2
and calculate the difference between SaO2 and
SjO2. In 11 cases in the non-surviving group, the
SaO2 value was 95.97±1.55%. In 12 cases in the surviving
group, the SaO2 value was 96.25±1.57%. The
SaO2 was not significantly different between the 2
groups (t=0.512, P>0.05). The SjO2 value of patients
in the surviving group was 68.84±4.68%, while that of the
non-surviving group was 84.98±2.81%. The SjO2 of
patients in the surviving group was significantly lower than that
of the non-surviving group (t=9.909, P<0.01). The
SaO2-SjO2 value of patients in the surviving
group was 27.30±5.58%, while that of the non-surviving group was
10.99±2.96%. The SaO2-SjO2 value of patients
in the surviving group was significantly higher than that of the
non-surviving group (t=8.633, P<0.01). The BIS value of patients
in the surviving group was 61.00±16.68, while that of the
non-surviving group was 8.00±10.39. The BIS value of patients in
the surviving group was significantly higher than that of the
non-surviving group (t=10.870, P<0.01).
 | Table I.Comparison of BIS, SaO2,
SjO2 and SaO2-SjO2 in the two
groups (mean ± SD). |
Table I.
Comparison of BIS, SaO2,
SjO2 and SaO2-SjO2 in the two
groups (mean ± SD).
| Group | Case (n) | SaO2
(%) |
SjO2* (%) |
SaO2-SjO2* (%) | BIS value |
|---|
| Non-surviving | 16 | 95.97±1.55 | 84.98±2.81 | 10.99±2.96 | 8.00±10.39 |
| Surviving | 17 | 96.25±1.57 | 68.84±4.68 | 27.30±5.58 | 61.00±16.68 |
| t-value | - | 0.512 | 9.909 | 8.633 | 10.870 |
| P-value | - | 0.612 | <0.01 | <0.01 | <0.01 |
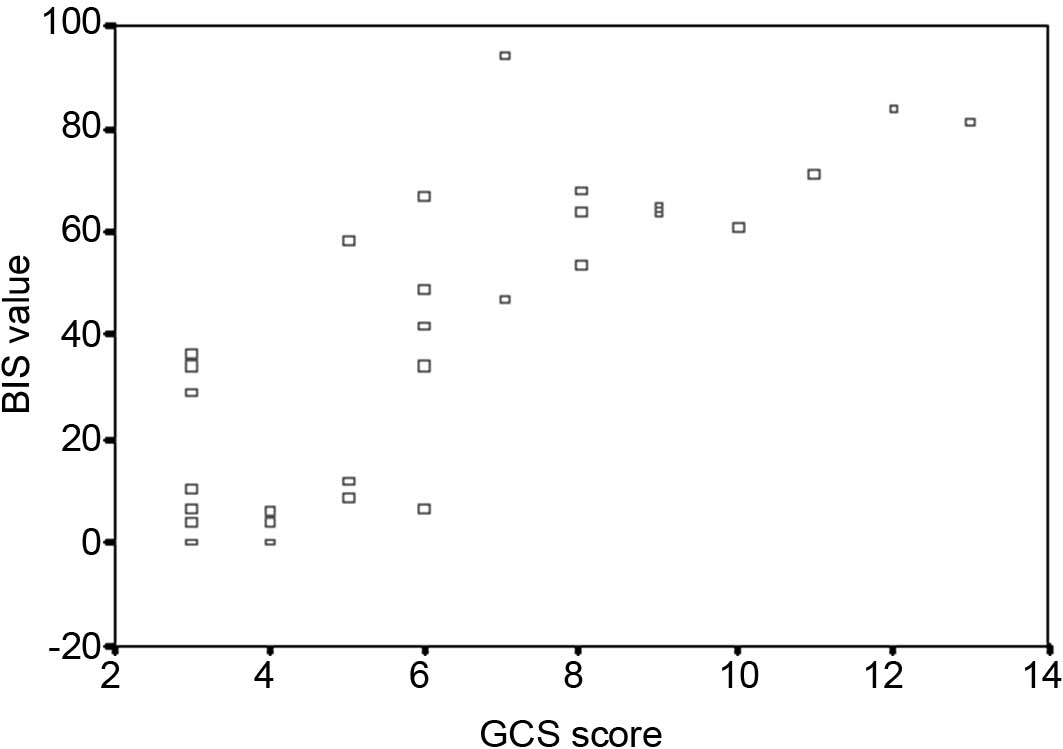
Correlation between BIS values and GCS
scores
A scatter diagram of the BIS value and GCS score is
shown in Fig. 1. Pearson
correlation analysis between BIS and GCS revealed a positive
correlation with a correlation coefficient of 0.821 and the
association was significant (P<0.001).
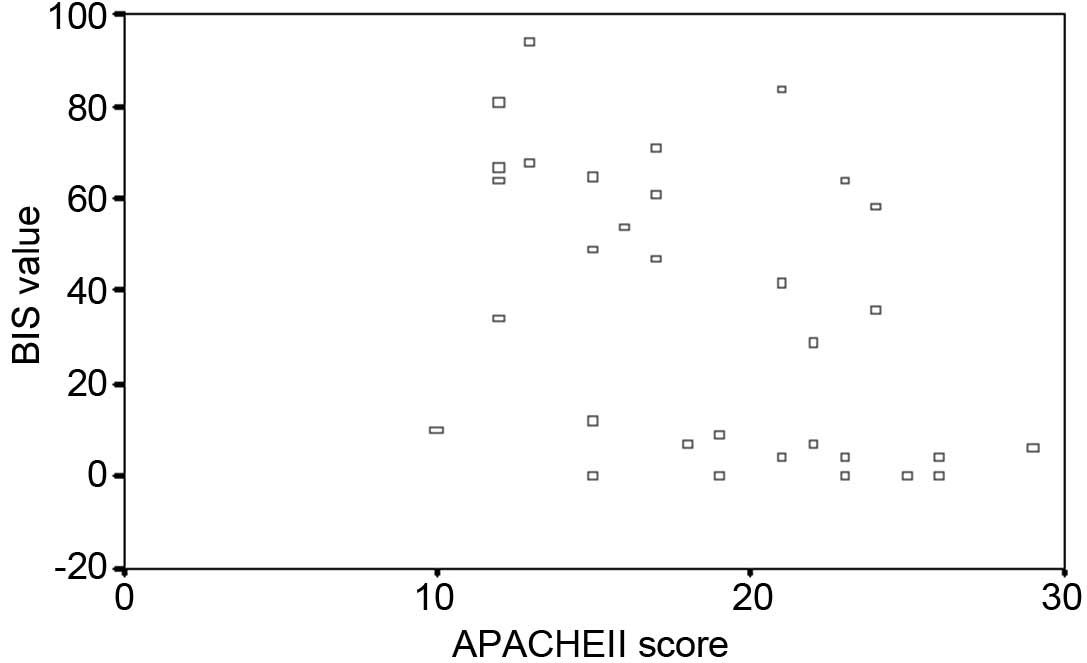
Correlation between BIS values and APACHE
II scores
A scatter diagram of the BIS value and APACHE II
score is shown in Fig. 2. Pearson
correlation analysis between BIS and APACHE II revealed a negative
correlation with a correlation coefficient of 0.434, and the
association was significant (P=0.012).
Discussion
It has been reported that the EEG shows extensively
independent activity ∼10 sec after cardiac arrest. Electrocerebral
activity with low voltage and high frequency appears at 15–20 sec
after chest cardiac massage (12).
Subsequently the electrocerebral activity reverts to normal with
the return of the sinus rhythm. Shibata et al(13) observed changes in the BIS value of
10 patients following cardiac arrest and noted that the BIS value
in patients with successful resuscitation was significantly higher
than in those who succumbed. When BIS values are persistently
<80, patients enter a vegetative state. Therefore, BIS is
considered to predict the prognosis of patients following cardiac
pulmonary cerebral resuscitation, although opinions differ
(14). Vivien et
al(15) observed the BIS value
and prognosis of 56 patients with severe coma (GCS≤5) and noted
that 12 patients with BIS values of 0 were validated to be brain
dead via EEG or cerebral angiography. Of the 44 patients with BIS
values of between 20 and 79 at admission to the ICU, the BIS value
gradually dropped to 0 in 27 patients within several hours or days
and progressed to brain death with clinical confirmation. A further
17 patients with an average BIS value >35 did not progress to
brain death. The authors proposed that BIS monitoring is useful for
predicting the occurrence of brain death in patients with severe
coma and provides experimental evidence for the diagnosis of brain
death. Fàbregas et al(16)
reported that BIS values aided the prediction of consciousness
recovery when studying cerebral injury.
Analysis of the 33 patients in the present study
indicated that the BIS values in the surviving group were
significantly higher than those in the non-surviving group. Further
analysis revealed that BIS values aided the prediction of the
prognosis. When the BIS value was persistently >80, patients
were more likely to be clear-minded. When the BIS value was
persistently <20 and showed progressive reductions, the patients
were unlikely to survive. When the BIS values were persistently
stable at 40–60, the majority of patients were in a vegetative
state. The results showed that the BIS value was able to predict
the prognosis of patients following cardiopulmonary resuscitation.
BIS mainly reflects the electrical activity of the cerebral cortex
and does not provide any evidence reflecting the electrical
activity of the brainstem. In the present study, two patients with
BIS values <40, remained alive in a vegetative state. The
possible reasons may be the ischemic and hypoxia tolerance time of
4–6 min for the cerebral cortex but 20–30 min for the brainstem
(17). Consequently, the
respiratory and vasomotor center remained intact and survived in a
vegetative state although the BIS values were low in certain
patients. The surviving group in the present study included
individuals who survived in a vegetative states and others with
good recovery of brain function. No subgroup analysis was conducted
due to the small number of cases. Enlarging the case number for
further subgroup analysis is likely to improve the evaluation of
the prognosis.
GCS and APACHE II scores are important indicators
for evaluating a patient’s condition in the ICU. It has been
reported that there is a good correlation between BIS and GCS
(18). In the present study, the
BIS value gradually increased with increased GCS scores and the
results showed a positive correlation between BIS and GCS. The
correlation remained unclear between the BIS and APACHE II score.
Although there were correlations between the BIS value and APACHE
II score and their association was significant (P<0.05), the low
correlation coefficient indicated a weak correlation. The APACHE II
score indicates the severity of illness which is mainly associated
with the physiological indicators of patients, while GCS scoring
reflects the degree of CNS injury. As such, GCS values and APACHE
II scores are different indicators. Since BIS reflects the
functional status of the cerebral cortex by analyzing the brain’s
electrical signals, it may be more closely correlated with the
GCS.
It has been reported that BIS values fall to 0 with
decreased electrocerebral activity when cardiac arrest results from
hypovolemia and rises when the blood pressure increases following
blood volume supplementation. The changes to BIS values are delayed
by 2 min compared with changes in EEG due to the time required for
calculating BIS values via brainwaves. However, changes to BIS
occur earlier than hemodynamic changes (19). Besides blood volume, there are
several important factors affecting BIS values, including
temperature (20,21), instruments in ICUs, levels of blood
sugar (22), ethnicity (2), physical therapy (23) and use of muscle relaxant (24–26)
or nerve blocker. It is necessary to identify a method for avoiding
the signal interference of BIS and provide an improved means of
monitoring ICU patients.
The blood circulating in the brain flows through the
jugular vein, thus SjO2 indicates the level of the
cerebral oxygen metabolism and reflects the recovery status of
cerebral circulation and function (11). According to the Fick formula
(SaO2 - SjO2 = CMRO2 / CBF x
CaO2), any factor that decreases the consumption of
cerebral oxygen decreases the SaO2-SjO2
value. The normal range of SjO2 is 54–75%. When
SaO2 is at 100%, the normal range of
SaO2-SjO2 is 25–46%. In patients with brain
death, the majority or all of the cells in the cerebral cortex are
dead and lose the ability to absorb and consume oxygen during the
hydrocephalus peak so the SaO2-SjO2 value is
lower than the normal range. BIS may also be low due to the
cessation of the brain’s electrical activity. The surviving group
in the present study showed higher SaO2-SjO2
values than the non-surviving group, indicating that cerebral
function and metabolism remained present, although there was
insufficient perfusion during the hydrocephalus peak and the cells
of cerebral cortex were oxygen deficient due to the consumption of
oxygen. The BIS value in the surviving group remained higher than
that of the non-surviving group. Therefore, there is a association
between the changes to the BIS value and the degree of oxygen
consumption in cerebral cells. The evaluation of a patient’s
prognosis is based on the BIS value which reflects the function of
the cerebral cortex. The combination of BIS and
SaO2-SjO2 values may predict the prognosis of
a resuscitated patient, but does not change the prognosis of the
patient.
An aggressive strategy of cerebral protection is
likely to decrease the injury or death of nerve cells, prevent
further deterioration and improve the prognosis in patients
following cardiac pulmonary resuscitation. The principles of
protecting the brain include applying mechanical ventilation,
maintaining the correct mean arterial pressure to improve cerebral
perfusion and actively lowering the patient’s temperature using an
ice pack or blanket. The present study demonstrated that there was
a correlation between the changes to the BIS value and the degree
of oxygen consumption in cerebral cells which reflected the
function of the cerebral cortex. At the the same time as
implementing cerebral protection strategies, further studies are
required to consider the BIS value as a guide for therapy.
Acknowledgements
This study was supported by a grant
from the Fundamental Research Funds for Nanjing City Medical
Science and Technology Development Project (YKK08099).
References
|
1.
|
Lehmann A, Karzau J, Boldt J, Thaler E,
Lang J and Isgro F: Bispectral index-guided anesthesia patients
undergoing aorto-coronary bypass grafting. Anesth Analg.
96:336–343. 2003.
|
|
2.
|
LeBlanc JM, Dasta JF and Kane-Gill SL:
Role of the bispectral index in sedation monitoring in the ICU. Ann
Pharmacother. 40:490–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Haenggi M, Ypparila-Wolters H, Bieri C, et
al: Entropy and bispectral index for assessment of sedation,
analgesia and the effects of unpleasant stimuli in critically ill
patients: an observational study. Crit Care. 12:R1192008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sackey PV, Radell PJ, Granath F and
Martling CR: Bispectral index as a predictor of sedation depth
during isoflurane or midazolam sedation in ICU patients. Anaesth
Intensive Care. 35:348–356. 2007.PubMed/NCBI
|
|
5.
|
Leary M, Fried DA, Gaieski DF, et al:
Neurologic prognostication and bispectral index monitoring after
resuscitation from cardiac arrest. Resuscitation. 81:1133–1137.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Stammet P, Werer C, Mertens L, Lorang C
and Hemmer M: Bispectral index (BIS) helps predicting bad
neurological outcome in comatose survivors after cardiac arrest and
induced therapeutic hypothermia. Resuscitation. 80:437–442. 2009.
View Article : Google Scholar
|
|
7.
|
Liu H and Liu Y: The bispectral index in
the ICU application. J Practical Med. 24:3091–3093. 2008.(In
Chinese).
|
|
8.
|
Chollet-Xémard C, Combes X, Soupizet F, et
al: Bispectral index monitoring is useless during cardiac arrest
patients’ resuscitation. Resuscitation. 80:213–216. 2009.PubMed/NCBI
|
|
9.
|
Myles PS, Daly D, Silvers A and Cairo S:
Prediction of neurological outcome using bispectral index
monitoring in patients with severe ischemic-hypoxic brain injury
undergoing emergency surgery. Anesthesiology. 110:1106–1115. 2009.
View Article : Google Scholar
|
|
10.
|
Dumans-Nizard V, Hamada S and Fischler M:
Bispectral index during cardiopulmonary resuscitation: a poor
indicator of recovery. Two very different cases. Anaesthesia.
65:196–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Edgren E, Enblad P, Grenvik A, et al:
Cerebral blood flow and metabolism after cardiopulmonary
resuscitation. A pathophysiologic and prognostic positron emission
tomography pilot study. Resuscitation. 57:161–170. 2003. View Article : Google Scholar
|
|
12.
|
Lasasso TJ, Muzzi DA, Meyer FB and
Sharbrough FW: Electroencephalographic monitoring of cerebral
fuction during asystole and successful cardiopumonary
resuscitation. Anesth Analg. 75:1021–1024. 1992.PubMed/NCBI
|
|
13.
|
Shibata S, Imota T, Shigeomi S, Sato W and
Enzan K: Use of the bispectral index during the early
postresuscitative phase after out-of-hospital cardiac arrest. J
Anesth. 19:243–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fatovich DM, Jacobs IG, Celenza A and
Paech MJ: An observational study of bispectral index monitoring for
out of hospital cardiac arrest. Resuscitation. 69:207–212. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Vivien B, Paqueron X, Le Cosquer P,
Langeron O, Coriat P and Riou B: Detection of brain death onset
using the bispectral index in severely comatose patients. Intensive
Care Med. 28:419–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Fàbregas N, Gambús PL, Valero R, Carrero
EJ, Salvador L, Zavala E and Ferrer E: Can bispectral index
monitoring predict recovery of consciousness in patients with
severe brain injury. Anesthesiology. 101:43–51. 2004.PubMed/NCBI
|
|
17.
|
Shen YM, Han JY, Zhao J, et al:
Cardiopulmonary resuscitation. Modern Critical Care Medicine. Zhao
N, Sa XM and Sun YL: 1st edition. Military Medical Science Press;
Beijing: pp. 1072010, (In Chinese).
|
|
18.
|
Wang Q, Wang GN and Zhou JX: The
bispectral index in the intensive care unit application. Reviews
and Lectures. 14:48–50. 2007.(In Chinese).
|
|
19.
|
Azim N and Wang CY: The use of bispectral
index during a cardiopulmonary arrest: a potential predictor of
cerebral perfusion. Anaesthesia. 59:610–612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tonner PH, Wei C, Bein B, Weiler N, Paris
A and Scholz J: Comparison of two bispectral index algorithms in
monitoring sedation in postoperative intensive care patients. Crit
Care Med. 33:580–584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Struys MM, Jensen EW, Smith W, et al:
Performance of the ARX-derived auditory evoked potential index as
an indicator of anesthetic depth: a comparison with bispectral
index and hemodynamic measures during propofol administration.
Anesthesiology. 96:803–816. 2002. View Article : Google Scholar
|
|
22.
|
Wu CC, Lin CS and Mok MS: Bispectral index
monitoring during hypoglycemic coma. J Clin Anesth. 14:305–306.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Brocas E, Dupont H, Paugam-Burtz C, Servin
F, Mantz J and Desmonts JM: Bispectral index variations during
tracheal suction in mechanically ventilated critically ill
patients: effect of an alfentanil bolus. Intensive Care Med.
28:211–213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Greif R, Greenwald S, Schweitzer E, et al:
Muscle relaxation does not alter hypnotic level during propofol
anesthesia. Anesth Analg. 94:604–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Vivien B, Di Maria S, Ouattara A, Langeron
O, Coriat P and Riou B: Overestimation of Bispectral Index in
sedated intensive care unit patients revealed by administration of
muscle relaxant. Anesthesiology. 99:9–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ge SJ, Zhuang XL, He RH, Wang YT, Zhang X
and Huang SW: Neuromuscular block with vecuronium reduces the
rapidly extracted auditory evoked potentials index during steady
state. Can J Anaesth. 50:1017–1022. 2003. View Article : Google Scholar
|