Introduction
Nerve fibers are distributed in the submucosa and
myenteric structure of normal intestines, which are called the
Meissner’s plexus and Auerbach’s plexus, respectively (1). The nervous plexus contains mature
neuroganglion cells which maintain intestinal peristalsis to
transport food (2). Without
neuroganglion cells, preganglionic fibers of the parasympathetic
ganglion from sacral spinal cord segments S2–S4 are not able to
form synapses and generate postganglionic fibers in the intestinal
tract, which causes preganglionic nerve fiber hyperplasia and
incoordination of intestinal systaltic and peristaltic movement,
resulting in difficulties in food transportation (3).
Urine experiences a similar sequence of migration
from production in the kidneys to secretion through the urethra.
The structures participating in urine transportation, including the
kidneys, ureter, bladder and urethra, are collectively called the
urinary tract. The urinary tract is composed of mucosa, myometrium
and an outer membrane (4). To
date, there is insufficient evidence on whether nerve fibers are
distributed in the urinary tract and whether there is a correlation
between nerve fiber activity and urine transportation. However, a
knowledge of nerve development and distribution in the urinary
tract is important for disease diagnosis and treatment. Therefore,
we designed the current study to observe the development and
distribution of autogenous nerves in the urinary tract of New
Zealand rabbits of varying ages through section analysis.
Materials and methods
Animals
Twenty New Zealand rabbits were selected aged 1, 4,
8 and 12 weeks. They were sacrificed by air injection in the ear
marginal and the urinary system, including the kidneys, ureter,
bladder and posterior urethra, was removed.
A further three pregnant rabbits were selected at 2,
3 and 3.5 weeks of gestation. They were sacrificed by air injection
in the marginal ear vein. Five fetal rabbits of each gestational
age were obtained by cesarean section. The fetal rabbits were
dissected and embryonic tissues, including the kidneys, ureter,
bladder and posterior urethra, were removed.
Immunohistochemistry
All organs were fixed in 0.4% formalin. Samples of
each urinary organ were taken as follows: i) kidney: parallel to
the renal pelvis; ii) ureter: upper-third of the ureter and
lower-third of the ureter; iii) bladder: upper half of the bladder
and lower half of the bladder including the trigone of the bladder;
iv) posterior urethra: proximal to the pubic symphysis.
Samples were embedded in paraffin and sections were
cut. Hematoxylin and eosin (H&E), protein gene product 9.5
(PGP9.5) and neuron-specific enolase (NSE) staining methods were
used following manufacturer’s instructions. All kits used were
purchased from Shanghai Changdao Biotech Co. Ltd. (Shanghai,
China).
Results
The H&E stained urinary organs were barely
visible to the naked eye and only a vague structure could be
observed microscopically at a gestational age of 2 weeks. At
gestational ages of 3 and 3.5 weeks, urinary organs began to form.
The glomeruli were observed to be immaturely developed and the
urinary tract was beginning to form.
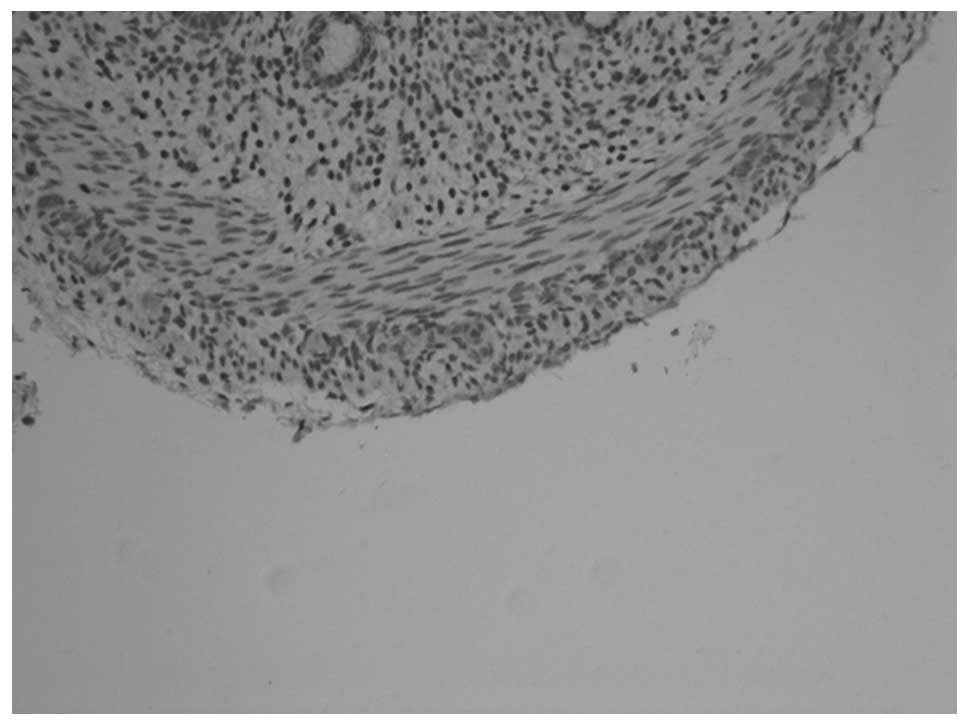
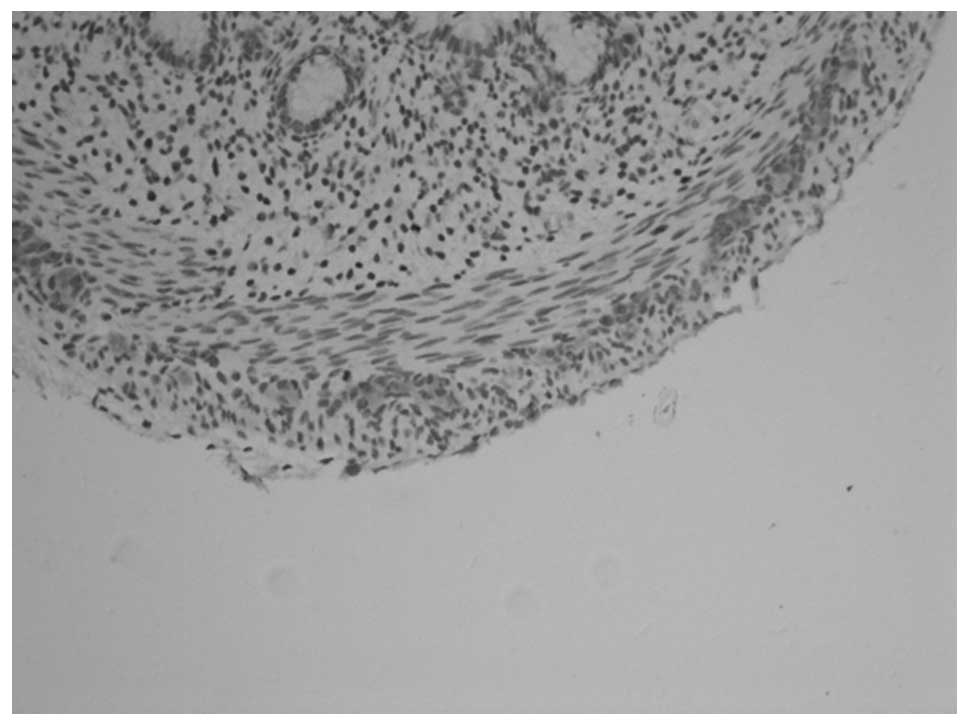
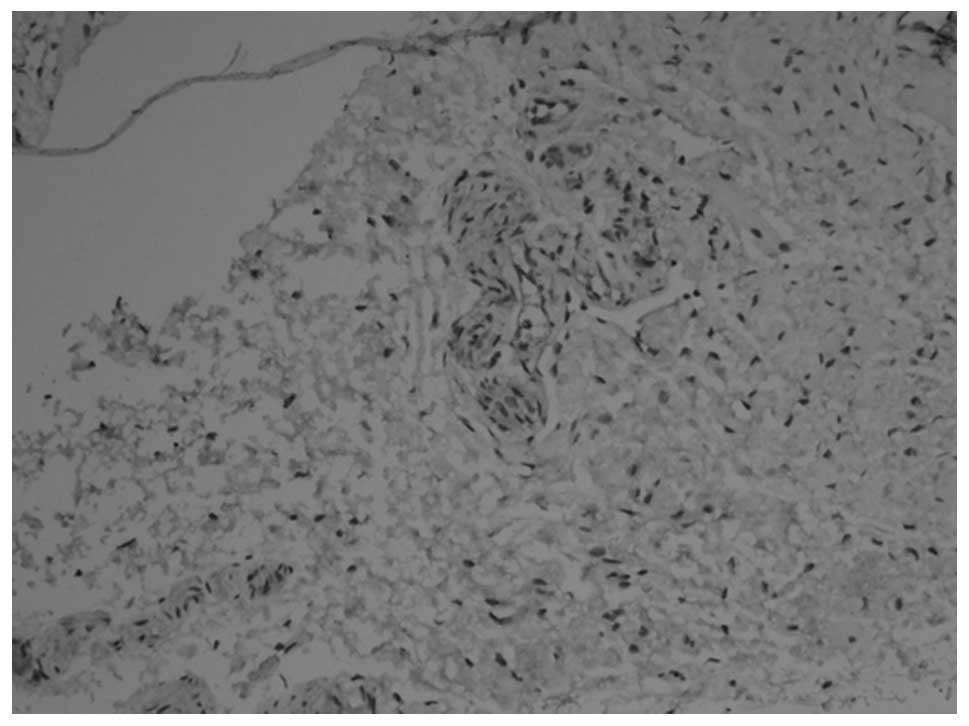
PGP9.5 and NSE revealed similar staining results. In
animals at gestational ages of 3 and 3.5 weeks, the myenteric
plexus and outer membrane of the nervous plexus were present in the
renal pelvis to the posterior urethra and neuroganglion cells were
sparsely distributed (Figs. 1 and
2). Animals aged 1 week
demonstrated sparse myenteric plexus distribution in the tissue
from the renal pelvis to the ureter and neuroganglion cells
disappeared. At 8 weeks, the myenteric plexus completely
disappeared; however, plexus remained at the site of the outer
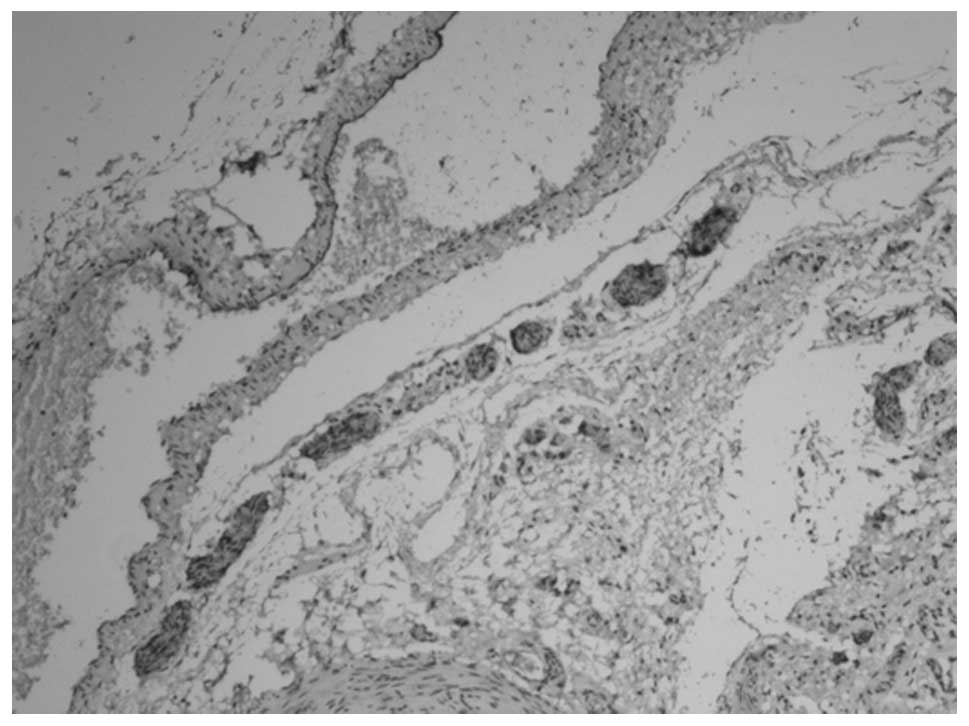
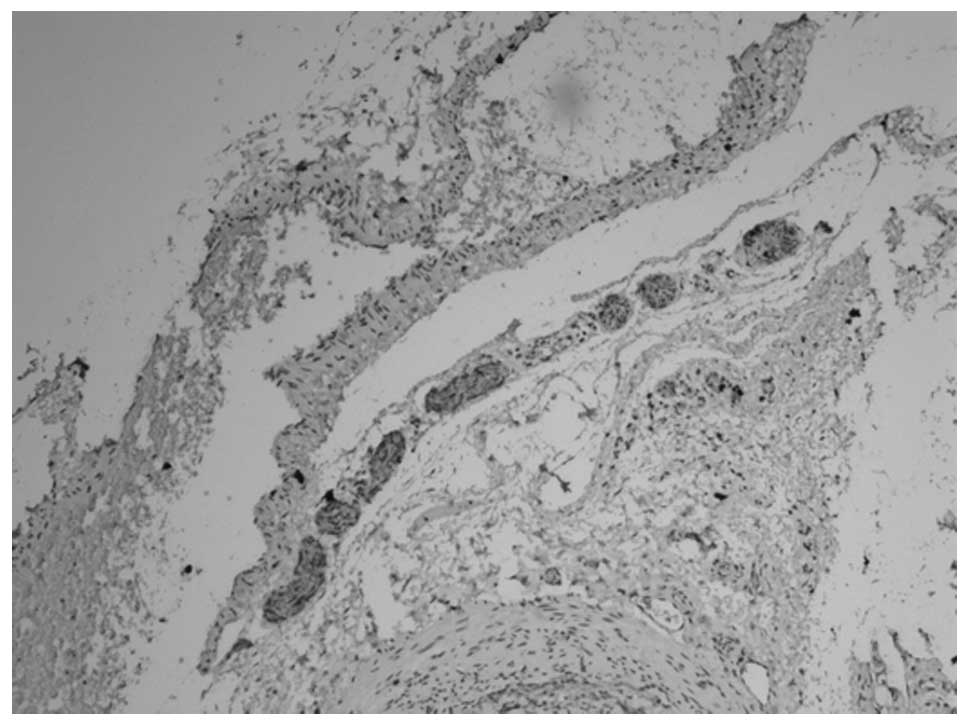
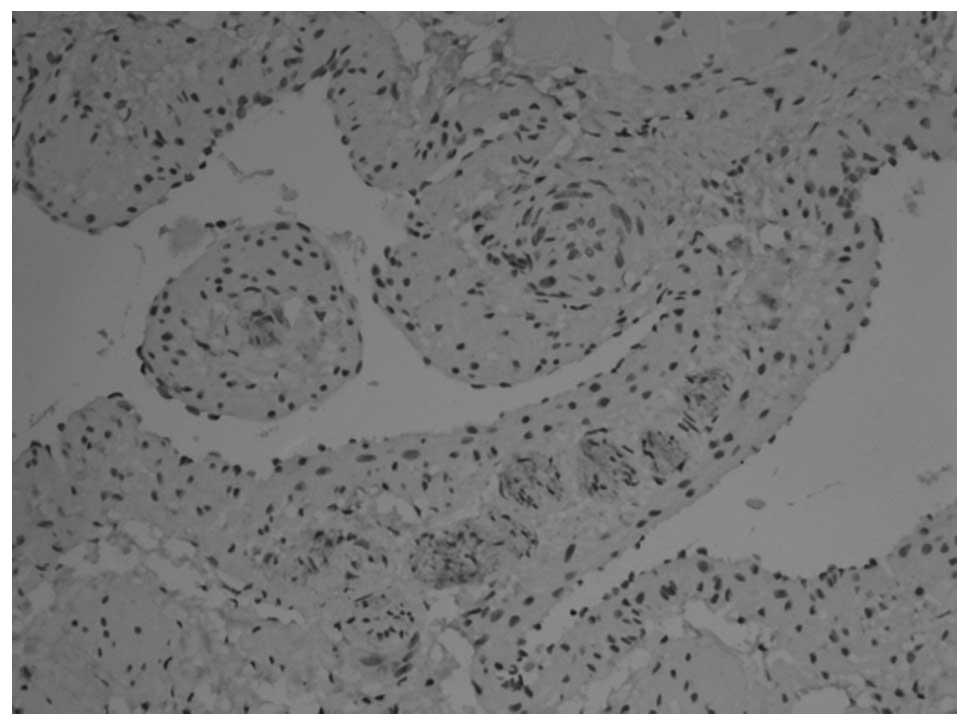
membrane (Figs. 3 and 4). At 12 weeks, the myenteric plexus
showed clear, positive staining in the tissue from the bladder to
the posterior urethra (Figs. 5 and
6) and the outer membrane of the
nervous plexus existed permanently.
Discussion
The main function of the urinary system is to
generate, transport and excrete urine through the urinary tract. To
a degree, difficulty in urine transportation is the main cause of
functional urinary obstruction (5). A number of organic diseases
presenting obstruction in the renal pelvis, ureter or posterior
urethral valve may cause further difficulties in urine
transportation (6). Primary and
secondary urine transportation irregularities may influence nerve
fiber development and distribution (7). Under normal conditions, the neural
regulation of the urinary system is complicated. The sympathetic
and parasympathetic nerves are the principal fibers that control
urinary function. Certain somatic nerves participate in controlling
bladder and urethral function, urine reservoir filling and
urination (8). Studies into nerve
development in the urinary tract and its function in the process of
urine formation and transportation are lacking. However, it is
important to understand the nerve distribution in the urinary
organs to comprehend the etiology and pathology of diseases.
Nerve fibers develop during the embryonic phase.
Obstacles to embryonic nervous development influence the function
of relative organs (9–12). In the sixth week of gestation in
the development of the human digestive tract, the neuroblasts in
the neural crest move downwards in the digestive tract wall to form
neuroganglion cells of the myenteric plexus. The myenteric
neuroganglion cells continue to move and form submucosal
neuroganglion cells. This movement is complete by the twelfth week
of embryonic development (13).
Compared with research on the digestive tract,
studies concerning nerve development in the urinary tract are
scarce. In the current study, we measured the nerve distribution in
the urinary tract wall of New Zealand rabbits at various
gestational ages, by immunohistochemistry. The distribution of the
nerve plexus and neuroganglion of the outer membrane may be the
residual of peripheral nerves, therefore the myenteric plexus
represents the autogenous nerve network of the urinary organs. Our
study revealed that in the urinary system of New Zealand rabbits,
the myenteric plexus of the upper urinary organs is formed when the
kidneys are in embryonic form. As the embryo develops, the nerve
plexus starts to degenerate and completely disappears in the adult
phase. The myenteric plexus of the lower urinary organs is present
continuously from a gestational age of 3 weeks to the adult phase,
and its neuroganglion cells are also present. This demonstrates
that the autogenous nerve development and distribution of urinary
organs is completely different from that of the digestive tract.
Additionally, the autogenous nerve development of the upper and
lower urinary organs are also different.
In the embryo, the upper and lower urinary tract
have different formation processes. In the fourth week of human
embryonic development, the ureteric bud protrudes from the
mesonephric duct and grows into renal blastema. In the fifth week,
it forms the renal pelvis (14).
The bladder originates from cloaca. During weeks 4–6 of human
embryonic development, the cloaca is divided into two parts by the
urogenital diaphragm. The ventral part grows into the bladder and
proximal urethra and the dorsal part grows into the hindgut
(15). We consider that different
origins of fetation result in the contrasting nerve distribution in
the upper and lower urinary tract. Judging from the development
period, the shaping of the urogenital sinus occurs earlier than the
development of intestinal neurons. Autogenous nerve development in
the bladder and posterior urethra from the cloaca has a close
correlation with the digestive tract so the distribution of the
myenteric plexus in the urinary system may be similar to that in
the intestines.
The myenteric plexus may be involved in the
functioning of the lower urinary organs. Physiological observations
indicate that the bladder and posterior urethra may actively
control urine transportation (16,17).
Contraction of the bladder and the opening of the posterior urethra
are synchronized to guarantee normal urination (18). In urine reservoir filling and
micturition, myenteric nerve fibers regulate and coordinate the
process (19,20). The renal pelvis and ureter are not
actively involved in urine transportation and their autogenous
nerve develops weakly and gradually degenerates after birth. In the
process of urine transportation, signaling in the muscle and
tissues ensure the normal systaltic and peristaltic action of the
organs (21). The uriniferous
tubule of the renal parenchyma is the site of fluid excretion and
absorption (22). A concentration
change in water-electrolyte levels in the microenvironment affects
the amount of urine and its composition. Therefore, the humoral
regulation is more important and almost has no autogenous nerve
distribution.
Autogenous nerve development and distribution in the
urinary tract is an important subject area. Our study has shown
that nerve development and distribution in the upper and lower
urinary tract are different, therefore it is difficult to clearly
explain the correlation between urination tract nerve distribution
and disease. This research is still in the early stages and further
research is required.
Acknowledgements
The authors are grateful to the
Science and Technology Commission of Shanghai Municipality for
financial support (No. 12ZR1419200).
References
|
1.
|
Sişu AM, Petrescu CI, Cebzan CC, et al:
Enteric nervous system development in cavitary viscera allocated to
the celiac plexus. Rom J Morphol Embryol. 49:63–67. 2008.PubMed/NCBI
|
|
2.
|
Mishalany H, Olson A, Khan F and Santos A:
Deficient neurogenic innervation of the myenteric plexus with
normal submucous plexus involving the entire small and large bowel.
J Pediatr Surg. 24:83–86. 1989. View Article : Google Scholar
|
|
3.
|
Corsois L, Boman F, Sfeir R, et al:
Synaptophysin expression abnormalities in Hirschsprung’s disease.
Ann Pathol. 24:407–415. 2004.(In French).
|
|
4.
|
Wu XR, Kong XP, Pellicer A, Kreibich G and
Sun TT: Uroplakins in urothelial biology, function, and disease.
Kidney Int. 75:1153–1165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Balster S, Schiborr M, Brinkmann OA and
Hertle L: Obstructive uropathy in childhood. Aktuelle Urol.
36:317–328. 2005.(In German).
|
|
6.
|
Klahr S: Obstructive nephropathy. Intern
Med. 39:355–361. 2000. View Article : Google Scholar
|
|
7.
|
Kubo T and Kawamura S: Anatomy and
function of the upper urinary tract. Nihon Hinyokika Gakkai Zasshi.
83:1759–1766. 1992.(In Japanese).
|
|
8.
|
Sugaya K, Nishijima S, Miyazato M and
Ogawa Y: Central nervous control of micturition and urine storage.
J Smooth Muscle Res. 41:117–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pacary E, Haas MA, Wildner H, et al:
Visualization and genetic manipulation of dendrites and spines in
the mouse cerebral cortex and hippocampus using in utero
electroporation. J Vis Exp. 26:41632012.
|
|
10.
|
Molina-Hernández A, Díaz NF and
Arias-Montaño JA: Histamine in brain development. J Neurochem.
122:872–882. 2012.
|
|
11.
|
Burzynski G, Shepherd IT and Enomoto H:
Genetic model system studies of the development of the enteric
nervous system, gut motility and Hirschsprung’s disease.
Neurogastroenterol Motil. 21:113–127. 2009.
|
|
12.
|
Heanue TA and Pachnis V: Enteric nervous
system development and Hirschsprung’s disease: advances in genetic
and stem cell studies. Nat Rev Neurosci. 8:466–479. 2007.
|
|
13.
|
Landman KA, Simpson MJ and Newgreen DF:
Mathematical and experimental insights into the development of the
enteric nervous system and Hirschsprung’s disease. Dev Growth
Differ. 49:277–286. 2007.PubMed/NCBI
|
|
14.
|
Sakurai H: Molecular mechanism of ureteric
bud development. Semin Cell Dev Biol. 14:217–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Körner I: Fetal bladder development. A
current overview. Urologe A. 46:1643–1646. 2007.(In German).
|
|
16.
|
Zilberman DE, Golomb J, Kitrey ND, et al:
Long-term urinary bladder function following unilateral refluxing
low loop cutaneous ureterostomy. Korean J Urol. 53:355–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fujihara A, Ukimura O, Iwata T and Miki T:
Neuroselective measure of the current perception threshold of
A-delta and C-fiber afferents in the lower urinary tract. Int J
Urol. 18:341–349. 2011. View Article : Google Scholar
|
|
18.
|
Yamaguchi O, Honda K, Nomiya M, et al:
Defining overactive bladder as hypersensitivity. Neurourol Urodyn.
26:904–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Satchell P and Vaughan C: Hypogastric
nerve activity to the feline bladder during slow filling. J Auton
Nerv Syst. 25:41–47. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Khadra MH, Satchell PM and Vaughan CW:
Sympathetic nervous system effects on feline bladder wall
compliance throughout continence. Acta Physiol Scand. 155:31–39.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kuvel M, Canguven O, Murtazaoglu M and
Albayrak S: Distribution of Cajal like cells and innervation in
intrinsic ureteropelvic junction obstruction. Arch Ital Urol
Androl. 83:128–132. 2011.PubMed/NCBI
|
|
22.
|
Waldrop JE: Urinary electrolytes, solutes,
and osmolality. Vet Clin North Am Small Anim Pract. 38:503–512.
2008. View Article : Google Scholar : PubMed/NCBI
|