Introduction
The cell membrane is a semipermeable membrane. Low
levels of penetrating fluid are likely to dissolve cells,
particularly red blood cells, and the excessive dissolution of red
blood cells results in kidney damage. In the process of metabolism
and a variety of physiological functions, a flow of active
molecules in and out of the cell occurs and the cell volume
changes. Excessive volume change leads to cellular dysfunction
(1). However, cells are partly
able to influence the changes in cell volume. The tolerance of
cells to low osmotic pressures and semipermeable membrane
permeability is different (2). To
date, with the exception of blood cells, changes to cells under
varying osmotic pressures have not been reported. In this study,
B95-8 cell growth, membrane changes and Epstein-Barr virus (EBV)
infection were investigated under varying osmotic pressures [0.90%
NaCl (330 mOsm/kg H2O), 0.36% NaCl (115 mOsm/kg
H2O), 0.27% NaCl (93 mOsm/kg H2O) and
distilled water]. In addition, under the above pressure conditions,
we investigated whether the virus was pumped out onto the cell
surface, to allow the virus to be effectively cleared by drugs.
Additionally, we investigated the removal of the human
immunodeficiency virus (HIV) from lymphocytes at a low osmotic
pressure.
Materials and methods
Materials
Electronic instruments
The following equipment was used: microscope
(Hitachi, Tokyo, Japan), H-500Steri-cycle (Hitachi), Sanyo MC0175
CO2 incubator (Sanyo, Osaka Moriguchi, Japan), SW-CJ-IF
super-clean single-sided platform (Aertai, Suzhou, China), inverted
biological microscope (Chongqing Optical and Electrical Instrument
Co., Ltd., Chongqing, China) and an advanced model 3900 osmometer
(Advanced Instruments, Norwood, MA, USA).
Solutions
The following solutions were prepared in a sterile
environment: 0.90% NaCl solution (330 mOsm/kg H2O,
isotonic solution), 0.36% NaCl solution (115 mOsm/kg
H2O, hypotonic solution), 0.27% NaCl solution (93
mOsm/kg H2O, hypotonic solution) and distilled water. In
addition, excess solution was used to measure the osmotic pressure
using the osmometer.
Stain
A solution of trypan blue (0.4%) was prepared with
normal saline.
B95-8 cells
B95-8 cells were obtained from the Chinese Medical
Science Institute. For cell recovery, the following procedure was
performed: the cryopreservation tubes were removed from the liquid
nitrogen tank and quickly placed in a 37°C water bath, with shaking
from time to time. The freezing tube was scrubbed with 75% ethanol.
The cell suspensions were then removed and injected into a
centrifuge tube, dropping the culture solution 10 times to mix. The
solution was centrifuged at low-speed (1,000 rpm) for 10 min, then
the supernatant was discarded and cells were washed repeatedly with
RPMI-1640 medium to remove the dimethyl sulfoxide. The culture
solution was transferred to a culture bottle and placed into the
CO2 incubator (37°C, 5% CO2). The following
day, the culture medium was replaced. The cells were collected
after culturing for 7 days. The number of cells was determined to
be 1×107/ml by electron microscopy (3).
Methods
B95-8 cells under various osmotic
pressures
Under sterile conditions, B95-8 cells were placed
into 10 ml centrifuge tubes containing 0.90% NaCl solution (330
mOsm/kg H2O, isotonic solution), 0.36% NaCl solution
(115 mOsm/kg H2O), 0.27% NaCl solution (93 mOsm/kg
H2O, hypotonic solution) or distilled water,
respectively. The cells were cultured at 37°C for 5 min and then
centrifuged at low-speed (1,000 rpm) for 5 min. The supernatant was
discarded and the step was repeated twice. The cells were then
centrifuged at 2,000 rpm for 5 min and the B95-8 cells collected.
The cells from each solution were then placed into three tubes. The
total solution in each tube was 0.3 ml and the density of the cells
was 5×106/ml. To each tube, 20 μl 0.4% trypan
blue was added for 10 min. The blood cells were counted on a
counting plate under a common microscope. The blue-stained cells
were dead and the cells without staining were living. In each tube,
500 cells were counted and the percentage of surviving cells was
calculated. The cells were placed in a 24-well plate to culture for
48 h in RPMI-1640 medium. The cells were fixed with 3%
glutaraldehyde fixation overnight then washed with buffer, acidized
with l% osmium tetroxide, dehydrated with an ethanol gradient,
embedded with epoxy resin 618, cut into sections using an LKB-V
slicer and stained using uranyl acetate and lead citrate (4).
HIV cells and low osmotic
pressure
This study was approved by the ethics committee of
Guangxi medical university, Nanning, China. After obtaining
informed consent from patients, 10 ml blood was extracted from
untreated HIV-positive patients. The blood was divided into three
tubes. Each tube contained 3 ml venous blood and was mixed with 3
ml Hanks’ fluid to dilute. Then, 3 ml diluted blood was added to 2
ml lymphocyte separation liquid and centrifuged at 2,000 rpm for 20
min. A flat capillary tube was used to absorb the single nuclear
cells, which were transferred into a short tube. More than five
times the volume of Hanks’ solution was added and the cells were
washed twice. The concentrated nucleated cells were placed in a
tube. Some of these cells were placed in a 24-well plate and
cultured for 48 h in RPMI-1640 medium. The remainder were fixed
with 3% glutaraldehyde and laid aside overnight to prepare for
electronic microscopic examination. The cells were fixed with l%
osmium tetraoxide, dehydrated using an alcohol gradient, embedded
in epoxy resin 618, cut into sections using an LKB-V slicing
machine and double stained with uranyl acetate and lead
citrate.
Results
Trypan blue staining
The survival rates of 500 cells under varying
osmotic pressures as revealed by trypan blue staining are shown in
Table I.
 | Table I.Survival rates of cells under
different osmotic pressures. |
Table I.
Survival rates of cells under
different osmotic pressures.
| Osmotic pressure | Cell survival rate
(%) |
|---|
| 0.90% NaCl (330
mOsm/kg H2O) | 92 |
| 0.36% NaCl (115
mOsm/kg H2O) | 89 |
| 0.27% NaCl (93
mOsm/kg H2O) | 91 |
| Distilled water | 2 |
No difference in the cell survival rate among the
different osmotic pressures was observed. Cells swelled with low
permeability in the low osmotic pressure tube. The blue cells were
considered to be apoptotic cells arising from the process of
cultivation. In addition, there were a small number of surviving
cells in the distilled water treatment tube.
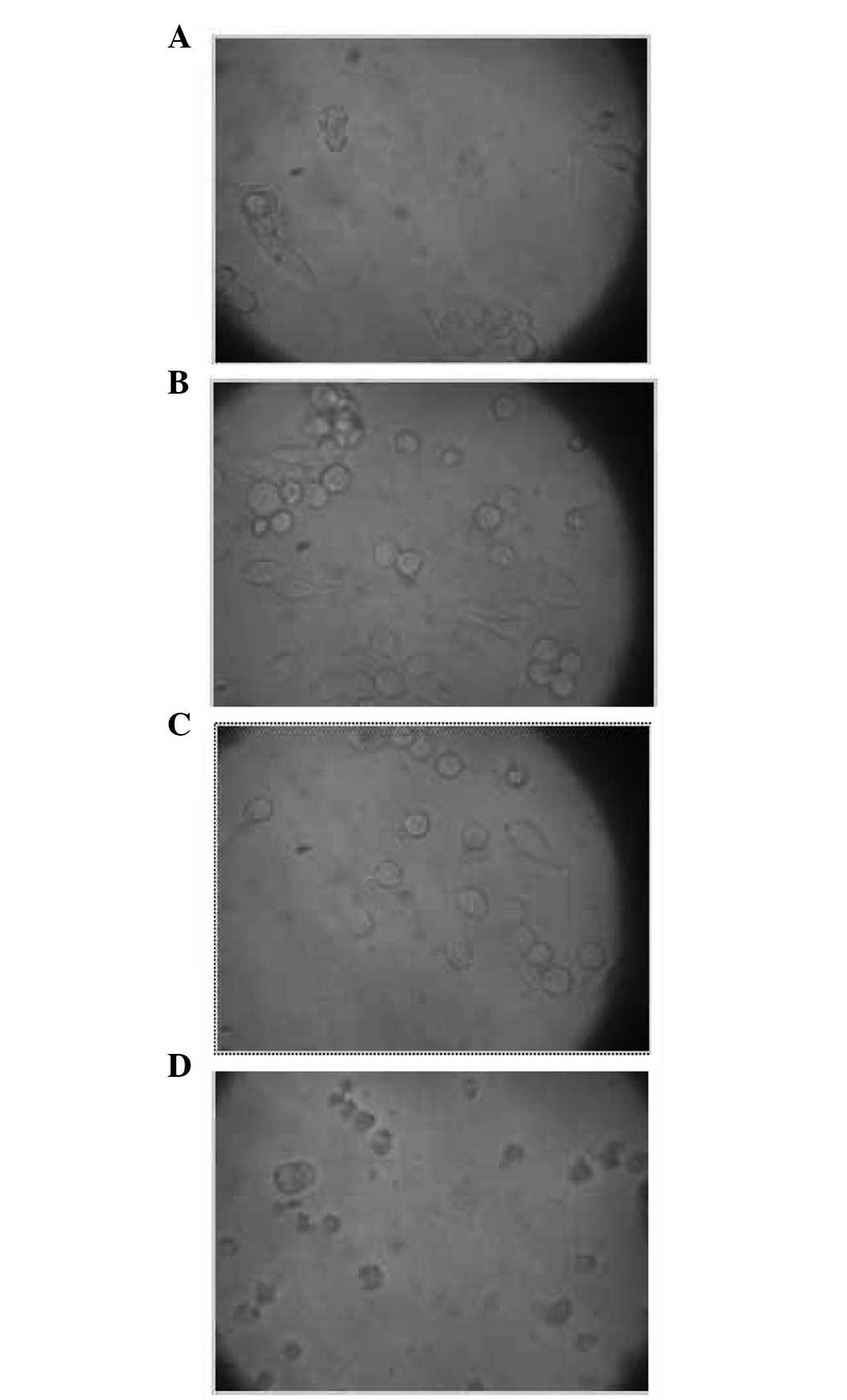
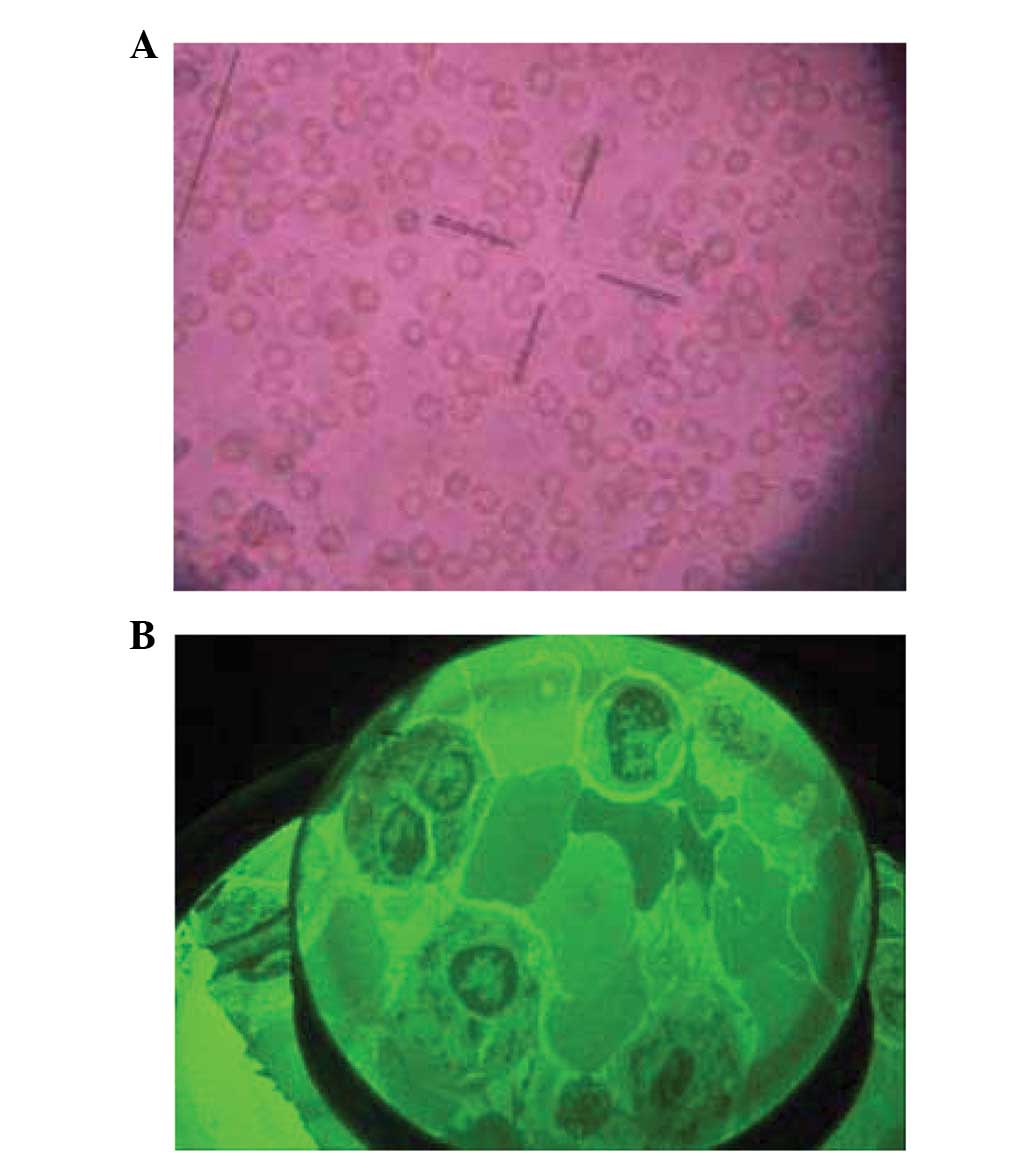
State of the cells under an inverted
microscope at different osmotic pressures
Cells were observed under the inverted microscope
with four different osmotic pressures, 0.90% NaCl solution (330
mOsm/kg H2O, isotonic solution), 0.36% NaCl solution
(115 mOsm/kg H2O, hypotonic solution), 0.27% NaCl
solution (93 mOsm/kg H2O, hypotonic solution) and
distilled water. After experiencing the altered osmotic pressure
for 48 h, B95-8 cells maintained normal activity. However, the
majority of cells treated with distilled water stopped growing
(Fig. 1).
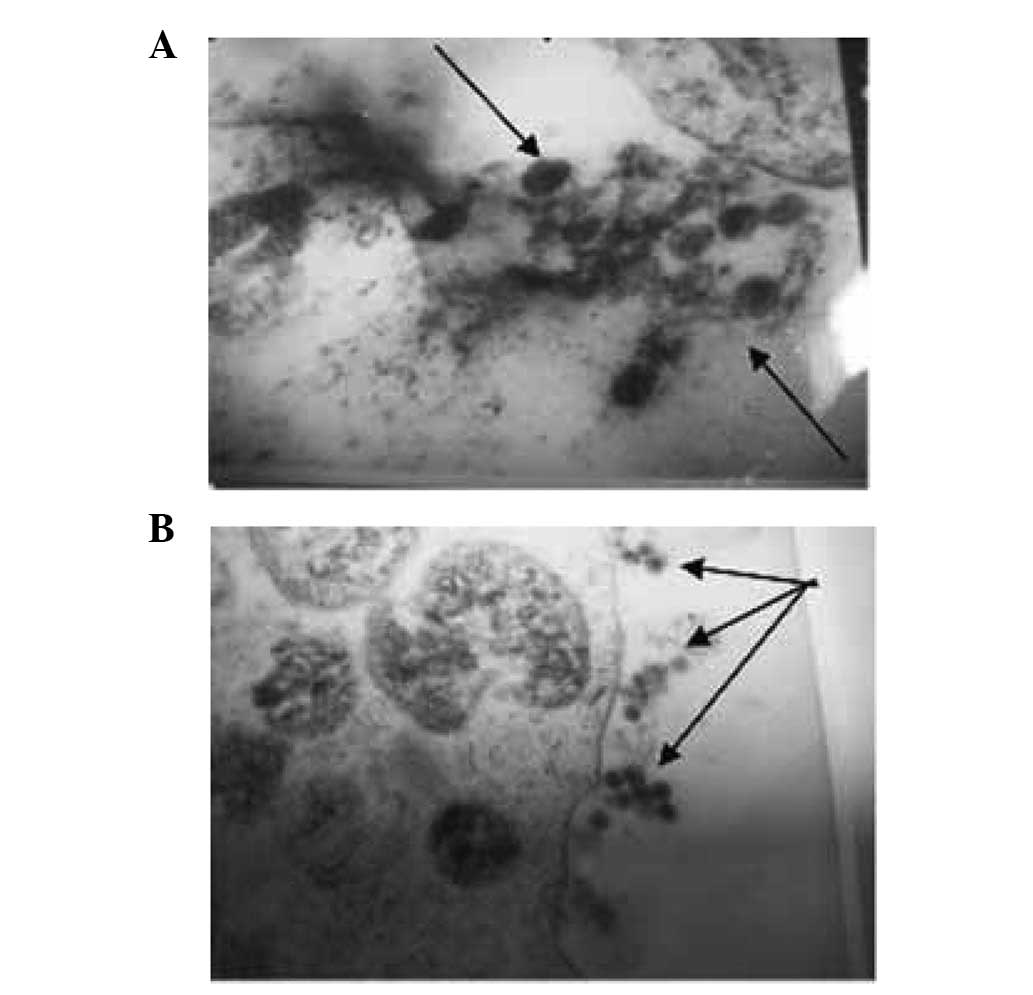
Cell states under different osmotic
pressures under a transmission electron microscope
Following treatment in the isotonic solution [0.90%
NaCl solution (330 mOsm/kg H2O)], EBV particles were
observed to be located inside and outside the cells. The whole cell
structure is shown in Fig. 2.
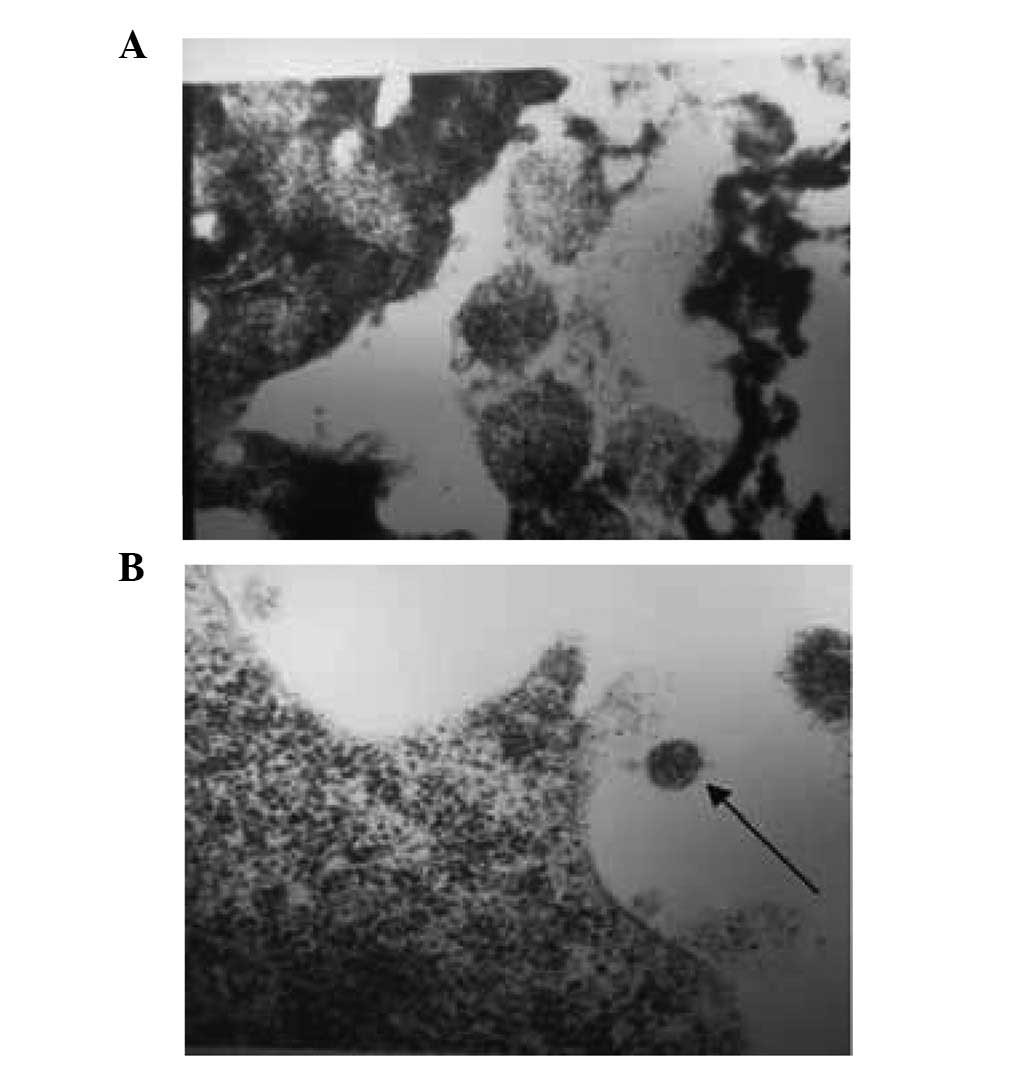
Following treatment in 0.36% NaCl solution (115 mOsm/kg
H2O), the organelle structure of the B95-8 cells
remained intact (Fig. 3). In the
0.27% NaCl solution (93 mOsm/kg H2O), EBV particles were
not observed in the B95-8 cells (Fig.
4). In addition, the intracellular organelles of the B95-8
cells were observed to have become vacuolated following treatment
with distilled water (Fig. 5).
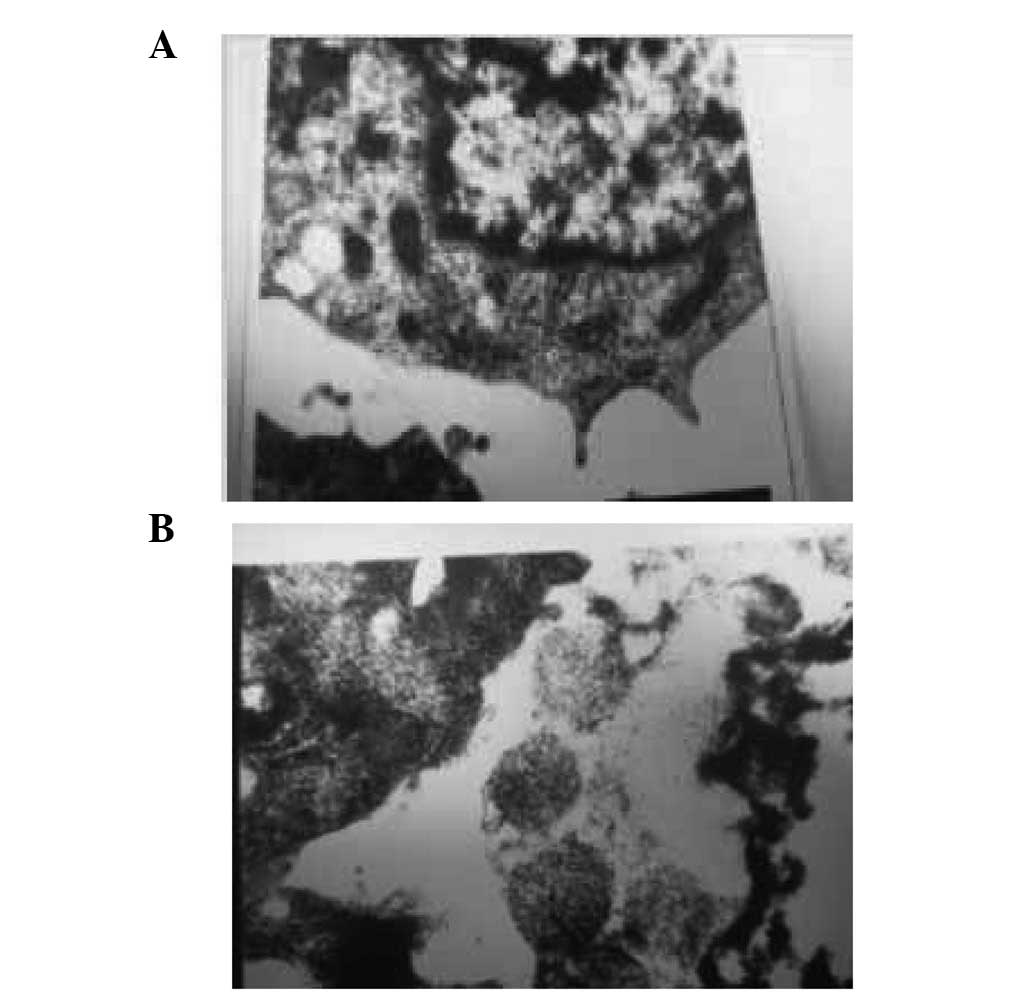
Pre-experiment of HIV virus particle
pumping from human lymphocytes under low osmotic pressure
We investigated whether HIV is removed from
lymphocytes under low osmotic pressure. We observed that in 0.30%
NaCl solution (104 mOsm/kg H2O), HIV virus particles
were not present in the CD4+ T cells. In addition, as
was observed for the removal of EBV from B95-8 cells, following
treatment in a hypotonic solution [0.30% NaCl (104 mOsm/kg
H2O)] for 48 h, some of the cells maintained normal
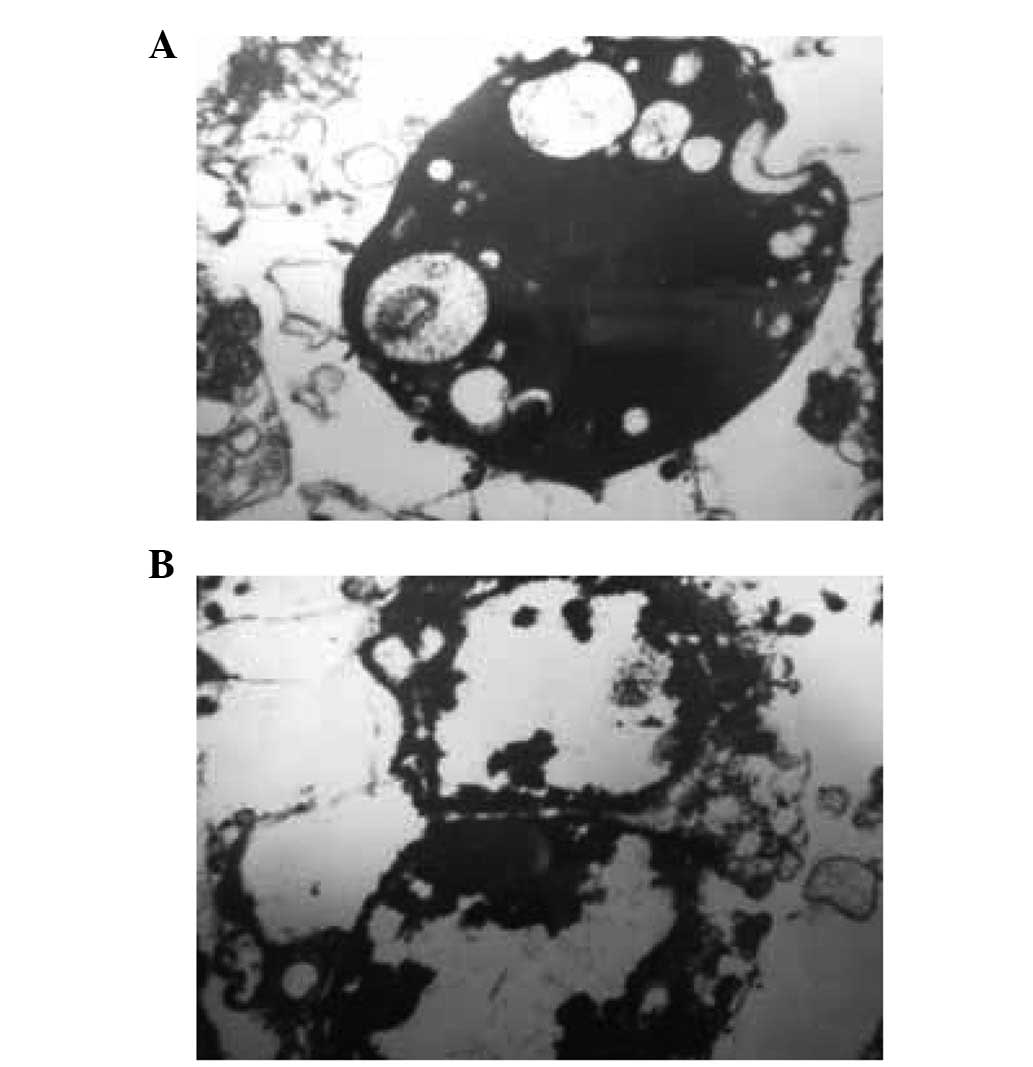
growth and activity (Fig. 6A).
Observation of cells, under an electron microscope, revealed that
the main organelles retained structural integrity and did not
present any changes (Fig. 6B). In
the two images, we observed that a number of red blood cells did
not dissolve following treatment in the hypotonic solution [0.30%
NaCl (104 mOsm/kg H2O)].
In the subsequent experiments using the
CD+4 cells, we observed that a number of the red blood
cells in the moderately hypotonic solution [0.45% NaCl (140 mOsm/kg
H2O)] began to burst and underwent hemolysis. The
membranes of the red blood cells in the more hypotonic solution
[0.27% NaCl (93 mOsm/kg H2O)] were almost completely
broken and dissolved.
Discussion
Osmosis is a common phenomenon in nature and is
important for maintaining the normal physiological function of the
human body. The semipermeable membrane is a selectively permeable
membrane and excessive penetration of liquid leads to cell lysis
(5). The current study aimed to
maintain the B95-8 host cell activity while pumping out the EBV
under variable osmotic pressure. Our results demonstrated that the
cells treated with a suitable osmotic pressure maintained normal
activity and the main structures in the cell’s complement system
were not altered. The cell underwent normal growth. In addition,
virus particles were pumped to the cell surface. B95-8 cells are
commonly used as the target cell for EBV. Under equal osmotic
pressure and low osmotic pressure, viral growth and maturation did
not lead to apoptosis and cell rupture. The virus was no longer in
the cell. In addition, at a low osmotic pressure, a number of virus
particles may have been collected by centrifugation or have been
pumped out of the cell. These results provide an important
theoretical basis for the effective removal of intracellular
viruses from cells.
Different cells have different hypotonic tolerances
and membrane permeability. Red blood cells are more sensitive at
low osmotic pressures. We conclude that, when treated with 0.30%
NaCl solution (104 mOsm/kg H2O), some of the cells
maintain normal activity following 48 h culture and under an
electron microscope, the structure of cell organelles maintain
integrity. However, a number of red blood cells were swollen. In
addition, the red blood cells began to rupture and dissolve in the
moderately hypotonic solution [0.45% NaCl (140 mOsm/kg
H2O)]. In the more hypotonic solution [0.27% NaCl (93
mOsm/kg H2O)], the B95-8 cells were almost completely
ruptured and dissolved. Therefore, in order to pump HIV out of the
cell, the exploration of a suitable low osmotic concentration is
required. Additionally, further research is required to determine
whether the virus that has been pumped out is capable of infecting
again.
Studies of osmotic pressure have only investigated
red blood cells. Osmotic pressure is divided into three levels:
absolute hypertonic, hypotonic and isotonic. According to our
experimental results, it is more reasonable that the cell osmotic
pressure be divided into 5 levels and that there are buffer zones
from the low osmotic pressure to the osmotic pressure and from the
osmotic pressure to the high osmotic pressure. Therefore, the
levels would be: i) high osmotic pressure: all intracellular fluid
passively penetrates outwards, so cell structure and function are
destroyed; ii) neutral-high osmotic pressure: a small amount of
intracellular fluid passively penetrates outwards and the cell wall
has a certain degree of shrinkage although the cell structure and
function are complete and the cell grows well after culturing; iii)
equal osmotic pressure: cell function and morphology do not change,
the exchange of substance is decided by the needs of the cell and
the exchange speed of intracellular and extracellular fluid is
slow; iv) neutral-low osmotic pressure: hypotonic solution flows
into the cell, cell swelling is observed, the cell structure and
function are complete, the cell grows well after culturing, and
some cell fluid, parasitic virus particles and the protein in the
cytosol produced before and after maturation of the virus are
pumped out and v) low osmotic pressure: cell structure and function
are damaged, most of the membrane is dissolved, organelles enter
the vacuole and cell death occurs.
A relatively stable internal environment is provided
by the cell membrane. The semipermeable membrane selectively
transports metabolites into and out of the cell, according to the
needs of the cell. There are four forms of liquid exchange: i)
liquid is blocked and cannot be exchanged; ii) cells are
selectively exchanged via the semipermeable membrane according to
the needs of the cell, but the speed is slow; iii)
low-molecular-weight molecules are exchanged quickly as in
hypotonic treatment and iv) barrier-free exchange. A relatively
large number of substances are quickly and controllably transported
to the intracellular environment. Therefore, it is possible to
block viral synthesis in the chromosomes. HIV-1 patients may be
able to receive early intervention therapy prior to symptom
formation. This study demonstrates the effect of the low osmotic
pressure theory.
Host cell function is not affected and the virus
particles are successfully removed from the cells. We consider that
relative to the host cell, as a foreign protein, the virus
particles do not have not a solid attachment to the host cell;
therefore, low osmotic pressure may be effectively used to pump out
the virus into the extracellular space.
Acknowledgements
The authors wish to thank everyone who
helped in the research. The study was supported by the Guangxi
Natural Science Foundation of China (no. 0447035).
References
|
1.
|
Shi LP, Zang YM, Hou XL and Wang J: Ion
channel mechanism of regulatory volume decrease in human epithelial
cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 24:356–360. 2008.(In
Chinese).
|
|
2.
|
Amiji MM and Sandmann BJ: What is osmosis?
Explanation and understanding of a physical phenomenon. Applied
Physical Pharmacy. McGraw Hill; New York, NY: pp. 54–57. 2002
|
|
3.
|
Cui Y, Kuang G and Yue H: Chinese herbal
medicine for inhibiting EB virus capsid antigen expression in cells
in vitro experimental study. Sichuan Cancer Treatment. 14:148–149.
2001.(In Chinese).
|
|
4.
|
Ling Z: Scanning electron microscopy. Cell
Ultrastructure and Electron Microscopy Shanghai Medical. University
Press; Shanghai: pp. 124–130. 2000
|
|
5.
|
Haynie DT: Gibbs free energy --
applications. Biological Thermodynamics. Cambridge University
Press; Cambridge: pp. 130–136. 2001
|