Introduction
Hedgehog proteins are crucial in the embryonic
development of animals, from insects to mammals (1). Furthermore, hedgehog proteins are
postnatally involved in physiological bone growth as well as in
fracture healing (2–4). In vitro, sonic hedgehog
protein (SHh) causes the proliferation and differentiation of
mesenchymal stem cells into the osteoblastic lineage by
upregulating bone morphogenetic proteins (BMPs) via SMAD signaling
(5). Previous studies have shown
that the combination of recombinant N-terminal SHh (N-SHh) with
BMP-2 synergistically induces the expression of osteogenic markers
in progenitor cells, whereas the transplantation of N-SHh into the
muscles of mice failed to induce ectopic bone formation after two
weeks (6). In turn, the
implantation of dermal fibroblasts expressing hedgehog proteins
into nude mice induced ectopic bone formation (7). The mechanism for hedgehog proteins in
bone formation in vivo remains unclear.
The expression of SHh by transduced fibroblasts
appears to be a reliable method for inducing ectopic bone
formation, since a further study demonstrated the closure of
calvarial bone defects in rabbits using SHh-expressing fibroblasts
(8). However, in clinical
practice, the use of genetically modified cells does not yet appear
to be adequate. Instead, the application of osteoinductive proteins
to porous synthetic bone substitute materials is a promising
approach.
Based on past experience, a combination of BMPs with
β-tricalcium phosphate (β-TCP), a bone substitute material, works
synergistically to yield new bone in animals (9) and has good release properties, as
well as inducing higher amounts of bone formation than are obtained
with a combination of BMP-2 and hydroxyapatite (10).
The application of BMP-2 to bone defects in humans
is already used in clinical practice. However, the benefit is not
clear and adverse reactions, including infection, pain, heterotopic
bone formation and immunogenic reactions, may be observed (11). One explanation for this limitation
is that BMP-2 as a monotherapy requires higher concentrations to
work than are required in physiological bone healing.
We hypothesized that the application of commercially
available N-SHh protein (alone or in combination with BMP-2) to
β-TCP would induce osteogenesis in bone defects in a superior
manner to a combination of β-TCP and BMP-2.
Materials and methods
Scaffold preparation
Scaffold
β-TCP is one of the most commonly used materials for
bone tissue engineering and it has osteoconductive properties
(12). β-TCP is completely
biodegraded over a period of up to two years, depending on the
host’s metabolism (13). β-TCP
bodies for small animal studies (chronOS; granule size, 0.7–1.4 mm)
were obtained from Synthes (Dübendorf, Switzerland). The bodies had
a porosity of ∼60%. The macropores had a diameter of ∼100–500
μm and the micropores were <5 μm. The weight of
0.5 cc chronOS (0.7–1.4 mm) was ∼0.5 g. Mesenchymal stem cells
adhered favourably to chronOS in vitro (14).
BMP-2
Recombinant human BMP was purchased from R&D
Systems (Wiesbaden, Germany). The human protein was used since BMPs
are highly conserved across animal species. In particular, mature
human, mouse and rat BMP-2 are 100% identical at the amino acid
sequence level.
SHh
Recombinant human N-SHh was also obtained from
R&D Systems. SHh proteins are highly conserved at the
N-terminus across vertebrates (99%) and studies support that they
are also conserved in their functional properties (15).
Composite preparation
The BMP-2 and N-SHh were dissolved in
phosphate-buffered saline, yielding a stock solution comprising 20
μg/ml BMP-2 and 50 μg/ml N-SHh. According to the
literature, these concentrations are only marginally effective and
thus were suited to examining whether a synergistic effect was
observed.
Next, the β-TCP was soaked with this solution. The
soaked β-TCP was dried under a hood overnight, then packaged in 50
μg (i.e. 2.5 μg N-SHh and/or 1 μg BMP-2)
portions. These portions were stored at −20°C until use. Previous
studies have shown that these preparations retain their
osteoinductive activity (9,10).
Surgical procedure
Animals
For the critical-sized defect model athymic nude
rats (RH-Foxn1rnu) were used so that immunological
reactions to the human N-SHh were entirely excluded. The animals
were purchased from Harlan Laboratories (Harlan Winkelmann GmbH,
Kreuzelweg, NM Horst, The Netherlands) This animal model is T-cell
deficient but has normal B-cell function.
The animals were divided into four groups (Table I). The first group served as a
control and the critical-sized femoral defects in this group were
filled only with inorganic bone substitute material (β-TCP). The
second group was a test group. The femoral defects of these animals
were filled with β-TCP combined with N-SHh. The third group also
served as a test group and the femoral defects were filled with
β-TCP impregnated with N-SHh and BMP-2. In the fourth group, the
defects were filled with β-TCP and BMP-2.
 | Table IGrouping of animals. |
Table I
Grouping of animals.
| Group number | Number of
animals | Filling of the
femoral critical sized defect |
|---|
| 1 | 10 | β-TCP only (bone
substitute material) |
| 2 | 10 | β-TCP + N-SHh |
| 3 | 10 | β-TCP + N-SHh +
BMP-2 |
| 4 | 10 | β-TCP + BMP-2 |
Surgery
Under general anesthesia (ketamine/xylazine i.p.)
and aseptic conditions, the anterolateral aspect of the right rat
femur was dissected. A plate was then positioned on the femur and
fixed with four screws, leaving two holes in the midshaft free.
Next, the screws and plate were loosened and two 5-mm osteotomies
of the mid shaft femur were performed with a liston-key. A 5-mm
empty segmental defect in a rat femur does not heal (defined as a
critical defect) as demonstrated in previous studies (16). The plate was then readapted to the
femur and the screws were tightened. Protein-impregnated scaffolds
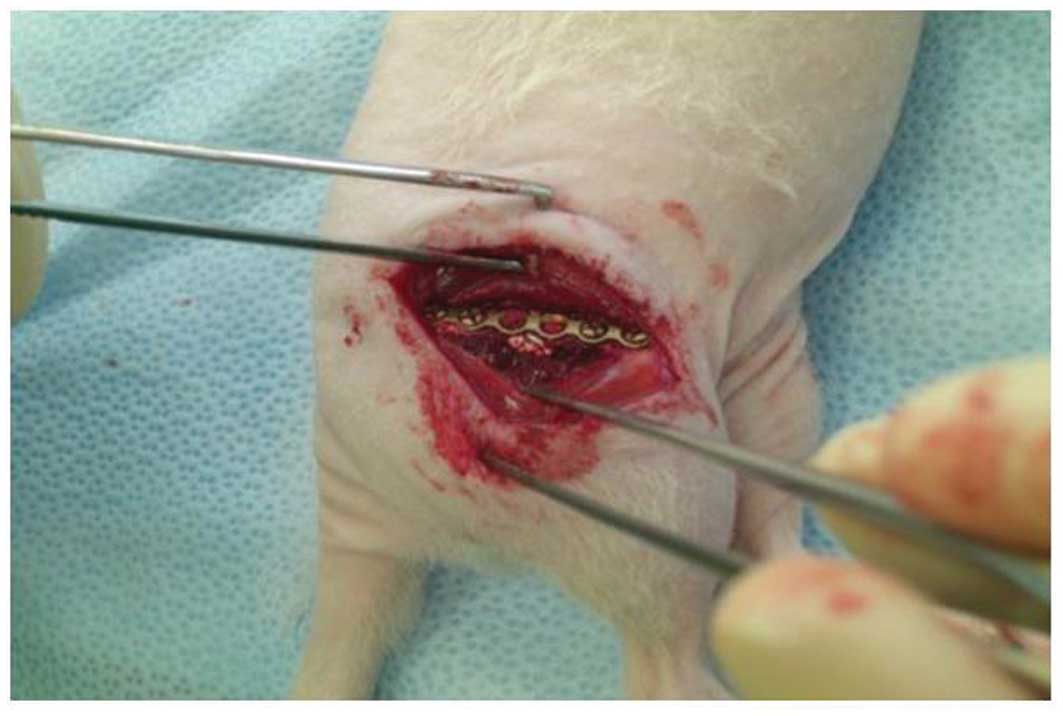
were implanted into the segmental defects (50 μg each) and
the wound was closed with absorbable suture material (Fig. 1). The animals received metamizole
in their drinking water as pain treatment over the subsequent days.
The rats were housed for eight weeks under standard conditions and
nutrition.
Osteosynthesis materials
The plates and screws were obtained from Synthes
(LCP compact hand). The smallest titanium plate (1.0) with twelve
non-locking holes was used. The plate was cut so that a
six-hole-plate was used for each femur. The screws were
self-cutting and had a diameter of 1.3 mm and a length of 6 mm. The
decision was made to stabilize the critical-sized defect with a
plate rather than an external fixator since plates appeared to be
more comfortable for the rats and also exhibited greater stability
(17).
Evaluation
The animals were sacrificed after eight weeks by
inhalation anesthesia and following i.p. pentobarbital injection.
The femora were explanted and freed from the ambient tissue. Since
it was not possible to remove all overgrown plates and screws
without destroying their structure, the decision was made to
evaluate all femora complete with the implanted osteosynthesis
material. Since these small plates were relatively flexible and
they were all tested under identical conditions, the obtained
results were considered to be relevant. Following explantation, the
subjective observations with regard to the stability were noted for
each femur: stable, unstable or insecure. Next, the explantation
was followed by conventional radiography. The results were rated by
two observers as consolidated or not consolidated (with regard to
uninterrupted callus or not).
Mechanical testing
The bones were stored at −20°C until mechanical
testing. Mechanical testing was performed by a standardized three
point bending test using a material testing device (Zwicki-line
5.0; Zwick-Roell, Ulm, Germany). The thawed bone was placed onto
the device to measure the flexural stability in an
anterior/posterior direction. The degree of displacement in mm at
the highest pressure reached was noted. From these data the
stiffness for each construct was calculated.
Histology
The femoral specimens were collected and decalcified
in 15% formic acid at 4°C. After 14 days the screws and plates were
removed. Following further processing the bones were
paraffin-embedded and cut. The slides were stained with hematoxylin
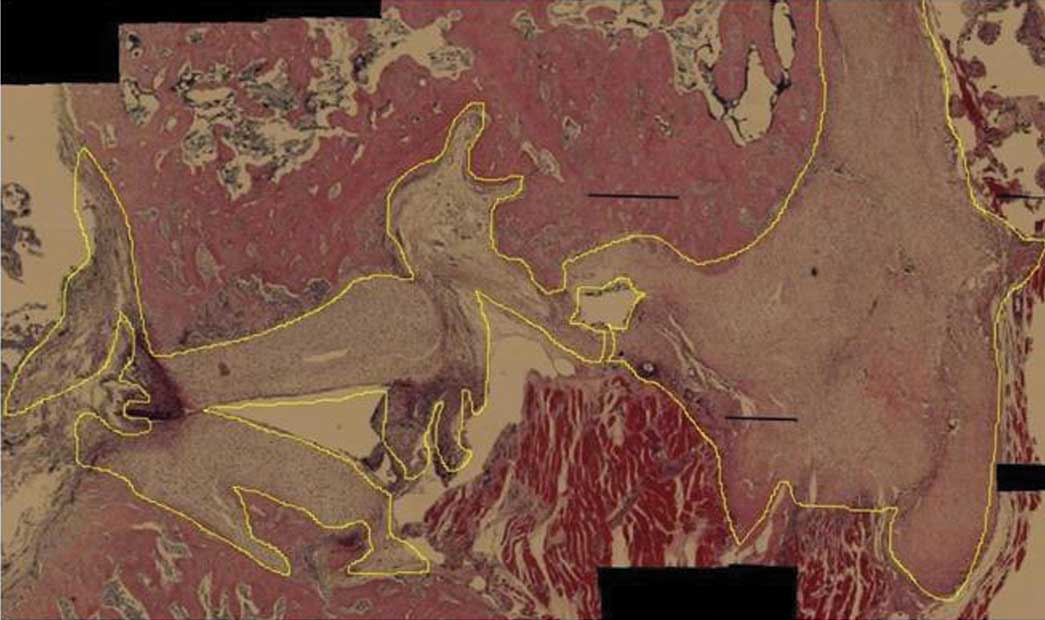
and eosin. Two observers identified the type of tissue adjacent to
the defect area. Next, the amounts of newly formed cartilage and
bone were determined using public domain ImageJ software
(http://rsb.info.nih.gov/ij/). All
samples were evaluated three times (same magnification for
all).
Statistical analysis
Sample size calculation
For determining the sample size, a mean stiffness of
15 N/mm was assumed in the control group (stabilized defects filled
with β-TCP alone) with a standard deviation of 10 N/mm (obtained
from preliminary results). The stiffness was expected to increase
to 30 N/mm under treatment with BMP-2 and SHh. The minimum number
of subjects required to detect a difference (under standard
assumptions, α, 0.05; β, 0.80) was therefore eight animals in each
group. To detect an even smaller difference of 13 N/mm the group
number was increased to 10 animals.
Normality testing
The data were first analyzed for normal distribution
by the D’Agostino-Pearson omnibus normality test. The majority of
groups did not pass this normality test (α, 0.05). For the
remaining groups, normality could not be assumed due to the small
sample size.
Analysis of variance (ANOVA)
The data were then analyzed by non-parametric ANOVA
(Kruskal-Wallis test), followed by the pairwise Dunn’s multiple
comparison test.
Results
Surgery and mechanical testing
The surgical procedures under general anesthesia
were performed without complications, although two rats died during
surgery due to major bleeding. During follow-up, three more animals
were sacrificed due to disabling swelling of the upper leg and
nerve injury. Another animal had unclear cachexia and was
sacrificed. These deceased animals were replaced by identically
treated animals to maintain 10 animals in each group. The remaining
animals did well and had no signs of impairment during the entire
eight weeks.
The first observation of the bones (stable, unstable
or insecure) was noted when the femora were explanted. Next, the
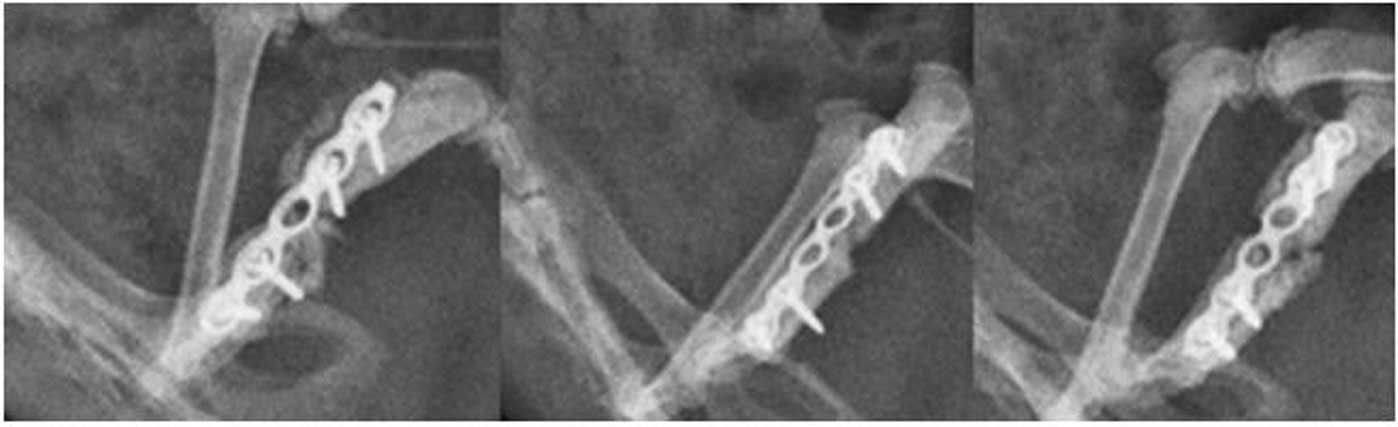
radiological evaluation was performed (Fig. 2). The femora in the N-SHh and
N-SHh/BMP groups were observed to have healed poorly. The femora of
the N-SHh and N-SHh/BMP groups appeared to be less stable and
radiologically consolidated than their counterparts with BMP-2 and
more so compared with the control with only β-TCP and no protein at
all (Table II).
 | Table IIValues of clinical (stable),
radiological (consolidated) and mechanical examination of the
explanted rat femora (± indicates standard deviation). |
Table II
Values of clinical (stable),
radiological (consolidated) and mechanical examination of the
explanted rat femora (± indicates standard deviation).
| Values | Group
|
|---|
| β-TCP | β-TCP + N-SHh | β-TCP + N-SHh +
BMP-2 | β-TCP + BMP-2 |
|---|
| Stable (%) | 60 | 40 | 40 | 60 |
| Consolidated (%) | 50 | 10 | 30 | 50 |
| F max (N) | 47.9±28.0 | 33.0±23.4 | 38.3±27.0 | 59.1±7.5 |
| Flexion (mm) | 3.2±1.2 | 3.5 ±1.2 | 3.4±1.7 | 3.0±1.5 |
| Stiffness (N/mm) | 16.5±8.5 | 12.3±10.3 | 13.4±9.7 | 26.3±2.0 |
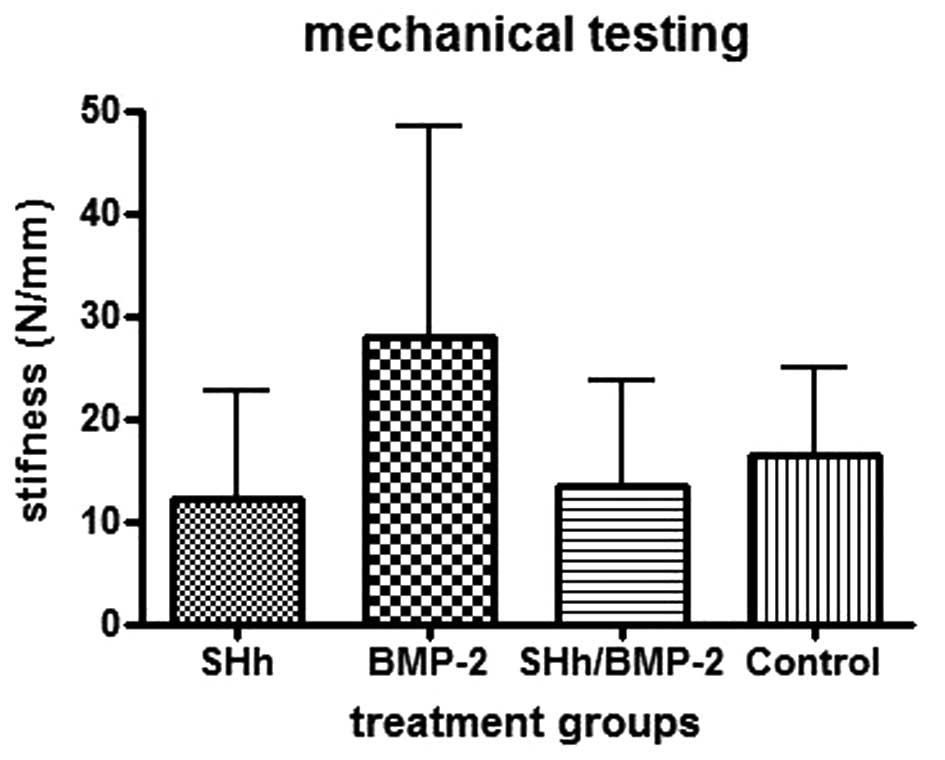
When the mechanical testing was performed, this
trend was observed further. The stiffness was calculated and the
femora with N-SHh and N-SHh/BMP-2 exhibited the lowest stiffness.
Femora that were combined with BMP-2 were more than twice as stiff
as the femora combined with N-SHh (Fig. 3).
Histomorphometry
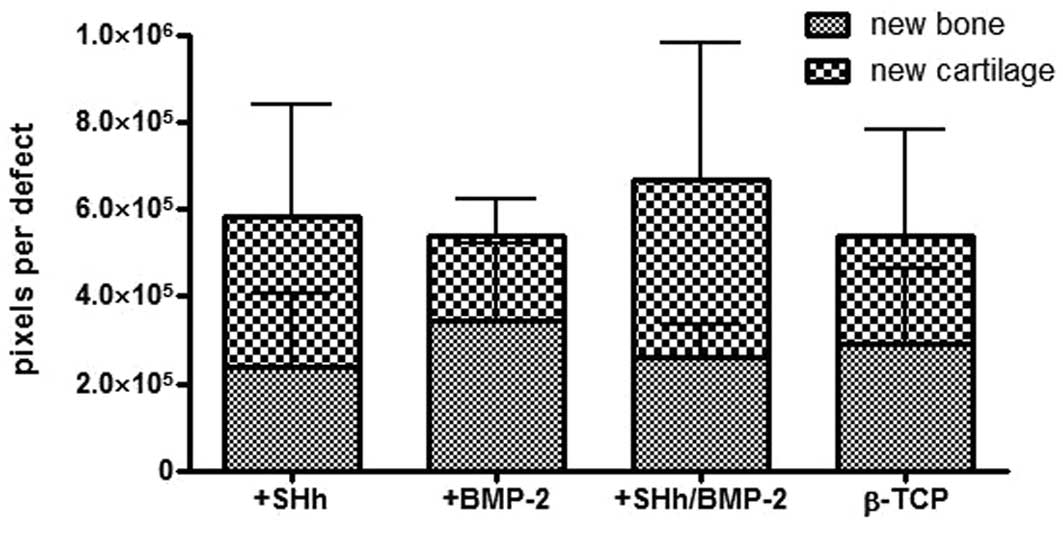
The amount of newly formed cartilage and bone was
calculated by ImageJ and noted as pixels per probe (whole defect
area, Figs 5 and 6). With regard to the formation of new
cartilage, the experimental group treated with BMP-2 and N-SHh
yielded the highest amount with 410,034 pixels per sample. The
animals with the N-SHh constructs had an average of 348,109 pixels
per sample. The group that served as a negative control (β-TCP
only) had an average of 221,426 pixels per sample. The BMP-2 group,
the positive control, had the lowest average with 193,938 pixels
per sample.
Newly formed bone was present at the highest levels
in the group treated with BMP-2 (344,550 pixels per sample),
followed by the control group (empty) with 291,301 pixels per
sample. Less bone was observed in the group treated with BMP-2 and
N-SHh (258,509) and the least amount of bone was observed in the
group treated with N-SHh (231,219). The amount of newly formed bone
among the groups reflected the stiffness trend observed in the
mechanical testing (Fig. 4).
Discussion
Bone defects in humans develop in various situations
and frequently represent major clinical problems in bone surgery.
The current treatment strategies include autologous bone
transplantation, allogenous bone transplantation or, for smaller
defects, filling with inorganic bone substitute materials.
Autologous bone transplantation is typically restricted by donor
site morbidity, limited amounts of harvested bone and longer or
more extensive surgical procedures. Allogenous bone transplantation
has the imminent risk of the transmission of infectious diseases
and therefore requires laborious and expensive preparation
procedures.
Synthetic inorganic bone substitutes, such as
hydroxyapatite or TCP, are widely used and extremely biocompatible,
particularly TCP which mimics the natural inorganic part of bone.
The major disadvantage is the lack of osteoinductive properties.
Therefore, the use of TCP is not adequate for diaphyseal or larger
metaphyseal bone defects. To overcome this, TCP may be augmented by
an osteoinductive protein, such as BMP-2.
In a rat model, the callus formation and bending
strength of a TCP + 25 μg rhBMP-2 bone graft was superior to
that of an autologous bone graft. Thus, TCP/rhBMP-2 composites have
the potential to become effective substitutes for autologous bone
grafts (18).
To demonstrate the effect of hedgehog signaling in
diaphy-seal bone defects, N-SHh impregnated β-TCP was implanted
into critical-sized femoral defects of athymic rats. The defect was
stabilized with the smallest available plate normally used in hand
surgery. β-TCP/BMP-2 composites were used as a control. After eight
weeks, the animals were sacrificed and the explanted femora were
evaluated by clinical, radiological, biomechanical and histological
examinations.
Notably, the N-SHh/β-TCP composites had lower
stiffness than the control group consisting of β-TCP without any
protein application. This effect was also observed in the
N-SHh/BMP-2/β-TCP composites. The BMP-2/β-TCP composites showed the
highest stiffness.
The results of the histomorphometrical analysis
showed the highest amounts of cartilage in the N-SHh and
N-SHh/BMP-2 groups. In this cartilage, bony islands were frequently
observed, indicating a current process of endochondral
ossification. The sum of the bone and cartilage was the highest in
the N-SHh/BMP-2 group, which supports our initial hypothesis.
In conclusion, the addition of N-SHh at a
concentration of 20 μg/ml delays bone healing when added to
bone substitute materials, even in combination with BMP-2. However,
the amount of ossifying cartilage was the highest in the N-SHh
groups, indicating a potential for robust bone healing. Further
studies should incorporate longer time spans for an adequate
healing period.
Acknowledgements
This study was supported with a
research grant by the AO Foundation (S-09-57W).
References
|
1
|
Fietz MJ, Concordet JP, Barbosa R, Johnson
R, Krauss S, McMahon AP, Tabin C and Ingham PW: The hedgehog gene
family in Drosophila and vertebrate development. Dev Suppl. 3–51.
1994.
|
|
2
|
Chung UI, Schipani E, McMahon AP and
Kronenberg HM: Indian hedgehog couples chondrogenesis to
osteogenesis in endochondral bone development. J Clin Invest.
107:295–304. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Day TF and Yang Y: Wnt and hedgehog
signaling pathways in bone development. J Bone Joint Surg Am.
90(Suppl 1): 19–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito H, Akiyama H, Shigeno C, Iyama K,
Matsuoka H and Nakamura T: Hedgehog signaling molecules in bone
marrow cells at the initial stage of fracture repair. Biochem
Biophys Res Commun. 262:443–451. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spinella-Jaegle S, Rawadi G, Kawai S,
Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V,
Blanchet AM, Adelmant G, Baron R and Roman-Roman S: Sonic hedgehog
increases the commitment of pluripotent mesenchymal cells into the
osteoblastic lineage and abolishes adipocytic differentiation. J
Cell Sci. 114:2085–2094. 2001.
|
|
6
|
Yuasa T, Kataoka H, Kinto N, Iwamoto M,
Enomoto-Iwamoto M, Iemura S, Ueno N, Shibata Y, Kurosawa H and
Yamaguchi A: Sonic hedgehog is involved in osteoblast
differentiation by cooperating with BMP-2. J Cell Physiol.
193:225–232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Enomoto-Iwamoto M, Nakamura T, Aikawa T,
Higuchi Y, Yuasa T, Yamaguchi A, Nohno T, Noji S, Matsuya T, Kurisu
K, Koyama E, Pacifici M and Iwamoto M: Hedgehog proteins stimulate
chondrogenic cell differentiation and cartilage formation. J Bone
Miner Res. 15:1659–1668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edwards PC, Ruggiero S, Fantasia J,
Burakoff R, Moorji SM, Paric E, Razzano P, Grande DA and Mason JM:
Sonic hedgehog gene-enhanced tissue engineering for bone
regeneration. Gene Ther. 12:75–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Urist MR, Lietze A and Dawson E:
Beta-tricalcium phosphate delivery system for bone morphogenetic
protein. Clin Orthop Relat Res. 187:277–280. 1984.PubMed/NCBI
|
|
10
|
Tazaki J, Murata M, Akazawa T, Yamamoto M,
Ito K, Arisue M, Shibata T and Tabata Y: BMP-2 release and
dose-response studies in hydroxyapatite and beta-tricalcium
phosphate. Biomed Mater Eng. 19:141–146. 2009.PubMed/NCBI
|
|
11
|
Garrison KR, Shemilt I, Donell S, Ryder
JJ, Mugford M, Harvey I, Song F and Alt V: Bone morphogenetic
protein (BMP) for fracture healing in adults. Cochrane Database.
Syst Rev. 16:CD0069502010.PubMed/NCBI
|
|
12
|
Bauer TW and Muschler GF: Bone graft
materials. An overview of the basic science. Clin Orthop Relat Res.
371:10–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niemeyer P, Krause U, Fellenberg J, Kasten
P, Seckinger A, Ho AD and Simank HG: Evaluation of mineralized
collagen and alpha-tricalcium phosphate as scaffolds for tissue
engineering of bone using human mesenchymal stem cells. Cells
Tissues Organs. 177:68–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schultheiss J, Seebach C, Henrich D,
Wilhelm K, Barker JH and Frank J: Mesenchymal stem cell (MSC) and
endothelial progenitor cell (EPC) growth and adhesion in six
different bone graft substitutes. Eur J Trauma Emerg Surg.
37:635–644. 2011. View Article : Google Scholar
|
|
15
|
Marigo V, Roberts DJ, Lee SM, Tsukurov O,
Levi T, Gastier JM, Epstein DJ, Gilbert DJ, Copeland NG, Seidman
CE, et al: Cloning, expression, and chromosomal location of SHH and
IHH: two human homologues of the Drosophila segment polarity gene
hedgehog. Genomics. 28:44–51. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohura K, Hamanishi C, Tanaka S and Matsuda
N: Healing of segmental bone defects in rats induced by a
beta-TCP-MCPM cement combined with rhBMP-2. J Biomed Mater Res.
44:168–175. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drosse I, Volkmer E, Seitz S, Seitz H,
Penzkofer R, Zahn K, Matis U, Mutschler W, Augat P and Schieker M:
Validation of a femoral critical size defect model for orthotopic
evaluation of bone healing: a biomechanical, veterinary and trauma
surgical perspective. Tissue Eng Part C Methods. 14:79–88. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niedhart C, Maus U, Redmann E,
Schmidt-Rohlfing B, Niethard FU and Siebert CH: Stimulation of bone
formation with an in situ setting tricalcium phosphate/rhBMP-2
composite in rats. J Biomed Mater Res A. 65:17–23. 2003. View Article : Google Scholar : PubMed/NCBI
|