Introduction
Prolonged ischemia may lead to irreversible tissue
injury. Reperfusion may also cause tissue injury and the composite
damage is known as ischemia-reperfusion (I/R) injury. Currently the
measures taken to protect the organs and tissues against I/R injury
are mainly ischemic preconditioning (IPC) and ischemic
postconditioning (IPO). In the protection of organs and tissues
from I/R injury, IPC and IPO have similar functions. It has been
reported that IPC (1–5) and IPO (6–10)
have protective effects on the myocardium, including the reduction
of infarct size, improvement of coronary blood flow and myocardial
reperfusion. This is achieved by activating the prosurvival kinases
PI3K-Akt, eNOS, NO and p70S6K, A adenosine receptors and protein
kinases, including Akt and Erk1/2, guanylate cyclase,
cGMP-dependent protein kinase (protein kinase G, PKG) and protein
kinase c (pkc) which results in pkc-ε opening the ATP-dependent
mitochondrial potassium (mito-KATP) channels (11). This inhibits the opening of the
mitochondrial permeability transition pore (mPTP) (12). Pharmacological preconditioning and
pharmacological postconditioning have functional similarities to
the associated phenomena, as well as IPC and IPO. It has been
reported that sevoflurane preconditioning improves ventricular
function and recovery from myocardial stunning and sevoflurane
postconditioning reduces reperfusion arrhythmias without affecting
the severity of myocardial stunning (13). However, the effects of diazoxide (a
selective mito-KATP channel opener) postconditioning in
the liver with ischemic reperfusion injury remain unclear.
In the present study, to clarify these issues, the
effects of diazoxide postconditioning on I/R-induced injury in rat
liver were investigated.
Materials and methods
Diazoxide and 5-hydroxydecanoate
(5-HD)
Diazoxide (a selective mito-KATP channel
opener) and 5-HD (a selective mito-KATP channel
inhibitor) were purchased from Sigma (St. Louis, MO, USA).
Diazoxide was dissolved and diluted with a solution of sodium
hydroxide (0.1 mol/l) while 5-HD was dissolved and diluted with
distilled water.
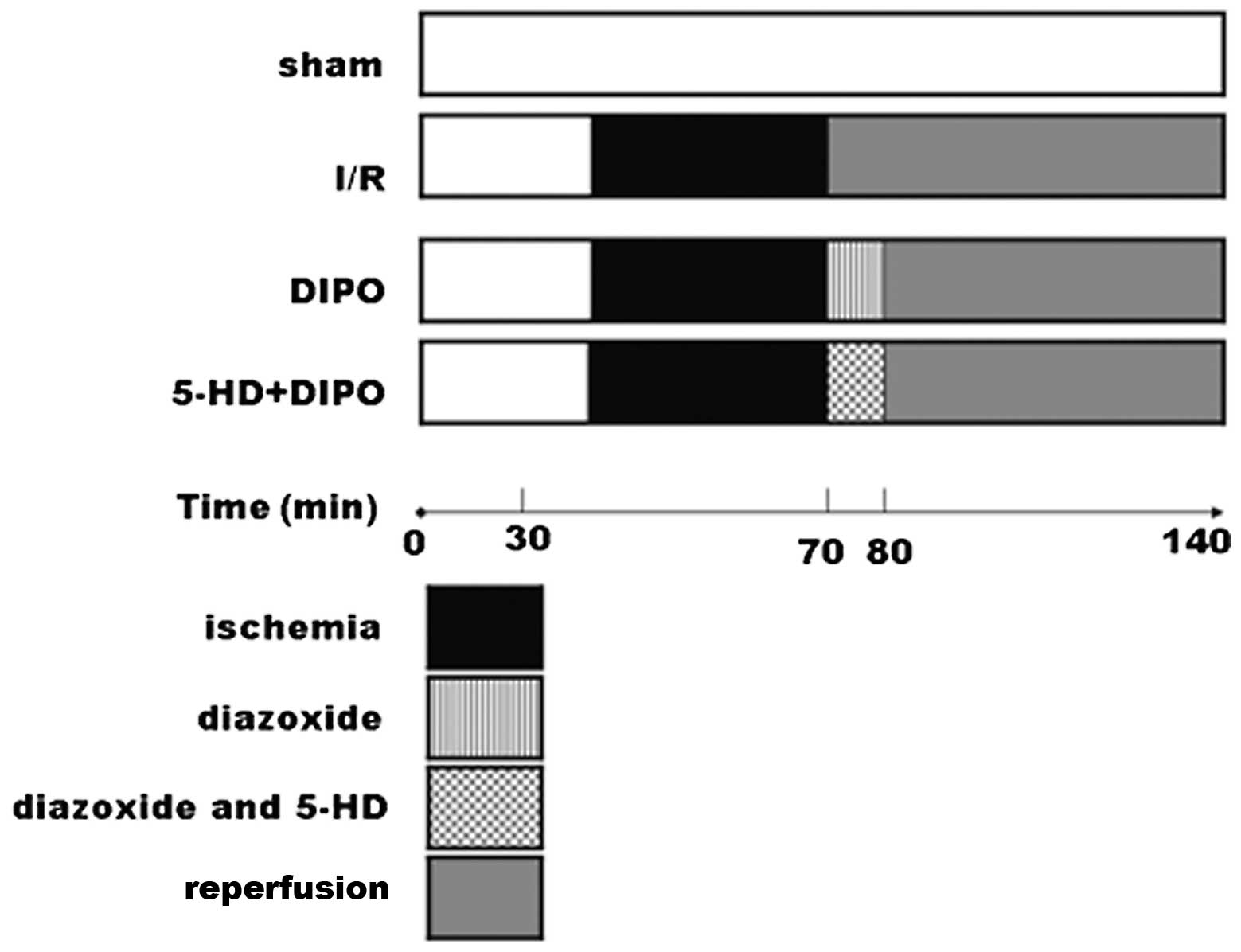
Experimental groups and protocols
Adult male Sprague-Dawley rats (weight, 200–250 g)
were used as the experimental animals. The rats were kept in a
temperature-controlled environment (25 to 30°C) and provided with a
standard diet with water ad libitum. Four groups were
studied (n=7/group): the sham-operated control group; I/R group
(occlusion of the porta hepatis for 60 min, followed by a
persistent reperfusion for 120 min); DIPO (diazoxide ischemic
preconditioning) group [occlusion of the porta hepatis for 60 min,
then treatment with diazoxide (30 μmol/l) for 10 min,
followed by a persistent reperfusion for 120 min], 5-HD+DIPO group
[occlusion of the porta hepatis for 60 min, then treatment with
diazoxide (30 μmol/l) and 5-HD (300 μmol/l) for 10
min, followed by a persistent reperfusion for 110 min]. After a
midline laparatomy incision, an atraumatic vascular clip was placed
on the vessels, blocking the portal venous and hepatic arterial
blood supply to the median and left lateral lobes of the liver and
resulting in ∼70% mouse liver I/R injury. After 60 min ischemia,
the diazoxide or diazoxide and 5-HD was injected through the tail
vein for 10 min to keep pace with the reperfusion. The
sham-operated animals underwent the same surgical procedure as the
other animals with the exception that the vessel clips were not
applied. Blood samples and liver tissues from each group were
obtained for analysis after reperfusion for 120 min (Fig. 1).
Serum liver function assay
Blood samples were obtained after reperfusion for
120 min. Serum alanine aminotransferase (ALT) and aspartate
transaminase (AST) levels were measured with a standard clinical
automated analyzer (ILab 600, Instrumentation Laboratory, Shimadzu
Co., Kyoto, Japan).
Protein expression levels of pkc-ε,
cytochrome-c (cyt-c), caspase-3 and bcl-2
The animal proteins were extracted from hepatic
tissues and quantified with the Bradford assay. Equal amounts of
protein (50 μg) were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). These
proteins were transferred onto polyvinylidene difluoride (PVDF)
membranes. The membranes were incubated overnight at 4°C with
rabbit polyclonal anti-pkc-ε (diluted 1:500), rabbit polyclonal
anti-cyt-c (diluted 1:500), rabbit polyclonal anti-caspase-3
(diluted 1:500) and rabbit polyclonal anti-bcl-2 (diluted 1:500)
separately, followed by the horseradish peroxidase-labeled
secondary antibody (diluted 1:2,000, Santa Cruz Biotechnology Inc.,
Santa Cruz, CA, USA). The membranes were re-incubated with β-actin
(β-actin; diluted 1:5,000, Santa Cruz Biotechnology Inc.) as a
control for protein loading. The detection procedures were
performed using an ECL advance western blotting detection kit, in a
GeneGnome system (Synoptics, Cambridge, UK). Band intensity volumes
were measured using Quantity One software (Bio-Rad, Hemel
Hempstead, UK).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Data were analyzed using ANOVA for multiple
comparisons. Comparisons between two groups were performed using a
t-test. All analyses were performed with the SPSS software (version
18.0, SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Physiological function of DIPO in hepatic
I/R injury
Reperfusion for 10 min with diazoxide, following
immediately after 60 min ischemia of the left liver lobes was
applied to the DIPO group to determine if DIPO was able to
attenuate I/R injury. Serum levels of ALT and AST (Fig. 2) were measured after 2 h of
reperfusion following 60 min of ischemia and were significantly
different among the groups. The ALT and AST levels in the I/R group
were significantly higher than those in the sham-operated control
mice. DIPO treatment significantly reduced the serum levels of ALT
and AST compared with those in the I/R group (Fig. 1). However, 5-HD may abrogate the
protective effect of DIPO (Fig.
2).
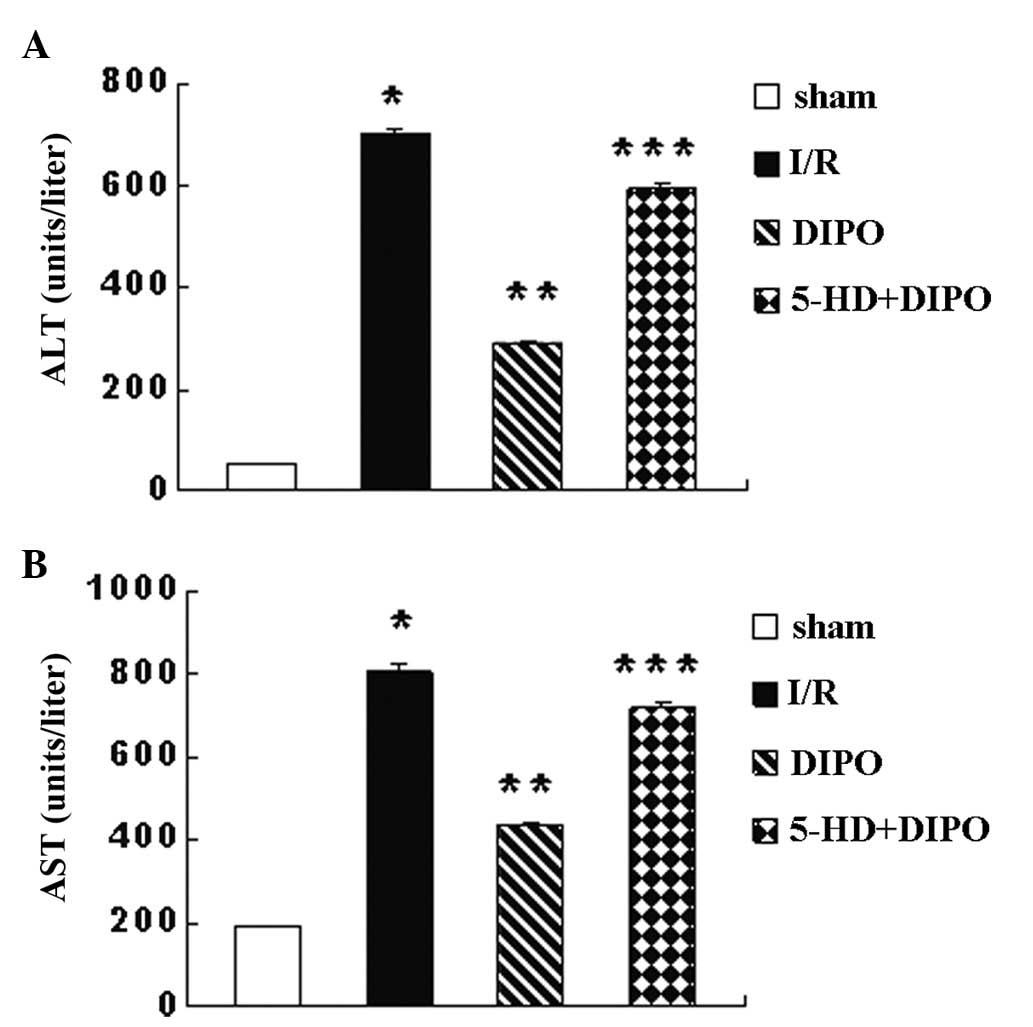 | Figure 2ALT and AST levels. After 60 min of
ischemia and 2 h of reperfusion, serum levels of (A) ALT and (B)
AST were determined. Compared with the sham group, the ALT and AST
levels in the I/R group were significantly increased. Compared with
the I/R group, the ALT and AST levels in the DIPO group were
significantly decreased. However, 5-HD eliminated the protective
effect of DIPO. Compared with the DIPO group, the ALT and AST
levels in the 5-HD+DIPO group were significantly increased.
*P<0.05 vs. sham; **P<0.05 vs. I/R;
***P<0.05 vs. DIPO (n=7). ALT, alanine
aminotransferase; AST, aspartate transaminase; I/R,
ischemia/reperfusion; DIPO, diazoxide ischemic postconditioning;
5-HD, 5-hydroxydecanoate. |
Protein expression levels of pkc-ε,
cyt-c, caspase-3 and bcl-2
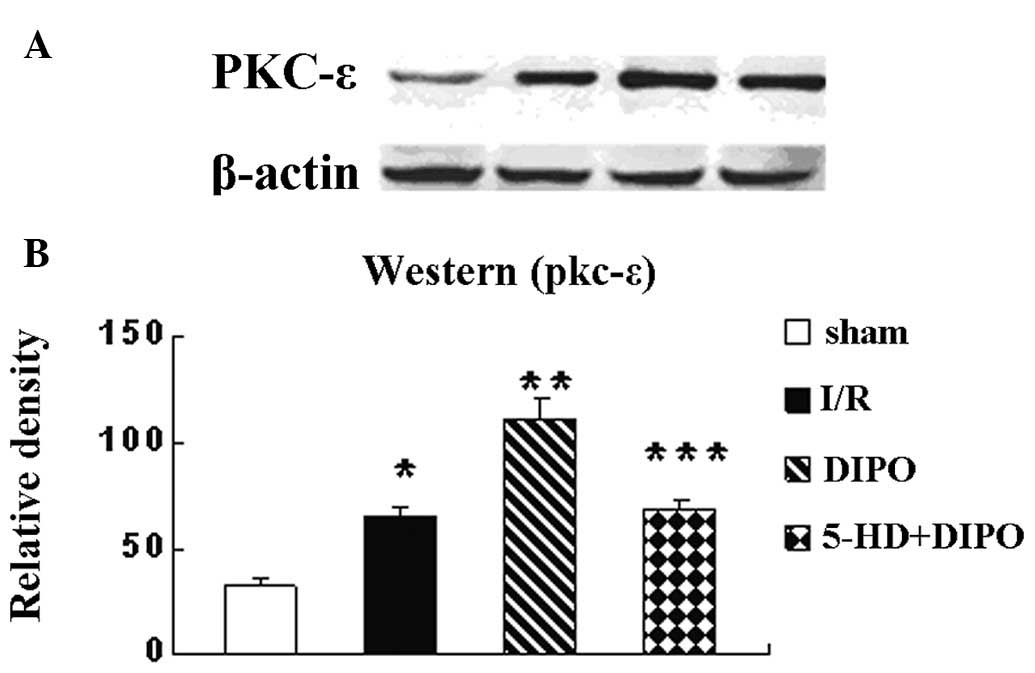
To assess how DIPO protects the liver from I/R
injury, the protein expression levels of pkc-ε were measured by
western blot analysis. The results revealed that the expression
levels of pkc-ε in the liver tissues were significantly increased
in the I/R group compared with those in the sham group, were
significantly higher in the DIPO-treated mice than in the I/R group
and 5-HD markedly abrogated the DIPO-induced increases in pkc-ε
expression (Fig. 3). It has been
previously reported that the apoptosis signaling pathway is
involved in the protective effect of IPO (14,15).
Whether DIPO alters the activation of the liver I/R-induced
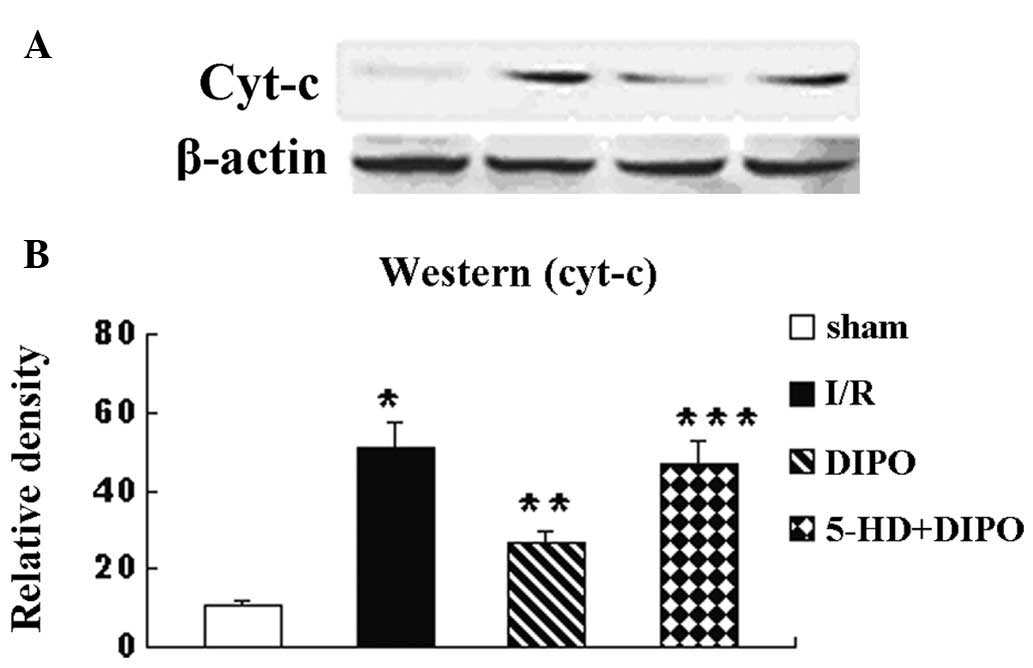
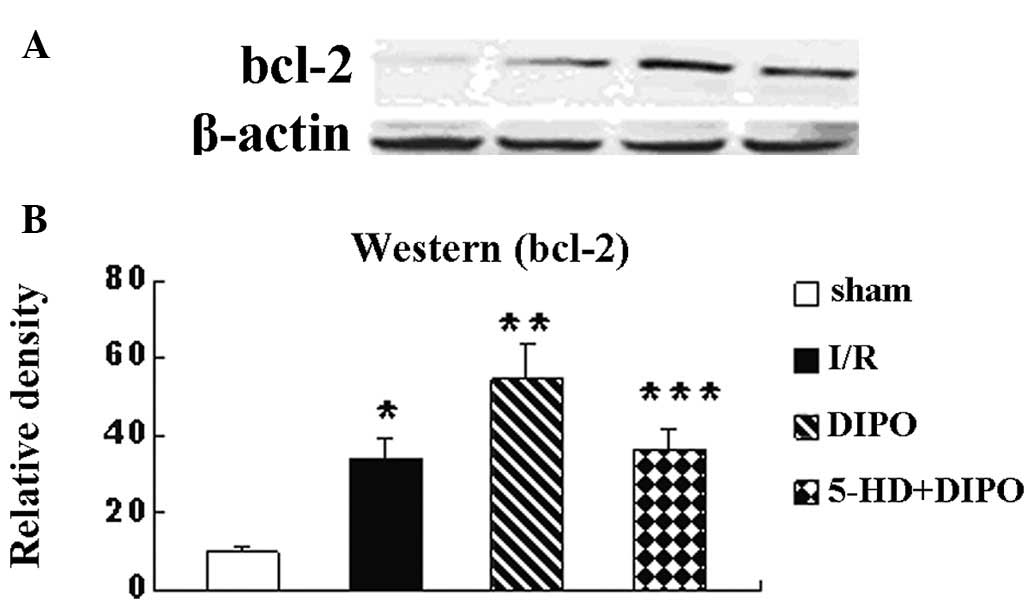
apoptosis signaling pathway was also investigated. The expression
levels of cyt-c, caspase-3 and bcl-2 were recorded (Figs. 4–6). The expression levels of cyt-c,
caspase-3 and bcl-2 in the I/R group were significantly increased
compared with those in the sham group. DIPO significantly increased
the expression levels of bcl-2 and decreased the expression levels
of cyt-c and caspase-3 compared with those in the I/R group.
Treatment with 5-HD markedly abrogated the DIPO-induced increases
in Bcl-2 expression and decreases in cyt-c and caspase-3
expression.
Discussion
The present study demonstrated for the first time in
live rat livers that DIPO protected the liver against I/R injury by
activating pkc-ε and opening mito-KATP channels. The
results indicated that DIPO significantly improved the function of
the liver. It improved the crucial indices of ALT and AST, which
reflect the function of liver, and may inhibit the apoptosis of
liver cells by inhibiting the apoptotic pathway. When 5-HD, the
mito-KATP channel blocker, was administered following
the DIPO surgery, it was revealed that the DIPO induced protection
was abrogated. Therefore, we suggest that mito-KATP is
crucial in I/R injury of the rat liver.
Previous studies have demonstrated that the opening
of mito-KATP channels may be involved in the
cardioprotective effects of IPC and IPO (16,17).
That the mito-KATP channel is the receptor responsible
for the cardioprotective actions of KATP channel openers
suggested that the mitochondrial level was significant in aspects
of the protective effect (18).
Another study demonstrated that mitochondrial protection by
diazoxide preconditioning reduced the permeability of the
mitochondria to exogenous cyt-c and maintained low outer membrane
permeability to nucleotides. It was also revealed that diazoxide
prevented increases in the permeability of the outer membrane to
nucleotides and cyt-c (19). The
release of cyt-c may activate the apoptotic pathway as an upstream
event of caspase activation (20).
It has also been demonstrated that preventing the activation of the
mitochondrial apoptotic pathway may inhibit apoptosis (15) and the participation of the
mitochondrial pathway has been demonstrated by the release of cyt-c
from mitochondria into cytoplasmic fractions and caspase-9 cleavage
(21). Caspase-9 activates
caspase-3 (15) which may cause
apoptosis. In that case, inhibiting the apoptosis by blocking cyt-c
release or caspase activation may be a therapeutic target. An
increasing number of studies have shown that bcl-2 family members,
particularly bcl-2, may inhibit apoptosis by blocking cytochrome c
release and inhibiting caspase-3 and -9 activation but not that of
caspase-12 (14,22). The present study demonstrated that
cyt-c release and active caspase-3 together indicated the
activation of the caspase-dependent pathway of apoptosis and bcl-2
overexpression in the DIPO group may inhibit this pathway which was
consistent with the previously mentioned findings.
The pkc family of signaling proteins, in particular
pkc-δ and pkc-ε, is commonly associated with the modulation of I/R
injury. Pkc-δ has been implicated as a key signaling element in the
cerebral and myocardial reperfusion injury processes (23–26).
Pkc-δ is associated with increased superoxide anion generation and
the enhanced release of pro-apoptotic factors and cyt-c (27,28).
Activation and trans-location of pkc-ε have been revealed to be
crucial in triggering the cardioprotective effects of IPC and IPO
(26,29–37).
It has been demonstrated that postconditioning decreased the
infarct size and was dependent on pkc signaling. Postconditioning
was associated with significantly higher pkc-ε levels in areas of
the myocardium at risk and selective isoform inhibition prevented
the infarct size reduction (26).
In addition, pkc-ε activity, possibly via oxygen radicals
originating from the mitochondrion, is necessary for the opening of
mito-KATP channels which may protect against reperfusion
injury (5,32,38,39).
In summary, postconditioning may promote the activation and
translocation of pkc-ε and limit the reperfusion-induced pkc-δ
translocation to mitochondria. In the present study it was
identified that, compared with the I/R group, the expression levels
of pkc-ε in the DIPO group were significantly increased and these
increases were abrogated by 5-HD. It is possible that the
protection the liver against I/R injury was through the activation
of pkc-ε which facilitated the opening of mito-KATP
channels.
The strategy of postconditioning with diazoxide is
relatively simple to perform, particularly during liver
transplantation, and may have the potential to be used in clinical
surgery where it may improve the survival rate of patients.
In summary, the findings of the present study
indicate that DIPO protects the liver from I/R injury by reducing
the serum levels of ALT and AST and opening mito-KATP
channels, activating and upregulating pkc-ε and inhibiting the
activation of the apoptotic pathway by decreasing the release of
cyt-c and the expression of caspase-3 and increasing the expression
of bcl-2.
Acknowledgements
The authors acknowledge the support of
the National Natural Science Foundation of China (No. 30530360 and
30772098).
References
|
1
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: a delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar
|
|
2
|
Hausenloy DJ, Mocanu MM and Yellon DM:
Cross-talk between the survival kinases during early reperfusion:
its contribution to ischemic preconditioning. Cardiovasc Res.
63:305–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu GS, Thornton J, Van Winkle DM, et al:
Protection against infarction afforded by preconditioning is
mediated by A1 adenosine receptors in rabbit heart. Circulation.
84:350–356. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leesar MA, Stoddard M, Ahmed M, et al:
Preconditioning of human myocardium with adenosine during coronary
angioplasty. Circulation. 95:2500–2507. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Costa AD, Garlid KD, West IC, et al:
Protein kinase G transmits the cardioprotective signal from cytosol
to mitochondria. Circ Res. 97:329–336. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsang A, Hausenloy DJ, Mocanu MM and
Yellon DM: Postconditioning: a form of ‘modified reperfusion’
protects the myocardium by activating the phosphatidylinositol
3-Kinase-Akt pathway. Circ Res. 95:230–232. 2004.
|
|
7
|
Darling CE, Jiang R, Maynard M, et al:
Postconditioning via stuttering reperfusion limits myocardial
infarct size in rabbit hearts: role of Erk1/2. Am J Physiol Heart
Circ Physiol. 289:H1618–H1626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao ZQ, Corvera JS, Halkos ME, et al:
Inhibition of myocardial injury by ischemic postconditioning during
reperfusion: comparison with ischemic preconditioning. Am J Physiol
Heart Circ Physiol. 285:H579–H588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heusch G: Postconditioning: old wine in a
new bottle? J Am Coll Cardiol. 44:1111–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vinten-Johansen J, Yellon DM and Opie LH:
Postconditioning: a simple clinically applicable procedure to
improve revascularization in acute myocardial infarction.
Circulation. 112:2085–2088. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jabúrek M, Costa AD, Burton JR, et al:
Mitochondrial PKC epsilon and mitochondrial ATP-sensitive
K+ channel copurify and coreconstitute to form a
functioning signaling module in proteoliposomes. Circ Res.
99:878–883. 2006.PubMed/NCBI
|
|
12
|
Costa AD, Jakob R, Costa CL, et al: The
mechanism by which the mitochondrial ATP-sensitive K+
channel opening and H2O2 inhibit the
mitochondrial permeability transition. J Biol Chem.
281:20801–20808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai AL, Fan LH, Zhang FJ, et al: Effects
of sevoflurane preconditioning and postconditioning on rat
myocardial stunning in ischemic reperfusion injury. J Zhejiang Univ
Sci B. 11:267–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu XC, Wang MW, Li SP and Wang HL:
Anti-apoptotic effect and the mechanism of orientin on
ischaemic/reperfused myocardium. J Asian Nat Prod Res. 8:265–272.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrer I and Planas AM: Signaling of cell
death and cell survival following focal cerebral ischemia: life and
death struggle in the penumbra. J Neuropathol Exp Neurol.
62:329–339. 2003.PubMed/NCBI
|
|
16
|
Fryer RM, Eells JT, Hsu AK, et al:
Ischemic preconditioning in rats: role of mitochondrial
KATP channel in preservation of mitochondrial function.
Am J Physiol Heart Circ Physiol. 278:H305–H312. 2000.PubMed/NCBI
|
|
17
|
Liu Y, Sato T, Seharaseyson J, et al:
Mitochondrial ATP-dependent potassium channels. Viable candidate
effectors of ischemic preconditioning. Ann NY Acad Sci. 874:27–37.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garlid KD, Paucek P, Yarov-Yarovoy V, et
al: The mitochondrial KATP channel as a receptor for
potassium channel openers. J Biol Chem. 271:8796–8799.
1996.PubMed/NCBI
|
|
19
|
Dos Santos P, Kowaltowski AJ, Laclau MN,
et al: Mechanisms by which opening the mitochondrial ATP-sensitive
K+ channel protects the ischemic heart. Am J Physiol
Heart Circ Physiol. 283:H284–H295. 2002.PubMed/NCBI
|
|
20
|
Kang PM, Haunstetter A, Aoki H, et al:
Morphological and molecular characterization of adult cardiomyocyte
apoptosis during hypoxia and reoxygenation. Circ Res. 87:118–125.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Li Q, Li Z, et al: The protection
of bcl-2 overexpression on rat cortical neuronal injury caused by
analogous ischemia/reperfusion in vitro. Neurosci Res. 62:140–146.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Wang J, Zhu S, et al: C-reactive
protein augments hypoxia-induced apoptosis through
mitochondrion-dependent pathway in cardiac myocytes. Mol Cell
Biochem. 310:215–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inagaki K, Hahn HS, Dorn GW II, et al:
Additive protection of the ischemic heart ex vivo by combined
treatment with delta-protein kinase c inhibitor and epsilon-protein
kinase C activator. Circulation. 108:869–875. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inagaki K, Chen L, Ikeno F, et al:
Inhibition of delta-protein kinase C protects against reperfusion
injury of the ischemic heart in vivo. Circulation. 108:2304–2307.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bright R, Raval AP, Dembner JM, et al:
Protein kinase C delta mediates cerebral reperfusion injury in
vivo. J Neurosci. 24:6880–6888. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zatta AJ, Kin H, Lee G, et al:
Infarct-sparing effect of myocardial postconditioning is dependent
on protein kinase c signalling. Cardiovasc Res. 70:315–324. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Churchill EN and Szweda LI: Translocation
of deltaPKC to mitochondria during cardiac reperfusion enhances
superoxide anion production and induces loss in mitochondrial
function. Arch Biochem Biophys. 439:194–199. 2005. View Article : Google Scholar
|
|
28
|
Murriel CL, Churchill E, Inagaki K, et al:
Protein kinase Cdelta activation induces apoptosis in response to
cardiac ischemia and reperfusion damage: a mechanism involving BAD
and the mitochondria. J Biol Chem. 279:47985–47991. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu GS, Cohen MV, Mochly-Rosen D and
Downey JM: Protein kinase C-epsilon is responsible for the
protection of preconditioning in rabbit cardiomyocytes. J Mol Cell
Cardiol. 31:1937–1948. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toma O, Weber NC, Wolter JI, et al:
Desflurane preconditioning induces time-dependent activation of
protein kinase C epsilon and extracellular signal-regulated kinase
1 and 2 in the rat heart in vivo. Anesthesiology. 101:1372–1380.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ludwig LM, Weihrauch D, Kersten JR, et al:
Protein kinase C translocation and Src protein tyrosine kinase
activation mediate isoflurane-induced preconditioning in vivo:
potential downstream targets of mitochondrial adenosine
triphosphate-sensitive potassium channels and reactive oxygen
species. Anesthesiology. 100:532–539. 2004. View Article : Google Scholar
|
|
32
|
Ping P, Zhang J, Qiu Y, et al: Ischemic
preconditioning induces selective translocation of protein kinase C
isoforms epsilon and eta in the heart of conscious rabbits without
subcellular redistribution of total protein kinase C activity. Circ
Res. 81:404–414. 1997. View Article : Google Scholar
|
|
33
|
Novalija E, Kevin LG, Camara AKS, et al:
Reactive oxygen species precede the epsilon isoform of protein
kinase C in the anesthetic preconditioning signaling cascade.
Anesthesiology. 99:421–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qiu Y, Ping P, Tang XL, et al: Direct
evidence that protein kinase C plays an essential role in the
development of late preconditioning against myocardial stunning in
conscious rabbits and that epsilon is the isoform involved. J Clin
Invest. 101:2182–2198. 1998. View
Article : Google Scholar
|
|
35
|
Yang Z, Sun W and Hu K: Molecular
mechanism underlying adenosine receptor-mediated mitochondrial
targeting of protein kinase C. Biochim Biophys Acta. 1823:950–958.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaljusto ML, Rutkovsky A, Stensløkken KO,
et al: Postconditioning in mouse hearts is inhibited by blocking
the reverse mode of the sodium-calcium exchanger. Interact
Cardiovasc Thorac Surg. 10:743–748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jones WK, Fan GC, Liao S, et al:
Peripheral nociception associated with surgical incision elicits
remote nonischemic cardioprotection via neurogenic activation of
protein kinase C signaling. Circulation. 120(Suppl 11): 1–9. 2009.
View Article : Google Scholar
|
|
38
|
Liu H, Zhang HY, Zhu X, et al:
Preconditioning blocks cardiocyte apoptosis: role of
K+ATP channels and PKCε. Am J Physiol Heart
Circ Physiol. 282:1380–1386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McPherson BC and Yao Z: Morphine mimics
preconditioning via free radical signals and mitochondrial
KATP channels in myocytes. Circulation. 103:290–295.
2001. View Article : Google Scholar : PubMed/NCBI
|