Introduction
Adverse drug reactions (ADRs) are a big challenge in
drug therapy, with cutaneous drug reactions accounting for a large
proportion. The clinical manifestations of drug eruptions are
highly variable. It is critical to recognize and deal with severe
cutaneous ADRs (SCADRs) as rapidly as possible as they are able to
cause life-threatening diseases, including Stevens-Johnson
syndrome, toxic epidermal necrolysis (TEN), generalized bullous
fixed drug eruption, acute generalized exanthematous pustulosis
(AGEP) and drug reactions with eosinophilia and systemic symptoms
(1,2).
The present study describes the rare case of a
female patient with medication-triggered epidermal necrolysis and
purulent bulla throughout her body, whose drug eruption was
successfully cured. To the best of our knowledge, no drug eruption
with purulent bulla and epidermal necrolysis has previously been
documented, and therefore the present study is the first case
report of its kind.
Case report
A 51-year-old female was referred to the Second
Affiliated Hospital, Hangzou, China, as a result of drug
anaphylaxis, which developed 3 days prior to admittance and became
aggravated rapidly. The present study was conducted in accordance
with the declaration of Helsinki and with approval from the Ethics
Committee of the Second Affiliated Hospital, School of Medicine,
Zhejiang University. Written informed consent was obtained from the
patient. The patient suffered a scalp trauma in a traffic accident
and was presented to a local hospital eight days prior to her
referral to the Second Affiliated Hospital, Hangzou. A single dose
of 1500 IU tetanus antitoxin (TAT) was prescribed immediately
following debridement. Subsequently, 8 g/day of intravenus
sulbenicillin was administered for a total of 5 days. Following
this, the patient suffered from moderate pruritus with a sudden
occurrence of generalized erythema and numerous easily ruptured
pustules the size of peas, however she had neither pain nor fever.
Although 10 mg/day of intravenus dexamethasone was administered,
the skin rash progressed rapidly with epidermal necrolysis,
generalized purulent bulla and erosion of the oral and vulval
mucosa in the succeeding 3 days. The patient suffered intense
causalgia. A patient history revealed no drug or food allergies and
no history of personal or familial psoriasis.
On admission after referral, the patient was alert
and cooperative. A physical examination showed no significant
abnormalities, with the exception of the skin eruptions and severe
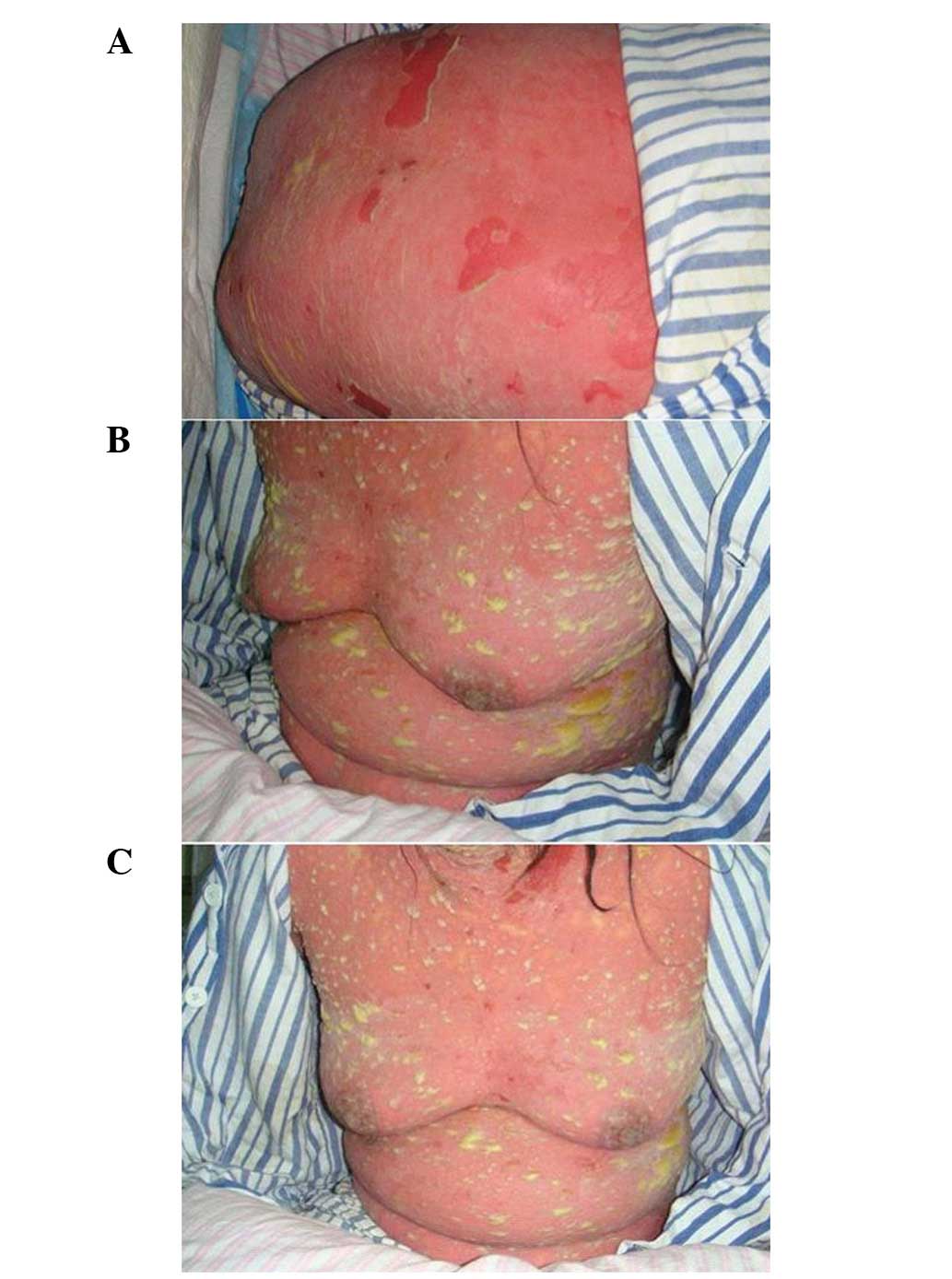
pitting edema on the lower extremities. The patient developed
diffuse bright red areas, non-follicular pustules and a generalized
purulent bulla all over the body, with epidermal necrolysis and the
detachment of 46% of the body surface area (BSA; Fig. 1). There was erosion in the oral and
vulval mucosa and Nikolsky’s sign was positive.
Laboratory studies revealed leukocytosis
(23.3×109/l) and neutrophilia (22.5×109/l).
With the exception of low albumin (3.03 mg/dl) and high C-reactive
protein (CRP; 287.3 mg/l) levels, the other blood chemical
examinations were normal. The blood and pus cultures were negative
for bacteremia. The search for specific IgE antibodies to inhalants
and food was performed by UniCAP RAST (Phadia, Uppsala, Sweden) and
was negative. A chest X-ray revealed no abnormal imaging results. A
skin biopsy was not performed as the idea was rejected by the
patient and her dependents, meaning that a written informed consent
was not obtainable. The clinical impression obtained was one of a
drug eruption with purulent bulla and epidermal necrolysis. The
patient was treated with intravenous methylprednisolone and
additional 0.4 g/kg/day intravenous immunoglobulin (IVIG) for 5
consecutive days. The quantities of purulent bulla markedly
decreased and the causalgia lessened on the third day of
hospitalization. The purulent bulla and epidermal necrolysis
disappeared on the fifth day. The dosage of methylprednisolone was
gradually decreased and the laboratory values were also improved in
the following days (Table I).
 | Table ILaboratory values and clinical
characteristics at 1, 2, 4, 8, 12 and 20 days post-admission. |
Table I
Laboratory values and clinical
characteristics at 1, 2, 4, 8, 12 and 20 days post-admission.
| Day |
|---|
|
|
|---|
| Clinical
characteristics | 1 | 2 | 4 | 8 | 12 | 20 |
|---|
| Body temperature
(°C) | 37.4 | 37.7 | 36.9 | 37 | 37.1 | 37 |
| White blood cell
count/mm3 | 23.3 | 17.7 | 16.1 | 10.2 | 9.7 | 8.5 |
|
Eosinophils/mm3 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 |
|
Neutrophils/mm3 | 22.5 | 17 | 15.1 | 8.3 | 8 | 6 |
| Aspartate
aminotransferase (IU/l) | - | 19 | 59 | 20 | 30 | 13 |
| Alanine
aminotransferase (IU/l) | - | 32 | 65 | 87 | 51 | 20 |
| Albumin (mg/dl) | - | 3.03 | 2.39 | 2.87 | 3.03 | 3.52 |
| C-reaction protein
(mg/l) | - | 287.3 | 175.7 | 57.6 | 22.9 | 3.5 |
| Prednisolone
(mg/day) | 80 | 160 | 120 | 80 | 60 | 28 |
Discussion
The patient was diagnosed with a drug eruption with
purulent bulla and epidermal necrolysis based on the following
criteria: i) the symptoms began 5 days after starting the TAT and
sulbenicillin treatments; ii) the presence of a generalized
purulent bulla, epidermal detachment of a large section of the body
surface area, erosions of the mucous membranes and a positive
result for Nikolsky’s sign; iii) the culture of the purulent bulla
content was negative for bacteria; iv) the symptoms were not
attributable to bacterial or viral infections; and v) the clinical
signs and laboratory abnormalities were responsive to
corticosteroids and IVIG. To date, pustular drug exanthema, which
refers in particular to AGEP (3),
is not common. Furthermore, TEN accompanied by AGEP has rarely been
reported (4). In the present case,
an acute onset of widespread erythema, pustules and purulent bulla,
leukocytosis, neutrophilia, a large area of epidermal necrolysis,
erosion of the mucous membranes of the cavitas oris and labium
minus and a positive Nikolsky’s sign over the whole body were
present following drug administration. The clinical features
appeared similar to AGEP and TEN to a certain extent, but the
widespread purulent bulla, which were >5 mm in diameter, emerged
on the disease onset and were not confluent with adjacent pustules,
meant that the diagnosis differed from that of AGEP. As there has
been no previously reported drug eruption displaying purulent bulla
and epidermal necrolysis, we suspect that this case represents a
new clinical pattern and have therefore named it purulent bullous
epidermal necrolysis.
A differential diagnosis should be made to
distinguish the condition of the patient in the present study from
that of other bullous diseases. Bullous impetigo may be excluded by
the negative results of the pus bacterial culture and the good
response to corticosteroids. It is unfortunate that a skin biopsy
was not performed, but from the typical clinical manifestation
observed, a diagnosis of other bullous diseases, including bullous
fixed drug eruption, drug-induced bullous pemphigus or drug-induced
bullous pemphigoid, may also be excluded.
It has been reported that AGEP is mostly caused by a
reaction to antibiotics, particularly the β-lactams and macrolides
(5). TEN is usually caused by
antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs),
anticonvulsants and antipodagrics (6,7). In
the present study, the patient suffered a drug eruption following
the administration of TAT and sulbenicillin. Although a previous
study has reported TEN caused by TAT (8), no reports of TAT causing purulent
eruptions or sulbenicillin-related TEN or purulent eruptions have
been documented. It is difficult to determinine whether a
particular medication is able to cause a specific eruption,
particularly when the patient is taking several drugs, due to the
lack of sensitivite and specific tests. Although drug provocation
testing is the best tool for dertermining the causal drug of a
non-immediate allergic reaction, it is dangerous and
contraindicated in severe cases, including those of AGEP and TEN
(9,10). In the present study the patient
refused any attempt to trace the causal drug.
Physicians who prescribe TAT or sulbenicillin should
be aware of this rare potential life-threatening complication.
References
|
1
|
Mockenhaupt M: Severe drug-induced skin
reactions: clinical pattern, diagnostics and therapy. J Dtsch
Dermatol Ges. 7:142–160. 2009.(In English and German).
|
|
2
|
Grover S: Severe cutaneous adverse
reactions. Indian J Dermatol Venereol Leprol. 77:3–6. 2011.
View Article : Google Scholar
|
|
3
|
Sidoroff A, Halevy S, Bavinck JN, Vaillant
L and Roujeau JC: Acute generalized exanthematous pustulosis (AGEP)
- a clinical reaction pattern. J Cutan Pathol. 28:113–119. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goh TK, Pang SM, Thirumoorthy T and Goh
SG: Acute generalised exanthematous pustulosis and toxic epidermal
necrolysis induced by carbamazepine. Singapore Med J. 49:507–510.
2008.PubMed/NCBI
|
|
5
|
Roujeau JC, Bioulac-Sage P, Bourseau C, et
al: Acute generalized exanthematous pustulosis. Analysis of 63
cases. Arch Dermatol. 127:1333–1338. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leenutaphong V, Sivayathorn A,
Suthipinittharm P and Sunthonpalin P: Stevens-Johnson syndrome and
toxic epidermal necrolysis in Thailand. Int J Dermatol. 32:428–431.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roujeau JC, Kelly JP, Naldi L, et al:
Medication use and the risk of Stevens-Johnson syndrome or toxic
epidermal necrolysis. N Engl J Med. 333:1600–1607. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polilov MI: Lyell’s syndrome caused by
antitetanus serum. Vestn Dermatol Venerol. 39:83–84. 1965.(In
Russian).
|
|
9
|
Torres MJ, Mayorga C and Blanca M:
Non-immediate allergic reactions induced by drugs: pathogenesis and
diagnostic tests. J Investig Allergol Clin Immunol. 19:80–90.
2009.PubMed/NCBI
|
|
10
|
Romano A, Di Fonso M, Papa G, et al:
Evaluation of adverse cutaneous reactions to aminopenicillins with
emphasis on those manifested by maculopapular rashes. Allergy.
50:113–118. 1995. View Article : Google Scholar : PubMed/NCBI
|