Introduction
Congestive heart failure (CHF) is a powerful,
independent predictor of atrial fibrillation (AF), with CHF
patients exhibiting a 6-fold increase in the relative risk of
developing AF (1). Research on the
abnormal ventricular electrophysiological properties associated
with heart failure (HF) have been performed, however, the mechanism
of electrical remodeling in atrial muscle presently remains
unknown. Furthermore, previous studies were mainly focused on
atrial electrical remodeling after the development of AF (2–9),
with only a few studies reporting on patients with organic heart
disease in sinus rhythm.
Li et al (10) studied a dog model with CHF induced
by ventricular pacing at 220–240 bpm for 5 weeks and discovered
that CHF significantly reduces the density of the L-type
Ca2+ current (ICa2+), the sensitive transient
outward K+ current (Ito1) and the slow
delayed rectifier K+ current (IKs) in atrial
myocytes without altering their voltage dependencies or kinetics.
The inward rectifier K+ current (Iki), the
ultra rapid rectifier K+ current (Ikur) and
the rapid delayed rectifier K+ current (Ikr)
were not altered by CHF, while the transient inward
Na+/Ca2+ exchanger (INCX) current
was increased. In addition, Cha et al (11,12)
discovered that CHF downregulates Ito1, IKs
and ICa2+, and upregulates INCX without
altering Iki. Clearly, these studies mainly focused on
the alteration of ion currents rather than the gene expression of
ion channels in atrial myocytes. Due to technical restrictions,
ionic currents in atrial myocytes were not able to be tested.
In this study, we have identified possible
alterations in atrial cellular ion currents and the molecular
mechanisms involved in HF. The underlying mechanisms predisposing
HF patients to AF have also been investigated. We demonstrated that
when compared with the normal cardiac function group, the mRNA
expression levels of Kv4.3α, KvLQT1 and L-Caα1c were
significantly reduced in patients with HF. The mRNA expression
levels of Kv1.5 were not evidently altered, while mRNA expression
levels of NCX increased significantly. These changes in gene
expression were in accordance with alterations in ion currents
demonstrated in the previous studies mentioned above (10–12).
Our results indicate that alterations in the gene expression of ion
channels may explain the molecular basis of altered ion currents in
the atrial myocytes of patients with HF.
Materials and methods
Patients
Thirty-six consecutive patients with sinus rhythm
(15 males and 21 females, aged 29–62 years old with a mean age of
44.7±6.94 years) undergoing coronary artery bypass surgery at the
Qianfoshan Hospital of Shandong University and the Jinan Central
Hospital Affiliated to Shandong University (China) were enrolled.
Among these, 18 cases presented with coronary heart disease, 5 with
dilated myocardial disease, 6 with rheumatic heart disease and 7
with congenital heart disease. Patients with an eject fraction (EF)
<0.4 were defined as the HF group (33.67±3.68%) while those with
EF >0.4 were defined as the normal cardiac function group
(60.28±4.98%). The study cohort was extremely homogeneous for age,
gender and overall etiology constitution. The study was approved by
the ethics committee of Shandong Provincial Qianfoshan Hospital,
Jinan, China. Written informed patient consent was obtained from
the patient’s family
Reverse transcription-PCR (RT-PCR)
The internal standard gene used was
glyceraldehyde-3-phosphate dehydrogenase (3-GAPDH) and the target
genes included the channel determinant genes of Ito1
(Kv4.3α), Iks (KvLQT1), Ikur (Kv1.5),
ICa2+ (L-Caα1c) and INCX (NCX).
Trizol® Reagent was purchased from Gibco BRL (Shanghai BioAsia
Biotechnology Co. Ltd., Shanghai, China). The RNA PCR kit (AMV)
Ver. 3.0 was purchased from Takara Bio, Inc. (Dalian, China). The
primer sequences were provided by Shanghai BioAsia Biotechnology
Co., Ltd. (Table I).
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Gene | Primer sequence | Fragment (bp) |
|---|
| GAPDH | F
5′-CCCATCACCATCTTCAGGAGCG-3′ | 411 |
| R
5′-GGCAGGGATGATGTTCTGGAGAGCC-3′ | |
| Kv4.3α | F
5′-CAGCAAGTTCACAAGCATCC-3′ | 649 |
| R
5′-AGCTGGCAGGTTAGAATTGG-3′ | |
| KvLQT1 | F
5′-AGCAGAAGCAGAGGCAGAAG-3′ | 370 |
| R
5′-GACGGAGATGAACAGTGAGG-3′ | |
| Kv1.5 | F
5′-AACGAGTCCCAGCGCCAGGT-3′ | 326 |
| R
5′-AGGCGGATGACTCGGAGGAT-3′ | |
|
L-Caα1c | F
5′-CTGGACAAGAACCAGCGACAGTGCG-3′ | 563 |
| R
5′-ATCACGATCAGGAGGGCCACATAG G-3′ | |
| NCX | F
5′-CTACCAAGTCCTAAGTCAGCAGC-3′ | 519 |
| R
5′-GATCCGAGGCAAGCAAGTGTAGA-3′ | |
Open-chest cardiac surgery was performed and 50–100
mg samples of right atrial appendages were rapidly collected with
sterilized Eppendorf tubes. The sample was frozen at −80°C
immediately following the addition of 0.5 ml TRIzol. The total RNA
was extracted using the TRIzol method. The extraction was dissolved
by adding 40 μl DEPC and then measured by a spectrophotometer, with
the optical density (OD) 260/OD280>1.8 and thereafter stored at
−20°C for detection. RT-PCR technology was applied for reverse
transcription and cDNA fragment amplification. The first-strand
cDNA was synthesized using AMV reverse transcriptase. Total RNA was
reverse transcribed in a final volume of 10 μl, containing the
following: 2 μl MgCl2 (25 mM); 1 μl 10X RT
buffer; 3.75 μl RNase free dH2O; 1 μl dNTP
mixture (10 mM); 0.25 μl RNase inhibitor; 0.5 μl AMV
reverse transcriptase; 0.5 μl oligo dT-adaptor primer; 1
μl RNA. The reverse transcription was then conducted as
follows: the reaction mixture was incubated at 30°C for 10 min,
annealed at 42°C for 30 min, followed by incubation at 99°C for 5
min and 5°C for 5 min. Following denaturation at 94°C for 2 min,
the samples were subjected to 30 cycles of denaturation at 94°C for
30 sec, annealing for 30 sec and extension at 72°C for 50 sec.
Then, 35 cycle PCR amplification was used with a 5-min extension
time (reaction solution 2). The 10 μl of amplified product
was electrophoresed in 1.5% agarose gel containing ethidium
bromide, examined and photographed under a UV transilluminator. The
intensity of each band was quantified using image analysis software
(TINA version 2.10, Raytest, Straubenhardt, Germany) and the
expression levels were calculated by measuring the OD of the target
gene and normalized to that of the amplified GAPDH.
Western blot analysis
All relevant proteins were harvested from tissue,
separated by 10% SDS/PAGE and then subjected to immunoblot
analyses. The primary antibodies against Kv4.3α, KvLQT1, Kv1.5,
L-Caα1c, NCX and actin were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA; anti-Kv4.3α, cat#
sc-11686, 1:200; anti-KvLQT1, cat# sc-365186, 1:200; anti-Kv1.5,
cat# sc-377110, 1:200; anti-L-Caα1c, cat# sc-166069,
1:200; anti-NCX, cat# sc-32881, 1:200; anti-actin, cat# sc-130301,
1:10,000). Secondary antibodies used in this study were donkey
anti-goat IgG-HRP (cat# sc-2020, 1:5,000, Santa Cruz Biotechnology,
Inc.), goat anti-rabbit IgG-HRP (cat# sc-2004, 1:5,000, Santa Cruz
Biotechnology, Inc.) and goat anti-mouse IgG-HRP (cat# sc-2005,
1:10,000, Santa Cruz Biotechnology, Inc.). Bound antibodies were
detected using the ECL system (Pierce Biotechnology, Inc.,
Rockford, IL, USA). The immunoblot experiments were repeated at
least 3 times. The mean normalized OD of detected Kv4.3α, KvLQT1,
Kv1.5, L-Caα1c or NCX protein bands relative to the OD
of the actin band from the same individual was calculated,
respectively.
Statistical analysis
Concise Statistics 2000 was used to perform the
statistical analyses. All numerical values are expressed as mean ±
SD. The t-test was performed for comparison of the experimental
group and the control group. P<0.05 was used to indicate a
statistically significant result.
Results
mRNA levels of genes involved in ion
channel regulation of HF patients
To investigate whether mRNA transcript levels of
genes involved in ion channel regulation were changed, right atrial
appendages were obtained from 18 HF patients and 18 individuals
with normal cardiac functions, who had undergone surgery. The total
RNAs were isolated and the mRNA levels were determined by RT-PCR.
The PCR products were separated on gels and the OD values of the
relevant bands of interest were measured to compare with the OD of
GAPDH bands. The mRNA expression levels of Kv4.3α,
KvLQT1, Kv1.5, L-Caα1c,
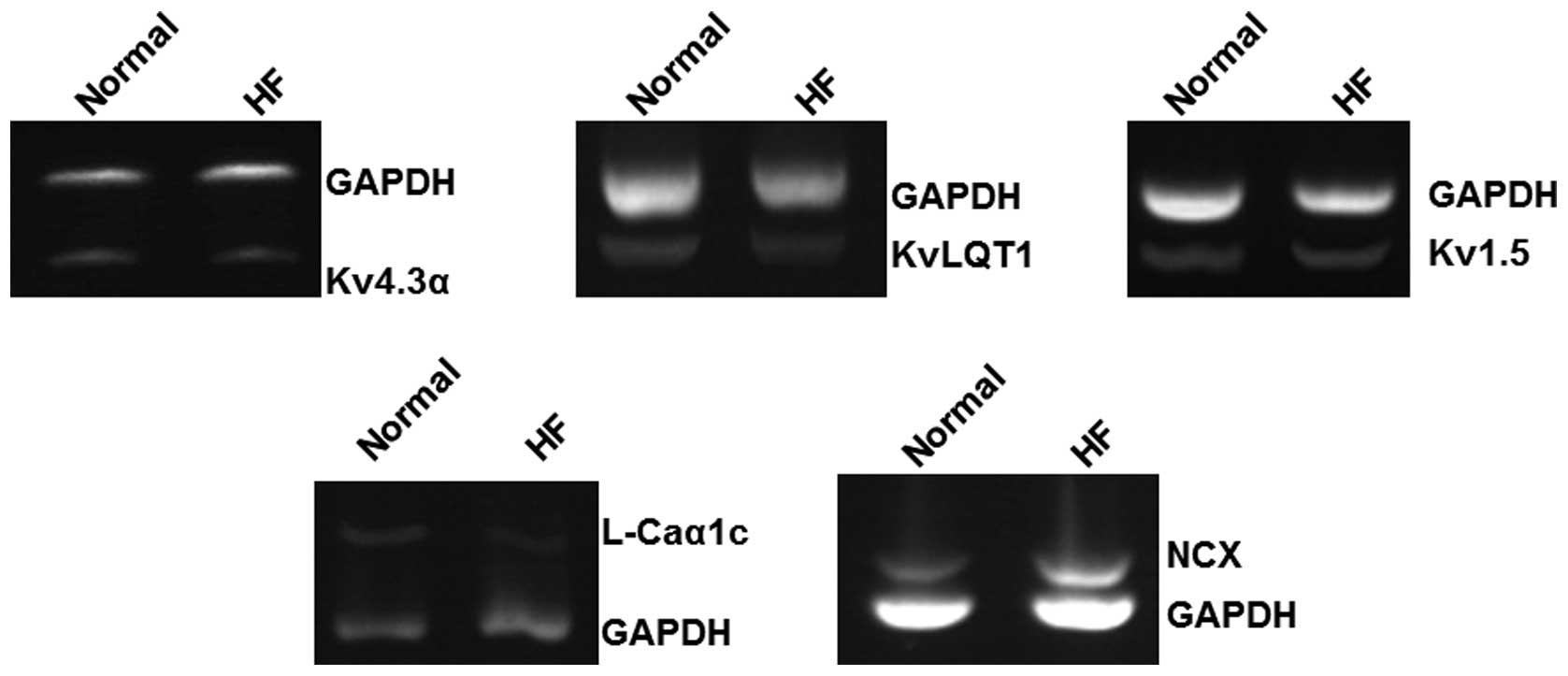
NCX and GAPDH are shown in Fig. 1. The amplification bands of the
target genes and the internal standard GAPDH gene are
consistent with theoretically expected sizes.
As shown in Table
II, compared with the mean value of mRNA levels in normal
individuals (0.34±0.07), the mRNA expression of Kv1.5 in the
HF group (0.30±0.05) was not significantly altered (P>0.05). The
mRNA expression levels of Kv4.3α, KvLQT1 and
L-Caα1c were significantly reduced in patients
with HF (P<0.01) in comparison with those detected in normal
individuals (0.83±0.07 versus 0.45±0.09, 0.56±0.04 versus
0.36±0.06, 0.42±0.09 versus 0.25±0.06, respectively). However, the
mRNA expression of NCX was significantly increased in HF
patients (0.31±0.07) compared with those detected in normal
individuals (0.19±0.05, P<0.01). These results suggest that mRNA
levels of some ion channel-related genes may be altered in HF
patients.
 | Table IImRNA expression of multiple ion
channels in the right atria of patients in the normal cardiac
function group, compared with the HF group (mean ± SD). |
Table II
mRNA expression of multiple ion
channels in the right atria of patients in the normal cardiac
function group, compared with the HF group (mean ± SD).
| Group | No. of patients | Kv4.3α/GAPDH | KvLQT1/GAPDH | Kv1.5/GAPDH |
L-Caα1c/GAPDH | NCX/GAPDH |
|---|
| Normal | 18 | 0.83±0.07 | 0.56±0.04 | 0.34±0.07 | 0.42±0.09 | 0.19±0.05 |
| HF | 18 | 0.45±0.09a | 0.36±0.06a | 0.30±0.05 | 0.25±0.06a | 0.31±0.07a |
Decreased expression of Kv4.3α, KvLQT1
and L-Caα1c but increased expression of NCX in HF
patients
To investigate whether levels of proteins encoded by
the Kv4.3α, KvLQT1, Kv1.5,
L-Caα1c and NCX genes were altered in
comparison to those in normal controls, total protein was harvested
from tissues, separated by 10% SDS/PAGE and then subjected to
immunoblot analyses. The cellular actin protein served as a loading
control. Representative blots are shown in Fig. 2A. The mean normalized OD of these
protein bands relative to the OD of the actin band from each
individual was calculated and subjected to statistical analyses.
Error bars represent the mean ± SD (P<0.05, Fig. 2B).
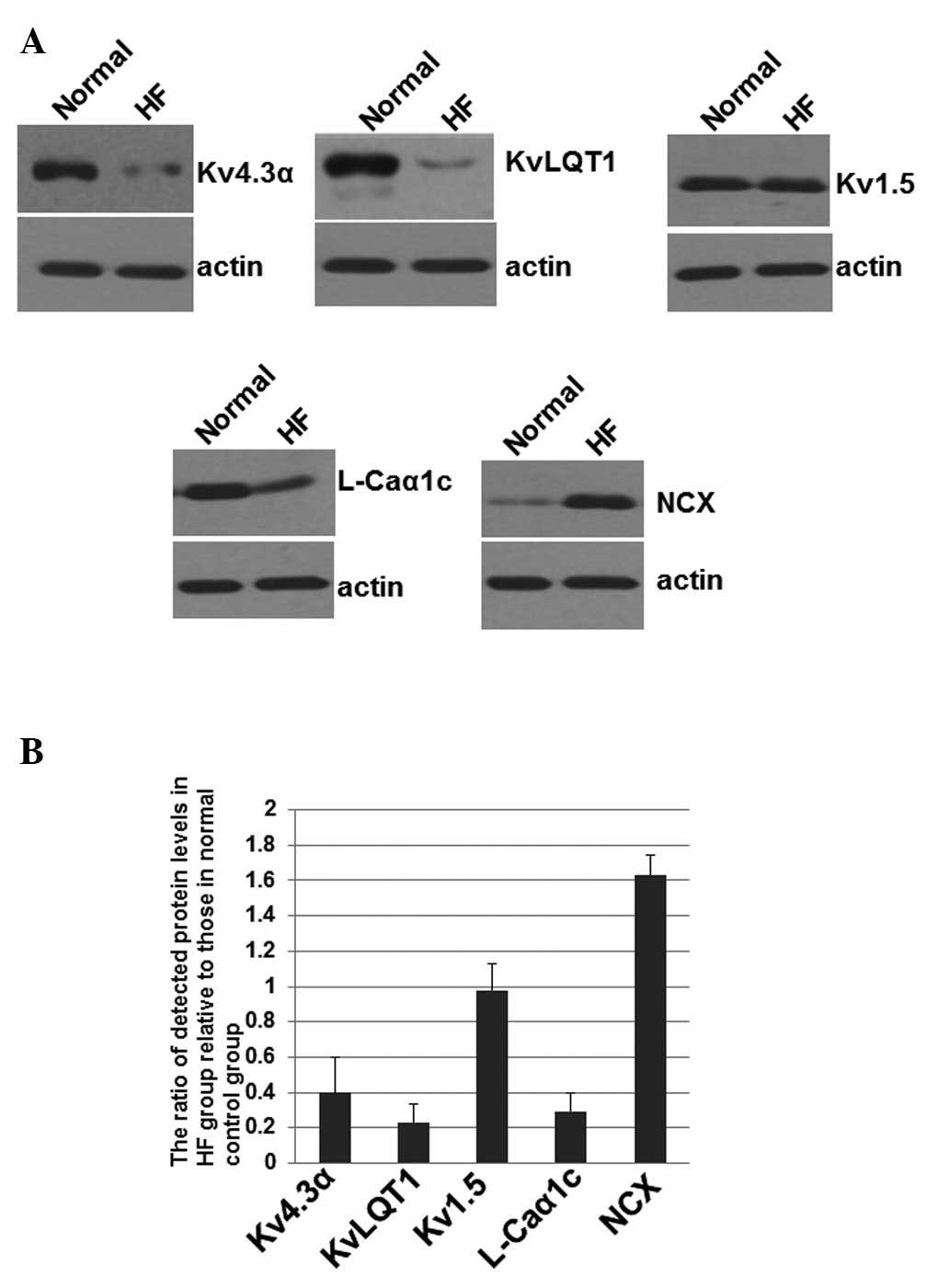 | Figure 2Western blotting of proteins encoded
by the Kv4.3α, KvLQT1, Kv1.5,
L-Caα1c and NCX genes. (A) Total protein
was harvested, separated by SDS/PAGE and subjected to immunoblot
analyses. The primary antibodies against Kv4.3α, KvLQT1, Kv1.5,
L-Caα1c, NCX and actin were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Bound antibodies were
detected using the ECL system (Pierce Biotechnology, Inc.,
Rockford, IL, USA). (B) The immunoblot experiments were repeated at
least 3 times. The mean normalized OD of detected Kv4.3α, KvLQT1,
Kv1.5, L-Caα1c or NCX protein bands relative to the OD
of the actin band from the same individual was calculated,
respectively. The mean ± SD was calculated. OD, optical density;
HF, heart failure. |
As shown in Fig.
2B, the levels of Kv4.3α, KvLQT1 and
L-Caα1c were significantly decreased to
0.41±0.21, 0.23±0.11 and 0.29±0.13, respectively, in the HF groups,
when the levels in the normal control group were artificially set
as 1. Kv1.5 levels were not altered significantly in the HF
group when compared with the normal group with a value of
0.98±0.15. However, expression levels of NCX were significantly
increased in HF patients (1.63±0.12) when compared with the normal
group (Fig. 2B). These results
suggest that the altered expression of Kv4.3α, KvLQT1,
L-Caα1c and NCX involved in ion channels of atrial
myocytes may be correlated with risk of AF in patients with HF.
Discussion
In this study, we have identified possible
alterations in atrial cellular ion currents and molecular
mechanisms involved in HF. The underlying mechanisms predisposing
HF patients to AF were also investigated. We demonstrated that when
compared with the normal cardiac function group, the mRNA
expression levels of Kv4.3α, KvLQT1 and L-Caα1c were all
significantly reduced in patients with HF. The mRNA expression
levels of Kv1.5 were not evidently altered, while mRNA expression
of NCX increased significantly. Thus, the changes of gene
expression were in accordance with the ion current alterations
observed in the aforementioned studies (10–12).
Ito1 has been identified as the major
component of phase 1 action potentials. Kääb et al (13) studied dog models with
pacing-induced HF, and discovered that the pharmacological
reduction of Ito by 4-aminopyridine in the control group decreased
the notch amplitude and prolonged the action potential duration
(APD), suggesting that downregulation of Ito1 in
pacing-induced HF is, at least partially, responsible for
prolongation of the action potential. Several compensatory
mechanisms have been proposed. It is particularly well known that
neurohormones are activated to promote long-term deterioration of
cardiac function and structure. Activation of the
renin-angiotensin-aldosterone system leads to an increase in the
circulation of AngII, and furthermore a decrease in the
Ito1(14), which
results in a significant difference in distribution and an increase
in repolarization dispersion, and finally induce cardiac arrhythmia
after combination with the receptor.
IKs is the major source for the
repolarization of the action potential (15). Previous evidence indicated that
Iks and the mRNA expression levels of KvLQT1 are reduced
in patients with HF (16). The
downregulation of IKs and Ito1 contribute to
the prolongation of APD, which contributes to the genesis of early
after depolarization (EAD) and delayed after depolarization (DAD)
as well as the physiological heterogeneity of cardiac myocytes
(17).
In addition, altered activity of NCX may also be
strongly correlated with the genesis of AF. The increased inward
INCX in the plateau phase is important to the production
of EAD. By contrast, the increased activity of NCX during the
diastolic spontaneous SR Ca2+ release period may lead to
a greater depolarization current and greatly increase DAD and the
propensity for triggered arrhythmias in HF patients (18–20).
DAD may induce triggered activity and subsequently promote
inducibility of sustained atrial tachycardia (21). The neuroendocrine system was
activated and the heart rate was increased with HF, leading to the
upregulation of inward ICa2+ and intracellular
Ca2+ overload, which provides feedback inhibition of
L-Ca2+ channels, a decrease in the inward movement of
Ca2+ and a shortened action potential plateau. The
overload of extracellular Ca2+ may further influence the
K+ channel and promote the electrical instability of cardiac cells,
which could induce arrhythmia and trigger activity.
In conclusion, alterations in the gene expression of
ion channels may provide the molecular basis of altered atrial
cellular ion currents in patients with HF, and furthermore, may
initiate atrial arrhythmia, particularly AF, by either trigger or
re-entry activity. HF-induced atrial ionic remodeling may be
important in the formation of AF substrate and contribute to the
potential mechanisms of AF in HF.
Acknowledgements
This study was supported by the
Shandong Provincial Health Department funded project (Grant No.
HC119).
References
|
1
|
Grönefeld GC and Hohnloser SH: Heart
failure complicated by atrial fibrillation: mechanistic,
prognostic, and therapeutic implications. J Cardiovasc Pharmacol
Ther. 8:107–113. 2003.PubMed/NCBI
|
|
2
|
Franz MR, Karasik PL, Li C, Moubarak J and
Chavez M: Electrical remodeling of the human atrium: similar
effects in patients with chronic atrial fibrillation and atrial
flutter. J Am Coll Cardiol. 30:1785–1792. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandozi C, Bianconi L, Villani M, et al:
Electrophysiological characteristics of the human atria after
cardioversion of persistent atrial fibrillation. Circulation.
98:2860–2865. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yue L, Melnyk P, Gaspo R, Wang Z and
Nattel S: Molecular mechanisms underlying ionic remodeling in a dog
model of atrial fibrillation. Circ Res. 84:776–784. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Wagoner DR, Pond AL, Lamorgese M,
Rossie SS, McCarthy PM and Nerbonne JM: Atrial L-type Ca2+ currents
and human atrial fibrillation. Circ Res. 84:428–436. 1999.
|
|
6
|
Krogh-Madsen T, Abbott GW and Christini
DJ: Effects of electrical and structural remodeling on atrial
fibrillation maintenance: a simulation study. PLoS Comput Biol.
8:e10023902012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hunter RJ, Liu Y, Lu Y, Wang W and
Schilling RJ: Left atrial wall stress distribution and its
relationship to electrophysiologic remodeling in persistent atrial
fibrillation. Circ Arrhythm Electrophysiol. 5:351–630. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Dong D and Yang B: Atrial
remodeling in atrial fibrillation and association between microRNA
network and atrial fibrillation. Sci China Life Sci. 54:1097–1102.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pang H, Ronderos R, Pérez-Riera AR,
Femenía F and Baranchuk A: Reverse atrial electrical remodeling: a
systematic review. Cardiol J. 18:625–631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li D, Melnyk P, Feng J, Wang Z, Petrecca
K, Shrier A and Nattel S: Effects of experimental heart failure on
atrial cellular and ionic electrophysiology. Circulation.
101:2631–2638. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cha TJ, Ehrlich JR, Zhang L, Shi YF,
Tardif JC, Leung TK and Nattel S: Dissociation between ionic
remodeling and ability to sustain atrial fibrillation during
recovery from experimental congestive heart failure. Circulation.
109:412–418. 2004. View Article : Google Scholar
|
|
12
|
Cha TJ, Ehrlich JR, Zhang L and Nattel S:
Atrial ionic remodeling induced by atrial tachycardia in the
presence of congestive heart failure. Circulation. 110:1520–1526.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kääb S, Nuss HB, Chiamvimonvat N, et al:
Ionic mechanism of action potential prolongation in ventricular
myocytes from dogs with pacing-induced heart failure. Circ Res.
78:262–273. 1996.
|
|
14
|
Mankad S, d’Amato TA, Reichek N, et al:
Combined angiotensin II receptor antagonism and
angiotensin-converting enzyme inhibition further attenuates
postinfarction left ventricular remodeling. Circulation.
103:2845–2850. 2001. View Article : Google Scholar
|
|
15
|
Charpentier F, Mérot J, Loussouarn G and
Baró I: Delayed rectifier K(+) currents and cardiac repolarization.
J Mol Cell Cardiol. 48:37–44. 2010.
|
|
16
|
Thomas D, Wimmer AB, Karle CA, et al:
Dominant-negative I(Ks) suppression by KCNQ1-deltaF339 potassium
channels linked to Romano-Ward syndrome. Cardiovasc Res.
67:487–497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu X, Rials SJ, Wu Y, et al: Left
ventricular hypertrophy decreases slowly but not rapidly activating
delayed rectifier potassium currents of epicardial and endocardial
myocytes in rabbits. Circulation. 103:1585–1590. 2001. View Article : Google Scholar
|
|
18
|
Pogwizd SM, Schlotthauer K, Li L, Yuan W
and Bers DM: Arrhythmogenesis and contractile dysfunction in heart
failure: Roles of sodium-calcium exchange, inward rectifier
potassium current, and residual beta-adrenergic responsiveness.
Circ Res. 88:1159–1167. 2001. View Article : Google Scholar
|
|
19
|
Pritchard TJ and Kranias EG: Junctin and
the histidine-rich Ca2+ binding protein: potential roles in heart
failure and arrhythmogenesis. J Physiol. 587:3125–3133.
2009.PubMed/NCBI
|
|
20
|
George CH: Sarcoplasmic reticulum Ca2+
leak in heart failure: mere observation or functional relevance?
Cardiovasc Res. 77:302–314. 2008.
|
|
21
|
Stambler BS, Fenelon G, Shepard RK, Clemo
HF and Guiraudon CM: Characterization of sustained atrial
tachycardia in dogs with rapid ventricular pacing-induced heart
failure. J Cardiovasc Electrophysiol. 14:499–507. 2003. View Article : Google Scholar : PubMed/NCBI
|