Introduction
Nanoparticles have been increasingly applied in
biomedical, pharmaceutical and clinical medicine. Among them,
nanosilver is widely used in clinical burns and dental and
urological practices (1–3). In cytotoxicity and animal
experiments, many studies demonstrated that nanosilver had no
toxicity, but had a high antibacterial activity (4–6).
Tian et al (7) identified
that local use of a nanosilver wound dressing not only accelerated
healing, but also improved the appearance of scars. Madhumathi
et al (8) and Ong et
al (9) showed that a
nanosilver/chitosan dressing effectively resists
Staphylococcus, colonic and other bacteria and shows good
hemostatic effects in the treatment of burn wounds.
Growth factors are a class of peptides or proteins
that are able to regulate cell growth and differentiation and
promote tissue healing (10).
Epidermal growth factors (EGFs) have achieved good clinical results
(11), but their in vivo
stability is poor; they are vulnerable to degeneration or
inactivation and are easily diminished in the blood circulation.
Development of a stable, safe and effective preparation has become
a challenging and practical focus for pharmacological research
(12). Existing studies have
demonstrated the role of controlled- or sustained-release
formulations that are prepared with nanoparticles as a carrier.
Carriers effectively protect drugs against inactivation and achieve
sustained, controlled or even targeted release, thus significantly
improving the curative efficacy and reducing toxic side-effects
(13).
Anti-inflammatory nanosilver combines with EGF to
create a sustained-release carrier that has resistance to infection
and sustained-release properties similar to those of EGF. The
resultant carrier is able to promote the repair of skin damage and
compensate for the wound infection-induced low activity when EGF is
used alone. Our research group has preliminarily determined the
optimal particle size and complex conditions for nanosilver and
EGF. In the present study, a sustained-release carrier was prepared
using a 20-nm nanosilver particle and EGF using the self-assembly
method (14) to elucidate the
biological effects and character of the carrier.
Materials and methods
Preparation and characterization of
the silver nanoparticle-EGF sustained-release carrier (NanoAg-EGF)
solution
A 5,000-ppm solution of silver nanoparticles (5 ml)
was magnetically stirred with 50 μg/ml EGF solution (10 ml)
and adjusted with HCl-Tris buffer to pH 7.0. The volume was set at
50 ml and scattered for 30 min with an ultrasonic dispersion
machine. The solution was then placed in a 37°C water bath
overnight for 12 h to obtain a 500-ppm solution of the
sustained-release carrier (final concentration of silver
nanoparticles, 500 ppm; final concentration of EGF, 10
μg/ml). The sustained-release solution (25 ppm) was
similarly prepared (final concentration of silver nanoparticles, 25
ppm; final concentration of EGF, 10 μg/ml). Freeze-dried EGF
powder (1 mg) was dissolved in sterile distilled water to prepare
the EGF-alone (10 μg/ml) and a 500-ppm solution of silver
nanoparticles (1 ml) was set at 10 ml to obtain 500-ppm solution of
silver nanoparticles and was set at 50 ml to obtain 100-ppm
solution of silver nanoparticles. All solutions were stored in a
sterile bottle in a refrigerator at 4°C for further use.
Transmission electron microscopy
The 500-ppm nanosilver particle solution and the
500-ppm NanoAg-EGF solution were observed with transmission
electron microscopy.
Ultraviolet visible (UV-VIS)
spectrophotometry
The 25-ppm sustained-release carrier solution was
centrifuged at 20,000 rpm for 2 h and the supernatant was then
collected for detection. Double distilled water served as a
reference sample adjusted to ‘A0.000’. The test sample was placed
into the cuvette followed by the ordered measurement of the
EGF-alone (10 μg/ml), NanoAg-alone (100 ppm), NanoAg-EGF (25
ppm) and sustained-release supernatant groups.
Cell proliferation experiments with
the NanoAg-EGF
The KMST6 skin fibroblast cell line was resuscitated
and added to a culture medium to obtain a triturated cell
suspension. The cell suspension was subpassaged at
5×105/ml and cultured in a 75-ml culture flask at 37°C
in a 5% CO2 incubator until passages 4–8. The suspension
was then seeded onto a 96-well cell culture plate with
100-μl/well at 37°C in a saturated humidity of 5%
CO2 for 24 h. The culture medium was discarded and the
sample was added to the EGF (10 mg/l), nanosilver solution (500
ppm), nanosilver-EGF (500 ppm) group, nanosilver-EGF combination
group (NanoAg+EGF) and control groups. Each group had eight double
wells; there were four repeated plates in each group with 100
μl of solution in each well. All samples were cultured at
37°C in a 5% CO2 incubator. One culture plate was taken
out at 12, 24, 36 and 48 h for an MTT colorimetric test. The cell
growth curve was then plotted.
Antibacterial tests with the
NanoAg-EGF
Five pathogenic microorganisms, namely
Staphylococcus aureus (ATCC 29213), Escherichia coli
(ATCC 25922), Pseudomonas aeruginosa (10102), Candida
albicans and Streptococcus pneumoniae, were incubated
with the culture medium (including the nutrient broth and agarose
media) at 4°C. Each bacterial species was repeatedly incubated on
five plates; the concentration of bacterial suspension was
estimated turbidimetrically and inoculated onto petri dishes at
concentrations of 5×105 to 5×106 cfu/ml. The
bacterial suspension was smeared onto the surface of a nutrient
agar plate and the petri dishes were dried at room temperature. The
sterile, dried filter paper (5-mm diameter) was collected and added
to 5 μl of the reagents to prepare the antibacterial slices.
The samples were cultured for 24 h in a 37°C incubator. The
diameter of the antibacterial ring was measured with compasses and
a caliper and the measurements were repeated three times.
Wound healing experiments with the
NanoAg-EGF
A wound was made on each side of the spinal cord in
15 rats, which were intramuscularly injected with 5 mg/kg
gentamicin (equivalent to the plasma concentration in human adults)
once daily for 3 days for the systemic anti-infective treatment.
The wounds were randomly assigned to the NanoAg-EGF, NanoAg-alone,
EGF-alone, NanoAg+EGF or normal saline control groups. Each wound
was administered its assigned treatment once daily. The drugs
infiltrated the whole wound by sterile syringe infusion and each
wound received 0.25 ml of drug per treatment. Images of the wounds
were captured on days 3, 7 and 12 and at the time of wound healing.
The non-healing area was calculated using a computer image analysis
system (CAD software). The healing rate was calculated as follows:
Healing rate = (initial wound area − nonhealing area) / initial
wound area × 100.
Statistical analysis
Measurement data are expressed as mean ± standard
deviation and were analyzed using SPSS 13.0 software. Differences
among the groups were compared using an analysis of variance.
Pairwise comparison was performed with the LSD test. P<0.05 was
considered to indicate a statistically significant result.
Results and Discussion
Characterization of the NanoAg-EGF
with transmission electron microscopy
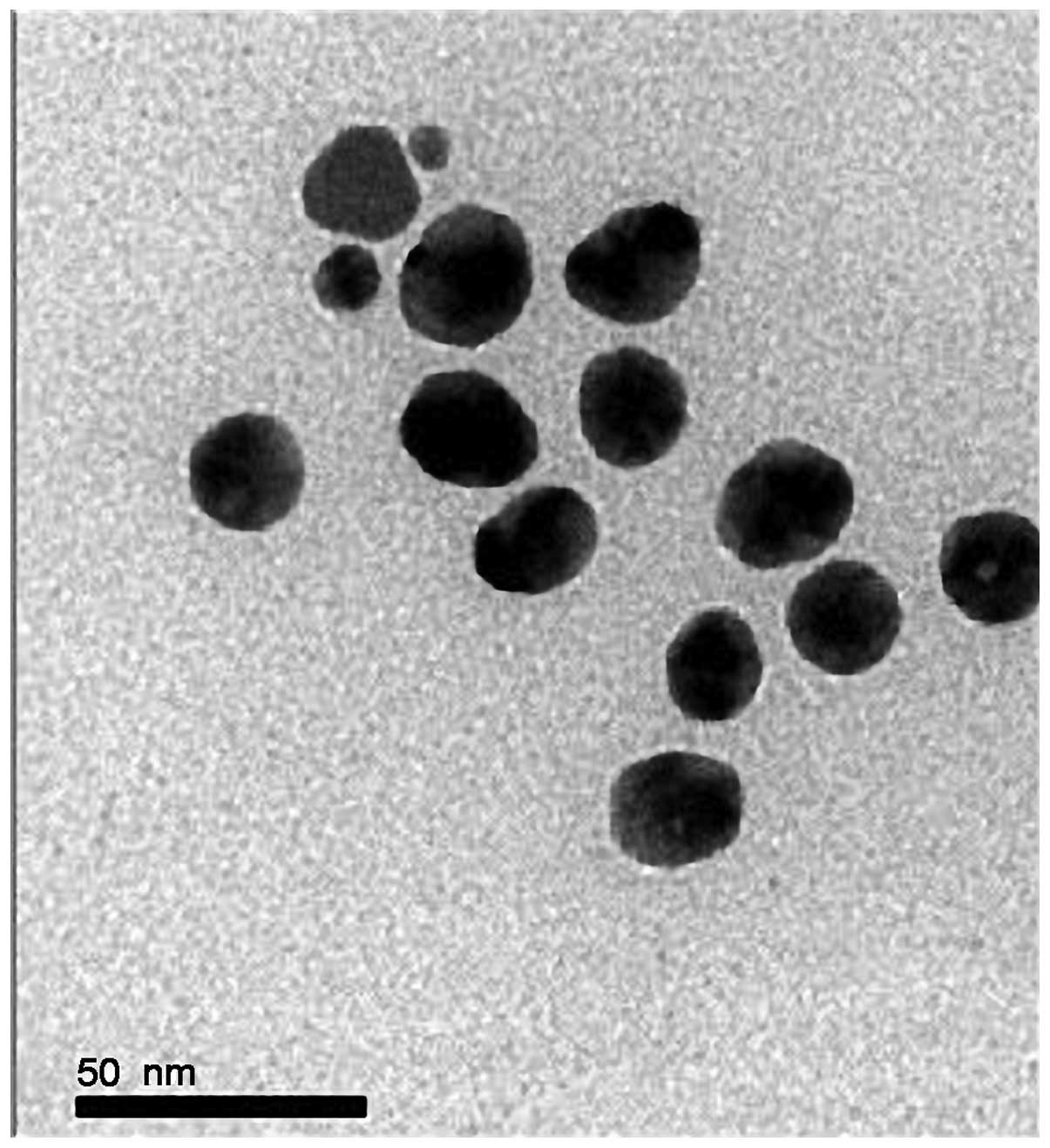
A transmission electron microscopic image of
nanosilver at 500 ppm is shown in Fig.
1. The characterization analysis showed that the silver
nanoparticles were spherical with uniform distribution, showing no
agglomeration or growth, and with a particle size of 15–25 nm. A
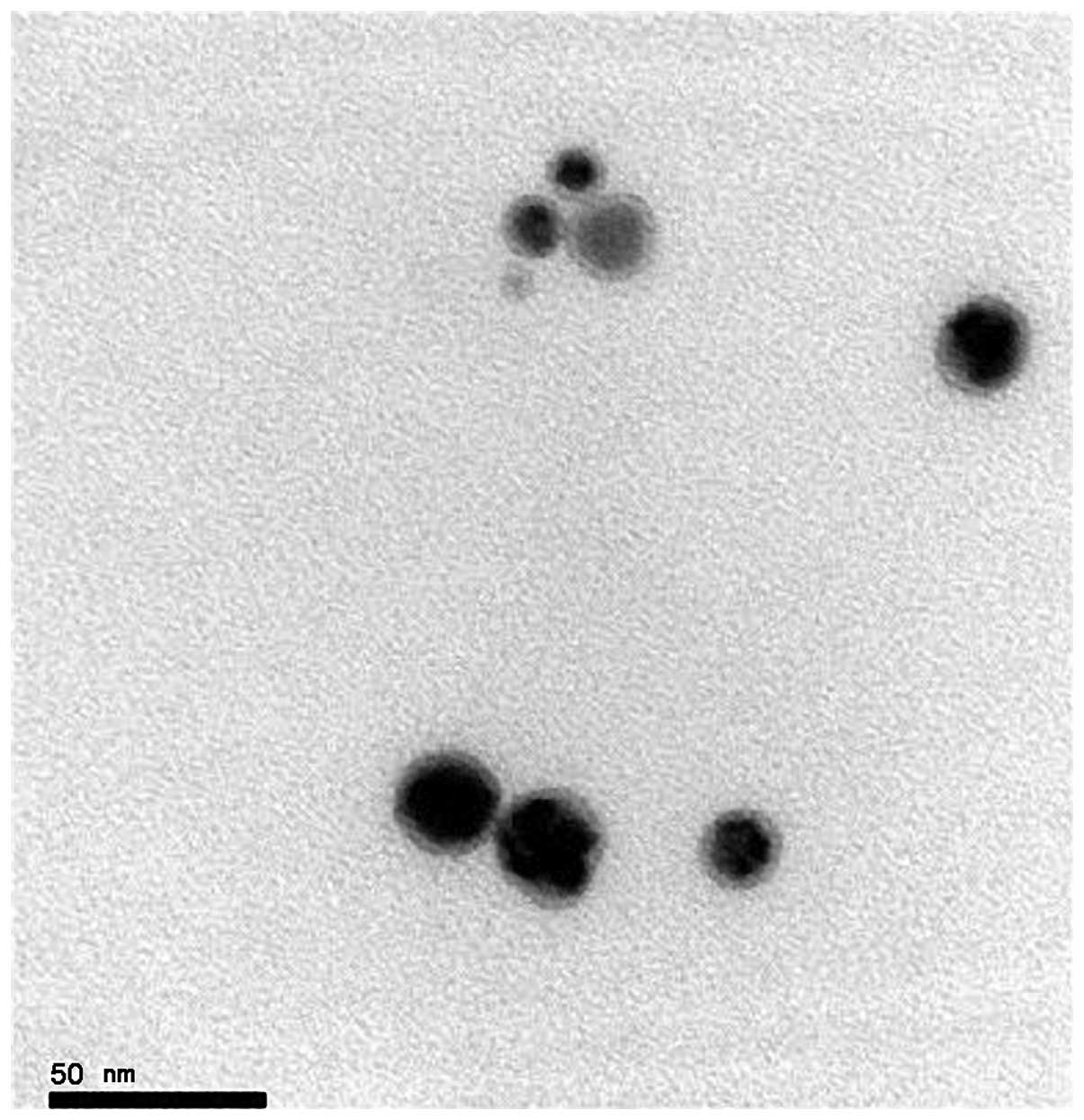
transmission electron microscopic image of the NanoAg-EGF is shown
in Fig. 2. Lightly stained EGF
covered the surface of the spherical silver nanoparticles and
formed a nebula-like shadow, which was surrounded by silver
nanoparticles. This is objective evidence of EGF adhesion on the
silver nanoparticles.
Peptides and proteins are increasingly being used in
clinical practice, and the preparation method using nanoparticles
has been developed (15) so that
they may serve as carriers of these peptide and protein drugs. The
use of biodegradable polymers or inorganic nanoparticles as
carriers of peptides and proteins, thus achieving a
sustained-release effect, is a current research focus (16–18).
UV-VIS characterization
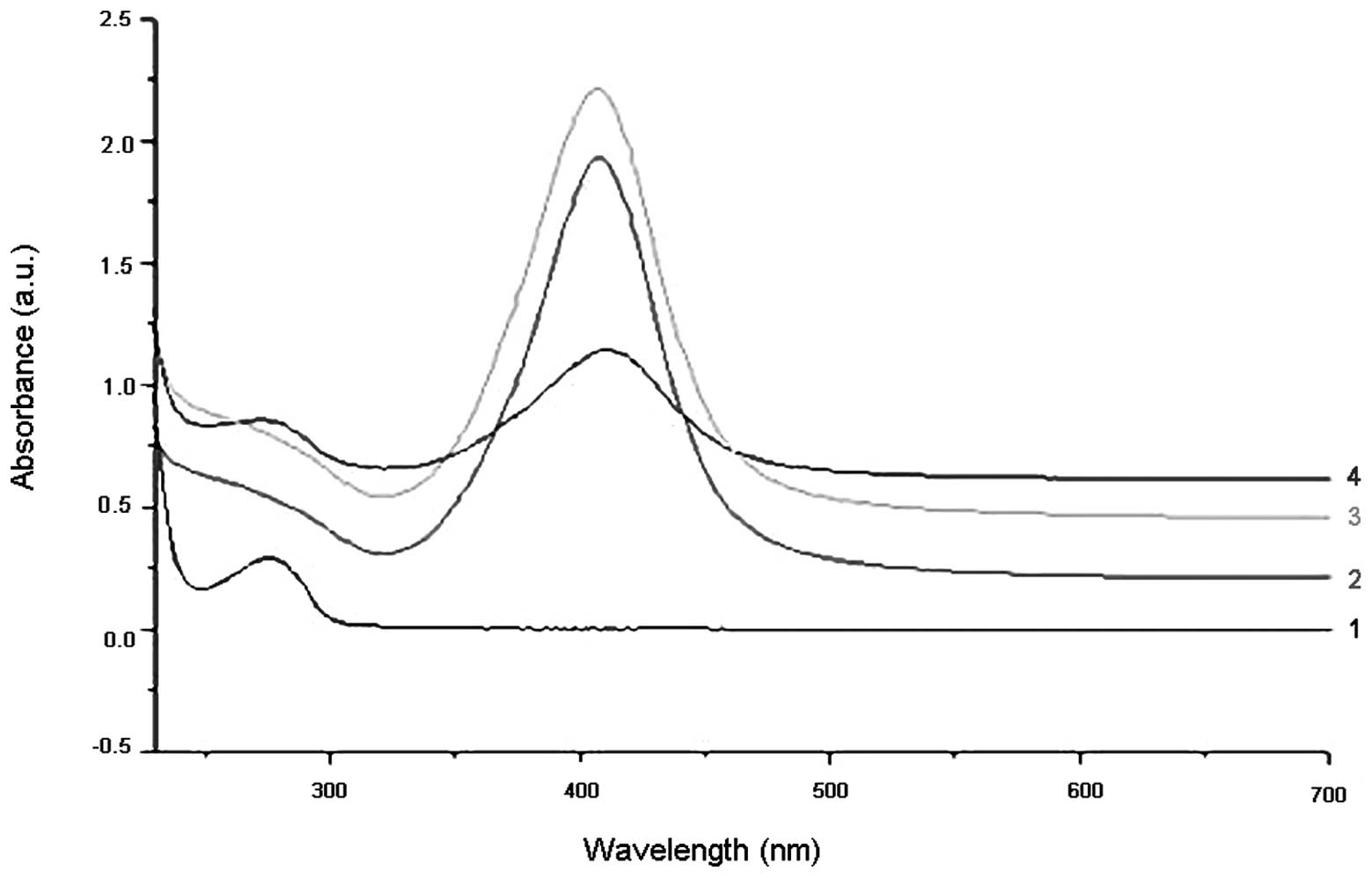
The UV-VIS absorption spectra of the NanoAg-alone,
EGF-alone and NanoAg-EGF solutions are shown in Fig. 3. The first absorption peak in curve
4 was exactly the same as that in curve 1, indicating that free EGF
was present in the NanoAg-EGF solution. The second absorption peak
of curve 4 shifted to the right compared with the absorption peak
in curves 2 and 3, with the UV-VIS absorption peak of the
NanoAg-EGF 5 nm away from that of the silver nanoparticles. This
evidence suggests that the EGF acting with the nanosilver in the
NanoAg-EGF solution produced a nanosilver-EGF complex.
According to the Mie theory (19,20),
the plasma absorption peak gradually red-shifts with increasing
nanoparticle size. When the size of the silver nanoparticles
increased, the plasma absorption peak red-shifted, which is strong
evidence for adhesion of nanosilver to EGF. EGF effectively
adsorbed to the surface of the silver nanoparticles, indicating
that the NanoAg-EGF was successfully prepared. This is consistent
with a previously described outcome (21) showing that nanosilver are able to
act with proteins, resulting in the alteration of their
spectrum.
NanoAg-EGF promotes cell
proliferation
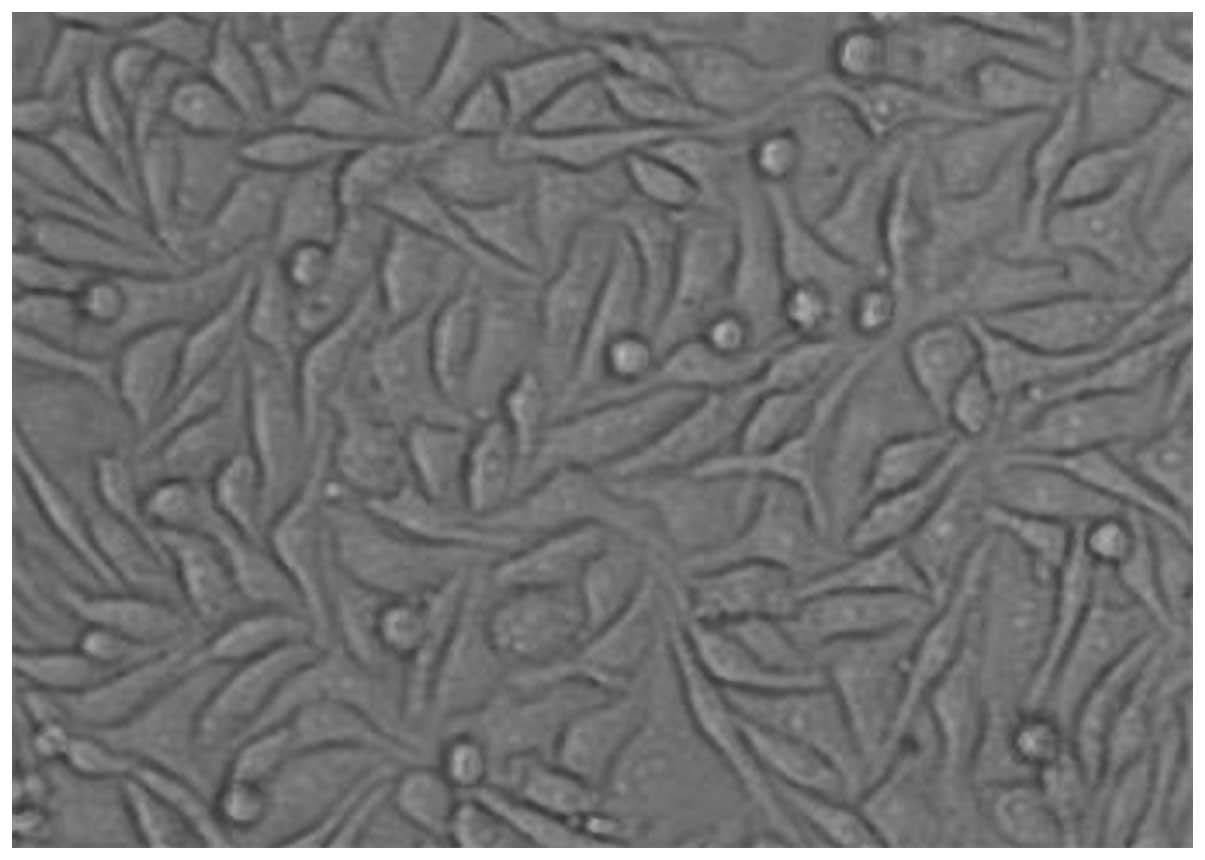
Growth of fibroblast cell culture
Under light microscopy, the number of dermal
fibroblasts was increased, the distribution was dense in the whole
field of vision and the cells were mostly spindle-shaped.
Hematoxylin-eosin staining showed a pink-stained cytoplasm and
blue-stained nuclei. The cells were fusiform-shaped with several
processes or star-shaped and flat. The outlines were clear and the
nuclei were oval. There were no significant differences in the
morphology of the treated cells (Fig.
4).
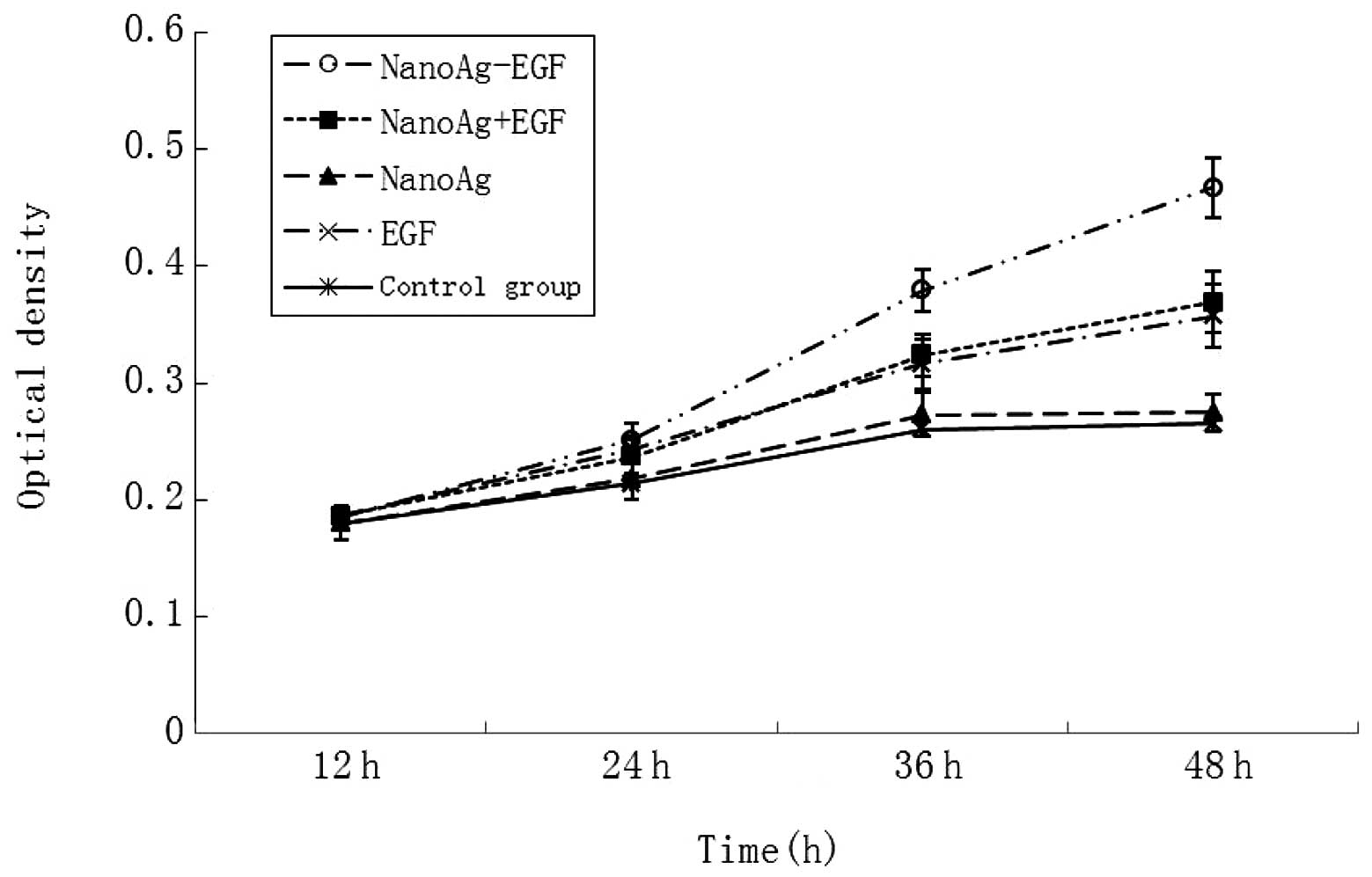
The absorbance value in each group was detected at
12, 24, 36 and 48 h (Fig. 5).
There was no significant difference in the absorbance value of the
human fibroblasts at 12 h (0.180±0.011 vs. 0.186±0.009; P>0.05).
Cell proliferation was apparent as time went by. Cell proliferation
in the groups containing EGF (i.e., the NanoAg-EGF, NanoAg+EGF and
EGF-alone groups) was significantly evident compared with that in
the nanosilver and control groups (P<0.05). At 36 and 48 h, cell
proliferation in the sustained-release carrier group was the most
evident. The absorbance values were 0.359±0.027 and 0.467±0.026,
respectively, which were significantly greater than those in the
combined (0.324±0.022 and 0.359±0.027) and EGF groups (0.316±0.019
and 0.357±0.016; P<0.05). This is evidence that cell
proliferation was faster and more stable following 24h in the
sustained-release carrier group. Accordingly, it is speculated that
the NanoAg-EGF is able to greatly promote cell proliferation and
that this ability is closely attributed to the sustained-release
effect of the silver nanoparticles on EGF.
In the experiments of the present study, the cell
proliferation was promoted to varying degrees in the groups, but no
significant difference was observed. This may be as the biological
effects on the promotion of cell proliferation remained in the
initial phase within a short duration of EGF action. Cell protein,
DNA and RNA synthesis was significantly increased, but no
quantitative change in cell number was identified. When fibroblasts
were treated with EGF, the cell cycle duration was ∼10 h and DNA
synthesis started at 8 h and became active. Subsequent to 24 h of
cell culture, the number of cells increased in the NanoAg-EGF,
EGF-alone and NanoAg+EGF groups with significant differences
compared with the NanoAg-alone and control groups. This provides
evidence that EGF promoted cell proliferation. Subsequent to 36 h
of cell culture, the number of cells in the NanoAg-EGF group was
significantly higher than that of the EGF-alone and NanoAg+EGF
groups, suggesting that the concentration of sustained-release
carrier was better than that of the EGF-alone and explaining the
cell proliferation at 36 and 48 h.
Antibacterial test of NanoAg-EGF
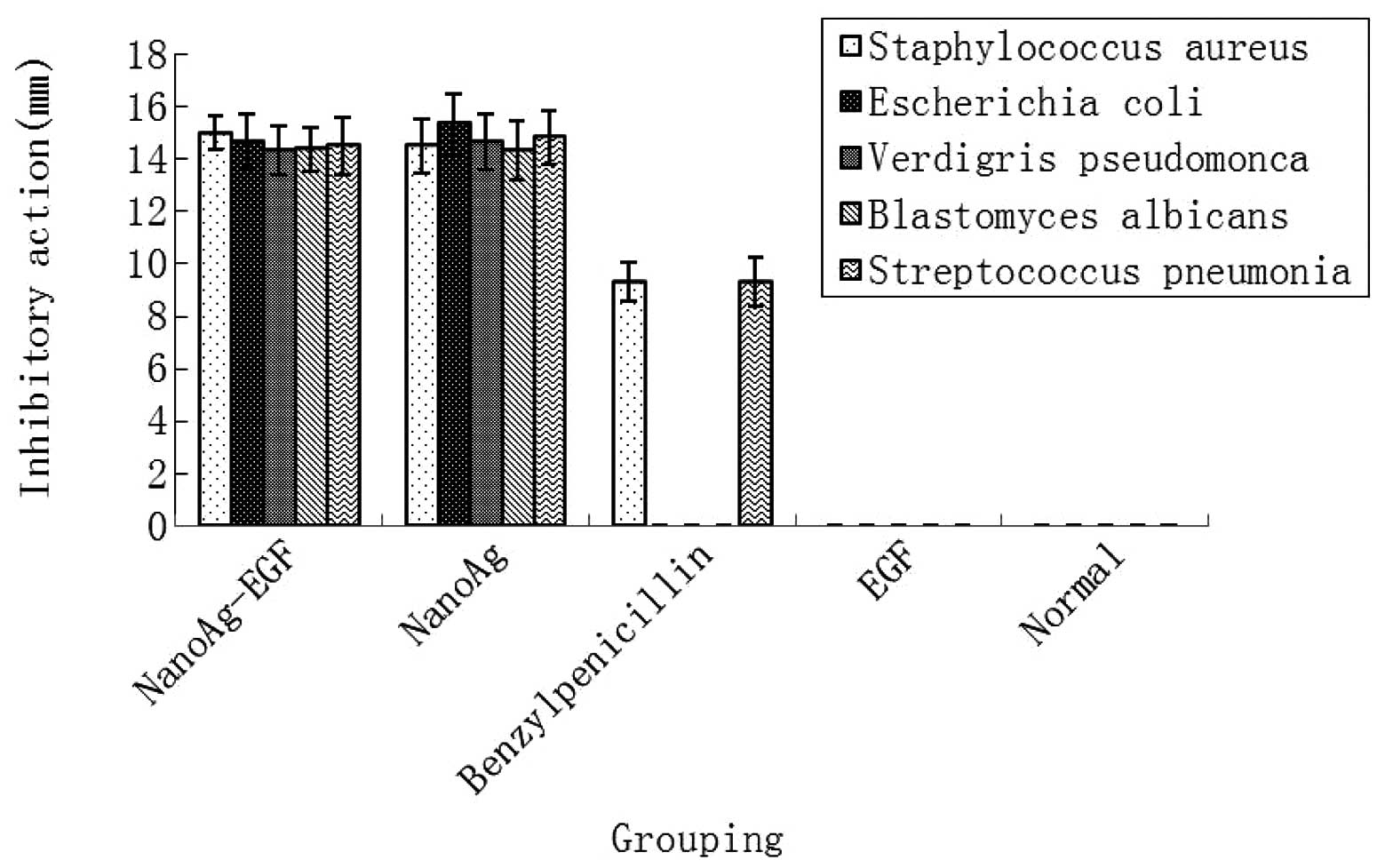
The inhibitory action of each treatment group was
compared in five pathogenic microorganisms. As shown in Fig. 6, the NanoAg-EGF and NanoAg-alone
groups showed good antibacterial properties against five pathogenic
microorganisms, namely Staphylococcus aureus, Escherichia
coli, Verdigris pseudomonas, Blastomyces albicans
and Streptococcus pneumoniae, with no statistically
significant difference in antimicrobial resistance between the two
groups (P>0.05). In the positive-control benzylpenicillin group,
weak antibacterial activity occurred only against Staphylococcus
aureus and Streptococcus pneumoniae and was
significantly lower than that in the NanoAg-EGF and NanoAg-alone
groups (P<0.05). In the normal saline control and EGF groups,
there was no antibacterial effect on the five pathogenic
microorganisms.
The antibacterial effect of the NanoAg-EGF was
determined. The NanoAg-EGF and nanosilver groups showed strong
inhibitory actions against the five pathogenic organisms, while the
EGF-alone and normal saline control groups showed no inhibitory
effects. In the positive-control group, benzylpenicillin sodium was
only resistant to Staphylococcus aureus and the
antibacterial effect was significantly lower than that of the
NanoAg-alone and NanoAg-EGF groups. There was no significant
difference between these two groups. The present experiments not
only validate the antibacterial effect of nanosilver, but also
confirm that nanosilver has a good inhibitory effect on
Staphylococcus aureus and Pseudomonas aeruginosa,
which readily demonstrate drug resistance.
NanoAg-EGF promotes wound healing
Morphological observations
The wounds in the rats of the NanoAg-EGF group were
cleaner than those in the other groups, with less leakage. The rats
also had a mental status that was close to that of normal rats, a
normal diet, vigorous activity and no hair loss. Wound healing in
the other groups was relatively poor or even difficult, with more
secretions and surrounding swelling, leading to formation of
chronic ulcers.
NanoAg-EGF promotes wound healing in
animal experiments
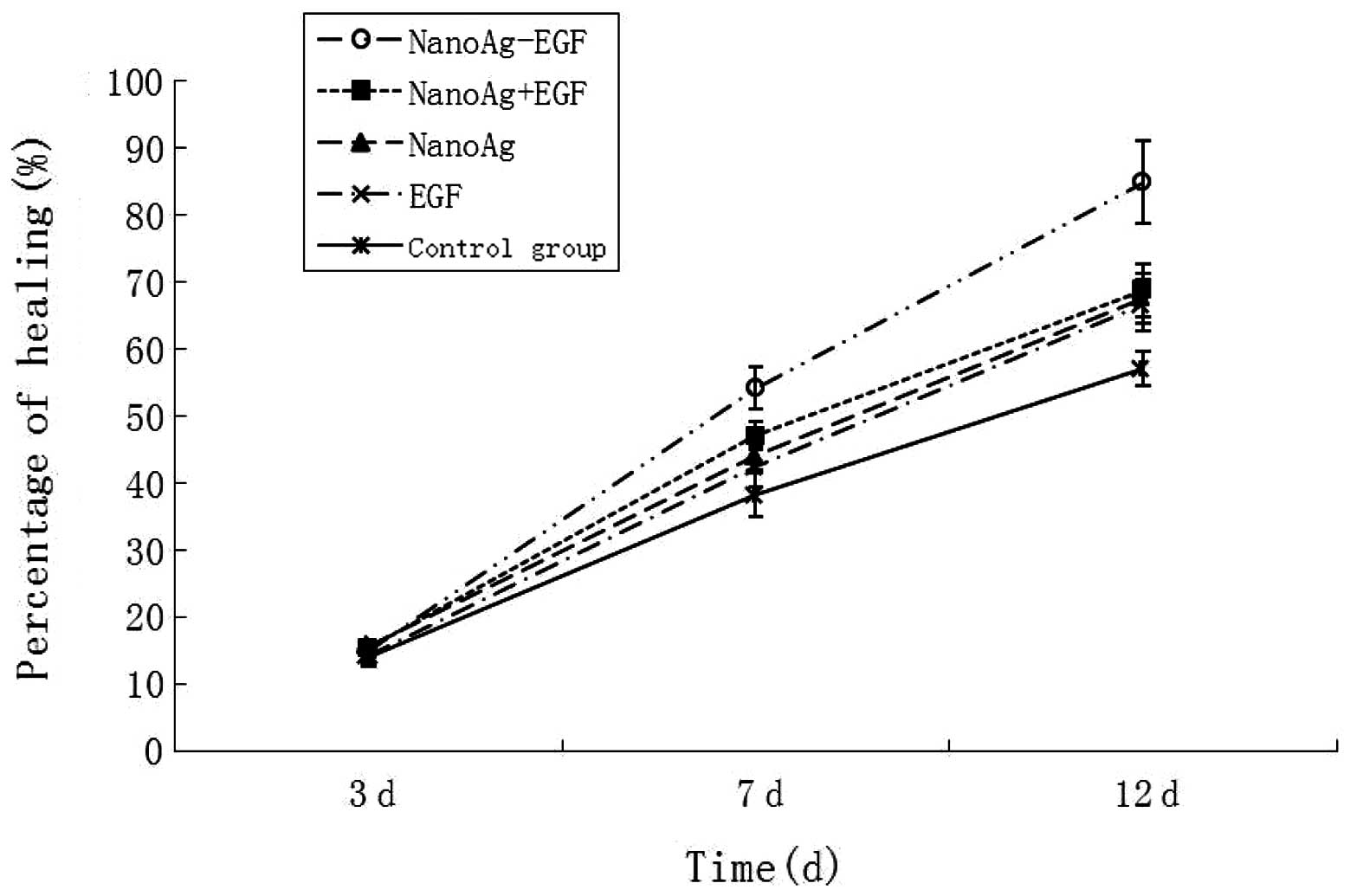
The statistical data on the wound-healing rate in
each group are shown in Fig. 7.
The wound-healing rate at 3 days post-treatment in each group
ranged from 14.105±1.098 to 15.814±1.518% with no significant
difference between the groups (P>0.05) at 7 and 12 days. The
healing rates in the NanoAg-EGF group were 54.19±3.1137 and
84.933±6.147%, respectively, which were significantly higher than
those in the other four groups (P<0.05). The wound-healing
duration in the NanoAg-EGF group was 14.75±1.603 days, which was
significantly shorter than that of the other four groups
(NanoAg+EGF group, 17.25±1.422 days; EGF-alone group, 20.167±1.697
days; NanoAg-alone group, 17.083±1.505 days; and control group,
20.333±1.303 days; P<0.05). Thus, the wound-healing rate and
duration were the highest and shortest, respectively, in the
NanoAg-EGF group.
Numerous measures are used to improve wound-healing
duration and quality, including infection control, active removal
of necrotic tissue, correction of metabolism and application of
exogenous growth factors. Wound healing is a key problem in plastic
surgery and related research, and therefore the issue of how to
speed up wound healing is evident in clinical research (22). In the present study, there was no
significant difference in the wound-healing rate of each group at 3
days post-surgery, indicating that EGF was ineffective in the
promotion of wound healing in the early inflammatory stage. Even if
anti-infection measures are performed in a timely manner, wound
edema and acute infection occur at 2 to 3 days post-trauma. Due to
the marked change in the surrounding environment, cells on the
wound surface remain in the shock stage and growth factors are not
available. In addition, a certain amount of time is required to
upregulate the exogenous EGF receptor, thus, no promotion of wound
healing was identified in the NanoAg-EGF group at 3 days in the
present study. The healing rate reached a peak in the NanoAg-EGF
group 7 days subsequent to the injury with significant differences
when compared with the other groups; the differences were most
significant with time. The wound-healing duration in the NanoAg-EGF
group was 4 to 5 days shorter than in the saline group and 2 to 3
days shorter than in the combination group. Although the
combination group showed a better ability to promote wound healing
at all time-points, there was no significant difference compared
with the EGF-alone and NanoAg-alone groups. Therefore, it is
speculated that combining the silver nanoparticles with EGF is not
able to lead to a qualitative change in wound healing. In the
NanoAg-EGF group, the healing rate was significantly higher than
that of the other groups at 7 days, suggesting that the new
formulations are able to avoid wound hydrolysis, induce a sustained
and steady release of EGF and protect factors from wound hydrolysis
and bacterial destruction prior to the adherent growth factor
detaching from the silver nanoparticles. When the amount of growth
factors on the wound surface decreases, the adherent growth factor
gradually becomes free from the nanosilver and then binds with
receptors that are able to repair cells and promote cell
proliferation. Therefore, the wounds maintain a relatively high
concentration of growth factors and wound healing is
accelerated.
In conclusion, the experimental findings of the
present study confirm that the described NanoAg-EGF solution is
able to disperse well, that EGF adheres to the surface of silver
nanoparticles and that growth factor activity and antimicrobial
resistance coexist and effectively promote wound healing. Further
studies are required to conclusively determine the clinical
application and significance of these results.
Acknowledgements
This study was supported by the
Program for New Century Excellent Talents in University
(NCET-11-0527), the Fundamental Research Funds for the Central
Universities (No. 2011JQ028), the HNSF funds of Hunan Province (No.
11JJ6085) and the Hunan Provincial Science and Technology Project
(Nos. 2011TT2041, 2008SK3114 and 2010SK3113).
References
|
1
|
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee
HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH
and Cho MH: Antimicrobial effects of silver nanoparticles.
Nanomedicine. 3:95–101. 2007. View Article : Google Scholar
|
|
2
|
Chekman IS, Ulberg ZR, Gorchakova NO,
Nebesna TY, Gruzina TG, Priskoka AO, Doroshenko AM and Simonov PV:
The prospects of medical application of metal-based nanoparticles
and nanomaterials. Lik Sprava. 3–21. 2011.PubMed/NCBI
|
|
3
|
Dallas P, Sharma VK and Zboril R: Silver
polymeric nanocomposites as advanced antimicrobial agents:
classification, synthetic paths, applications, and perspectives.
Adv Colloid Interface Sci. 166:119–135. 2011.
|
|
4
|
Arora S, Jain J, Rajwade JM and Paknikar
KM: Cellular responses induced by silver nanoparticles: In vitro
studies. Toxicol Lett. 179:93–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ai J, Biazar E, Jafarpour M, Montazeri M,
Majdi A, Aminifard S, Zafari M, Akbari HR and Rad HG:
Nanotoxicology and nanoparticle safety in biomedical designs. Int J
Nanomedicine. 6:1117–1127. 2011.PubMed/NCBI
|
|
6
|
Teow Y, Asharani PV, Hande MP and
Valiyaveettil S: Health impact and safety of engineered
nanomaterials. Chem Commun (Camb). 47:7025–7038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian J, Wong KK, Ho CM, Lok CN, Yu WY, Che
CM, Chiu JF and Tam PK: Topical delivery of silver nanoparticles
promotes wound healing. Chem Med Chem. 2:129–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Madhumathi K, Sudheesh Kumar PT, Abhilash
S, Sreeja V, Tamura H, Manzoor K, Nair SV and Jayakumar R:
Development of novel chitin/nanosilver composite scaffolds for
wound dressing applications. J Mater Sci Mater Med. 21:807–813.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ong SY, Wu J, Moochhala SM, Tan MH and Lu
J: Development of a chitosan-based wound dressing with improved
hemostatic and antimicrobial properties. Biomaterials.
29:4323–4332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie HQ, Zhou JD, Luo CQ, Chen Y, Xia K and
Chen DJ: Construction of the eukaryotic expression plasmid
containing human epidermal growth factor gene with signal peptide.
Zhongguo Yi Shi Za Zhi. 8:189–191. 2006.(In Chinese).
|
|
11
|
Kiyohara Y, Komada F, Iwakawa S, Hirai M,
Fuwa T and Okumura K: Improvement in wound healing by epidermal
growth factor (EGF) ointment. II. Effect of protease inhibitor,
nafamostat, on stabilization and efficacy of EGF in burn. J
Pharmacobiodyn. 14:47–52. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Değim Z, Çelebi N, Alemdaroğlu C, Deveci
M, Öztürk S and Özoğul C: Evaluation of chitosan gel containing
liposome-loaded epidermal growth factor on burn wound healing. Int
Wound J. 8:343–354. 2011.PubMed/NCBI
|
|
13
|
Vaiana CA, Leonard MK, Drummy LF, Singh
KM, Bubulya A, Vaia RA, Naik RR and Kadakia MP: Epidermal growth
factor: layered silicate nanocomposites for tissue regeneration.
Biomacromolecules. 12:3139–3146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janjua M, Nudurupati S, Singh P and Aubry
N: Electric field-induced self-assembly of micro- and nanoparticles
of various shapes at two-fluid interfaces. Electrophoresis.
32:518–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhen M, Wang ZS and Zhu YW: Preparation of
silver nanoparticle via active template under ultrasonic. Zhong Nan
Da Xue Xue Bao Yi Xue Ban. 6:2006.(In Chinese).
|
|
16
|
Tan ML, Choong PF and Dass CR: Recent
developments in liposomes, microparticles and nanoparticles for
protein and peptide drug delivery. Peptides. 31:184–193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
del Pozo-Rodríguez A, Delgado D, Solinís
MA and Gascón AR: Lipid nanoparticles as vehicles for
macromolecules: nucleic acids and peptides. Recent Pat Drug Deliv
Formul. 5:214–226. 2011.PubMed/NCBI
|
|
18
|
Harde H, Das M and Jain S: Solid lipid
nanoparticles: an oral bioavailability enhancer vehicle. Expert
Opin Drug Deliv. 8:1407–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Papoff F and Hourahine B: Geometrical Mie
theory for resonances in nanoparticles of any shape. Opt Express.
19:21432–21444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhandari A, Hamre B, Frette Ø, Stamnes K
and Stamnes JJ: Modeling optical properties of human skin using Mie
theory for particles with different size distributions and
refractive indices. Opt Express. 19:14549–14567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai DH, Davila-Morris M, DelRio FW, Guha
S, Zachariah MR and Hackley VA: Quantitative determination of
competitive molecular adsorption on gold nanoparticles using
attenuated total reflectance-Fourier transform infrared
spectroscopy. Langmuir. 27:9302–9313. 2011. View Article : Google Scholar
|
|
22
|
Zhou JD, Chen DJ, Chen Y, Li P, Li GF, He
QY, Chen TF, Zhu J, Peng H, Xia K and Luo CQ: Human VEGF121 gene
transfected adult dermal fibroblasts in vitro. Zhonghua Shi Yan Wai
Ke Za Zhi. 21:1539–1541. 2004.(In Chinese).
|