Introduction
Mild cognitive impairment (MCI) is currently
considered an early stage of dementia, which has no effective
treatment. Reducing the progression of cognitive decline at the MCI
stage may be an important strategy for preventing conversion to
dementia (1). Carotid stenosis is
known to be an independent risk factor in the transformation
process of MCI to dementia (1–3).
Cerebral perfusion deficiency caused by hemodynamic changes and
cerebral emboli plays two key roles in this process (4–6).
Cerebral emboli and hypoperfusion are ameliorated by angioplasty
(7,8). Carotid artery stenting (CAS) has been
shown to prevent the occurrence of strokes safely and effectively
in multi-center studies (9,10);
however, the effects of CAS on cognitive outcome in patients with
carotid artery stenosis are controversial (11,12).
A number of factors may lead to the variation in cognitive
responses observed in the clinic, including differences in baseline
cerebral perfusion status, detrimental effects on procedural
emboli, temporary flow interruption and the beneficial effect of
improved cerebral hemodynamics.
In this prospective study, we aimed to investigate
the effect of CAS on neurocognitive function in patients with
carotid stenosis and MCI.
Patients and methods
Subjects
A total of 240 inpatients with carotid stenosis and
MCI were consecutively selected from the Department of Neurology,
Daping Hospital, Chongqing from January 2008 to January 2011. They
were assigned to a treatment group (CAS + drugs therapy, 167 cases)
or a control group (simple drug therapy, 73 cases) according to
patient preference. Eligibility requirements were: i) patients aged
55 years and older; ii) patients with symptomatic carotid stenosis
>50% or asymptomatic carotid stenosis >70%, measured
according to the North American Symptomatic Carotid Endarterectomy
Trial (NASCET) criteria or its noninvasive equivalent (13); and iii) patients who were diagnosed
with MCI. Exclusion criteria were: i) evidence of other significant
stenosis (>50%) in the major arteries of the head or neck; ii)
evidence of an acute cerebral infarction requiring emergency
thrombolysis and/or emergency stent placement; iii) patients who
had experienced a recent stroke (within 4 weeks, given the
potential impact on cognitive function); iv) a history of previous
subarachnoid or cerebral hemorrhage; v) a concomitant neurological
disorder potentially affecting cognitive function, including severe
Parkinson’s disease; vi) being unable to comply with the study
assessment; vii) a mental illness or a score on the Hamilton
Depression Rating Scale (HDRS) >17; viii) drug abuse; and ix)
moving away or declining to participate.
Protocol approvals and patient
consent
This study was approved by the Institutional Review
Board of the Third Military Medical University and all subjects and
their care-givers provided informed consent. Registration number:
ChiCTR-ONRC-12001879.
Baseline data
At inclusion, data were collected on presenting
symptoms, demographic characteristics and vascular risk factors
(VRFs). Demographic data was composed of age, gender and
educational level (lower educational level refers to an education
time ≤6 years; higher educational level refers to an education time
>6 years). The VRFs included hypertension, diabetes,
hyperlipidemia, prior ischemic event, coronary artery disease,
atrial fibrillation, current smoking habit and daily alcohol
consumpton. The severity of carotid stenosis was grouped into
moderate stenosis (51–69%) and severe stenosis (≥70%) by digital
subtraction angiography (DSA) or computed tomography (CT)
angiograms according to the NASCET method (13). The score on the National Institutes
of Health Stroke Scale (NIHSS) (14) was assessed at baseline and at 1 day
and 6 months after the procedure.
Neuropsychological examinations
(NPEs)
Cognition was assessed in the week preceding the
procedure and 6 months after the procedure. NPEs were performed by
two trained clinical neuropsychologists, who were blind to the
outcome of the treatment. Mini-Mental State Examination (MMSE) and
the Barthel Index of Activities of Daily Living (ADL), which were
validated previously in elderly Chinese individuals (15,16),
were used. The subjects with an abnormal MMSE score were assessed
with HDRS to measure emotional status (17). Subsequently, a set of
neuropsychological tests were applied, including Montreal Cognitive
Assessment (MOCA), which identifies substantially more cognitive
abnormalities following a transient ischemic attack (TIA) and
stroke than the MMSE, to identify deficits in executive function,
attention and delayed recall (18); Fuld Object Memory Evaluation (FOME)
to detect extensive cognitive dysfunction mainly composed of memory
(19); rapid verbal retrieval
(RVR) to detect the function of semantic memory (20); and Wechsler Adult Intelligence
Scale (WAIS) to evaluate immediate memory and function of graphical
recognition (21).
Diagnosis of MCI
The clinical diagnosis of MCI was conducted
according to the established Petersen criteria (22), including: i) subjective complaint
of memory deficits; ii) abnormal memory functioning for age [tests
claim 1.5 standard deviation (SD) below normative values]; iii)
absence of dementia according to the diagnostic examination [MMSE
≥24 in subjects with higher educational level; MMSE ≥20 in subjects
with lower educational level; Clinical Dementia Rating (CDR) ≤0.5];
and iv) normal everyday functioning on ADL (<40). Subjects with
depressive disorder were excluded (23).
Treatment process and clinical
follow-up
CAS was performed in the week after the patients
were assigned to the treatment group, by routine use of an umbrella
stent. Technical success was defined as implantation of a stent
with a residual stenosis ≤30%; however, for patients with stenosis
>90%, to reduce the risk of high perfusion syndrome
postoperatively (24), residual
stenosis was extended to ∼60%. Aspirin and clopidogrel were
continued for 3 days before the procedure until 6 months after
successful intervention. Patients in the control group were treated
with the same oral medication as the treatment group. VRFs were
carefully controlled in the two groups by management of blood
pressure and blood glucose, as well as use of statins. Complete
neurologic examinations were performed by an independent
neurologist before, 1 week after and 6 months after treatment.
Restenosis (restenosis rate >50%), ipsilateral ischemic events,
neurologic sequelae, intracranial hemorrhages and mortalities were
recorded. Follow-up clinical and ultra-sound examinations were
scheduled at 6 months after therapy.
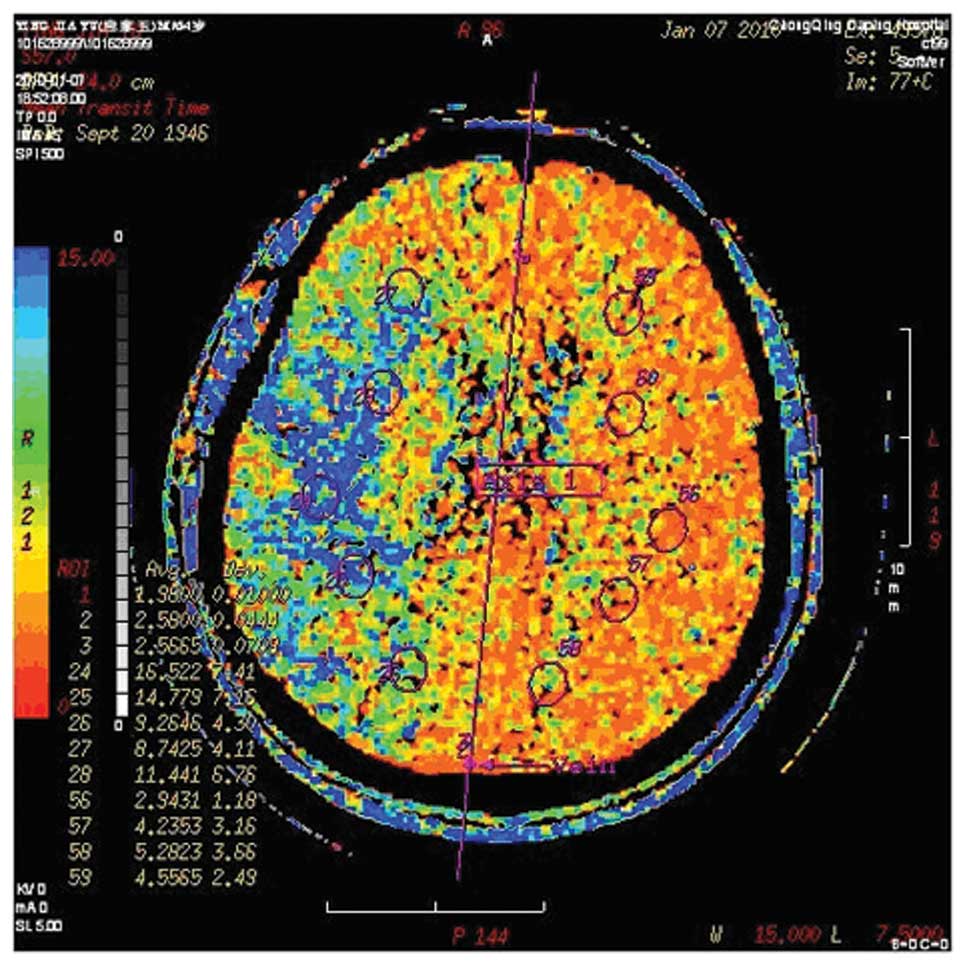
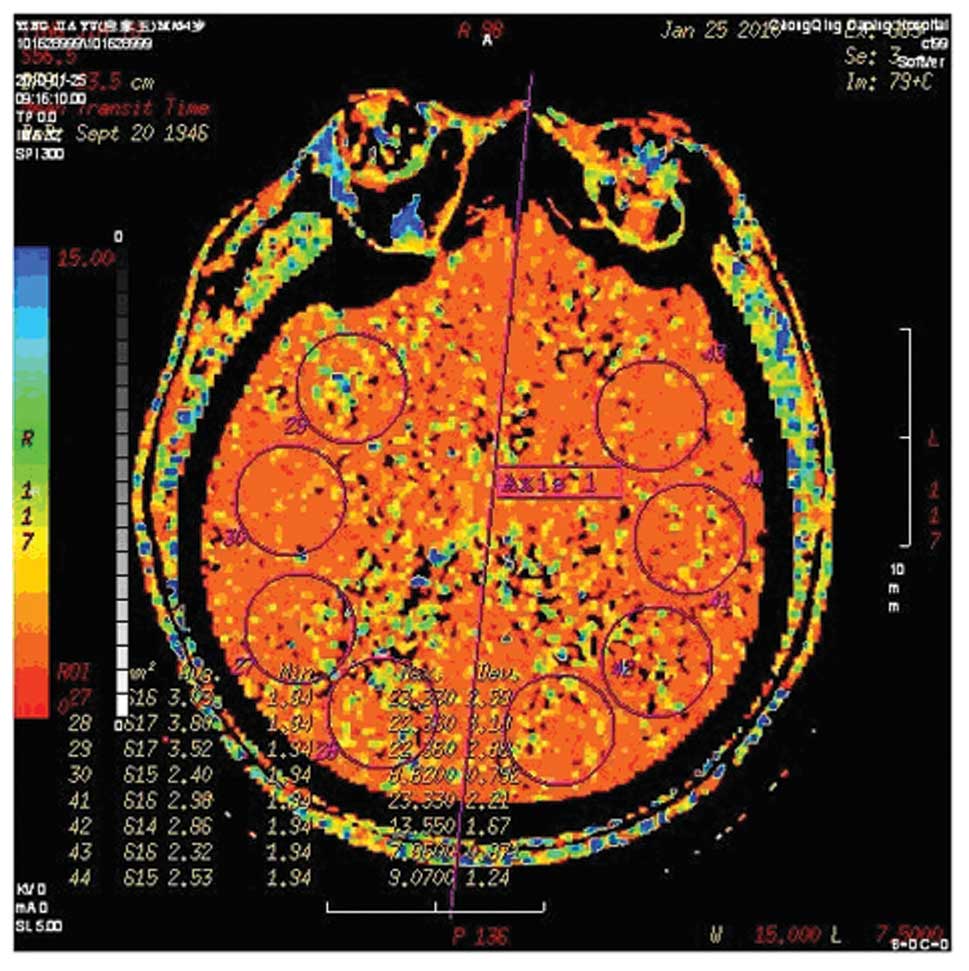
Computed tomography perfusion (CTP)
Brain CTP and CT angiography using a LightSpeed VCT
64-slice Scanner (GE Healthcare, Milwaukee, WI, USA) were scheduled
in before and 3 weeks after treatment. Assessment of cerebral
perfusion (at inclusion and follow-up) was performed by two
independent investigators who were unaware of the clinical and
angio-graphic outcomes. CTP data were analyzed on an advanced
workstation (Advantage 4.2, GE Healthcare). Cerebral blood volume
(CBV), cerebral blood flow (CBF), time to peak (TP) and mean
transit time (MTT) were calculated. A grading system was used for
qualitative assessment of the brain perfusion of the region of
interest: 0, complete perfusion; 1, hypoperfusion with preserved
CBV (lower CBF, delayed TP, increased MTT, decreased flow and
normal or elevated CBV); and 2, hypoperfusion with decreased CBV.
Improvement in brain perfusion after the procedure was defined as
at least a 1 categorical number decrease in the region of interest
according to the grading system (25). Figs.
1 and 2 are images of a
patient who had significant improvement in ipsilateral brain
perfusion following right carotid stenting.
Statistical analysis
Continuous data are presented as mean ± SD. Discrete
data are presented as counts and percentages. T-tests were used for
continuous and normally distributed data and Chi-square analyses
for comparing groups of categorical data. Paired continuous data
were compared by the Wilcoxon signed rank sum test. Pearson’s
correlation coefficients were used to assess the correlation
between the change in brain perfusion and the changes in the
results of neuropsychological tests. A two-sided P-value <0.05
was considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS 18.0 for Windows
(SPSS, Inc., Chicago, IL, USA).
Results
Among the 240 patients registered in this study, 208
patients (144 in the CAS group and 64 in the control group)
finished the NPEs and analysis of cognitive scores after treatment
and 6 months of follow-up. The other 32 patients were excluded due
to the following reasons: stenosis of 11 patients at the time of
angiography did not conform to the enrollment criteria (<50%
carotid stenosis in 4 cases and severe intracranial stenosis in 7
cases); 2 patients were unable to take the medicine on time at the
proper dosage; 3 patients had severe dysphasia, hearing and visual
impairment and worsening illness precluding evaluation; 3 patients
refused NEP assessment; 2 patients in the CAS group crossed over to
the control group and 1 patient in the control group crossed over
to the CAS group and then left the study. Additionally, 4 patients
(2 in the CAS group and 2 in the control group) were disabled due
to complications and 6 patients (4 in the CAS group and 2 in the
control group) refused to follow-up and left the study. The
pretreatment CTP examinations were performed in 155 of the 218
patients (71%) and the post-treatment scan in 120 patients
(58%).
Technical success was achieved in all patients in
the CAS group. Following stent placement, the severity of carotid
stenosis decreased to 21% (0–60%) vs. 68% (50–96%) preoperatively.
The stenosis was left-sided in 62.5% of patients. In the 6 month
follow-up, we observed stent restenosis in 4 patients (2.8%),
ipsilateral cerebral infarction in 3 patients (2.1%) and
ipsilateral TIA in 4 patients (2.8%). Of the 64 patients in the
control group, 2 patients (3.1%) had ipsilateral cerebral
infarction and 3 patients (4.7%) had ipsilateral TIA.
The patients in the two groups did not differ with
regard to baseline characteristics, educational level, VRFs and
NPEs prior to the procedure (Table
I).
 | Table IBaseline patient characteristics
(n=208). |
Table I
Baseline patient characteristics
(n=208).
| Characteristics | CAS group
(n=144) | Control group
(n=64) | P-value |
|---|
| Age (years) | 67.0±7.8 | 69.3±7.7 | 0.59 |
| Females | 48 (33.3) | 22 (34.4) | 0.88 |
| Lower education level
(≤6 years) | 57 (39.6) | 24 (37.5) | 0.78 |
| Hypertension | 97 (67.4) | 43 (65.6) | 0.98 |
| Diabetes
mellitus | 42 (29.2) | 22 (34.4) | 0.45 |
| Hyperlipidemia | 66 (45.8) | 26 (40.6) | 0.49 |
| Prior ischemic
event | 79 (54.9) | 36 (56.3) | 0.85 |
| Coronary artery
disease | 26 (18.1) | 10 (15.6) | 0.67 |
| Atrial
fibrillation | 4 (2.8) | 2 (3.1) | 0.89 |
| Smoking habit | 34 (23.6) | 14 (21.9) | 0.78 |
| Daily alcohol
consumption | 30 (20.8) | 16 (25.0) | 0.50 |
| Severe carotid
stenosis (>70%) | 61 (42.4) | 24 (37.5) | 0.51 |
| Left carotid
stenosis | 90 (62.5) | 38 (59.4) | 0.67 |
| NIHSS score | 0.72±1.16 | 0.69±1.15 | 0.87 |
| ADL | 23.0±2.7 | 23.1±2.8 | 0.88 |
| MMSE | 24.6±1.7 | 24.7±1.5 | 0.65 |
| MOCA | 23.7±1.7 | 23.8±1.5 | 0.60 |
| FOME | 13.8±2.2 | 14.2±2.3 | 0.17 |
| RVR | 25.7±2.1 | 26.0±1.9 | 0.45 |
| WAIS-DS | 6.7±2.1 | 6.8±2.0 | 0.86 |
Table II shows
neurocognitive and neurologic functions at baseline and after 6
months in the CAS and control groups. In the CAS group, we observed
significant improvements in the MMSE (before, 24.6±1.7 vs. after,
24.8±1.9; P=0.016), MOCA (before, 23.7±1.7 vs. after, 24.1±2.0;
P=0.006), FOME (before, 13.8±2.2 vs. after, 14.0±2.3; P=0.031) and
WAIS-DS (before, 6.7±2.1 vs. after, 6.9±2.3; P=0.040). The change
in MOCA was the most significant and RVR (before, 25.7±2.1 vs.
after, 25.9±2.3; P=0.201) also exhibited an increasing trend. In
comparison, all test parameters were decreased at follow-up in the
control group, however the reductions were not statistically
significant. NIHSS and ADL values were similar in the two groups at
the 6 month follow-up compared with baseline results.
 | Table IINeuropsychologic test scores at
baseline and follow-up. |
Table II
Neuropsychologic test scores at
baseline and follow-up.
| CAS group (n=144)
| Control group
(n=64)
|
|---|
| Test | Baseline | 6 months after
stenting | P-value | Baseline | 6 months after
medication | P-value |
|---|
| MMSE | 24.6±1.7 | 24.8±1.9 | 0.016 | 24.7±1.5 | 24.5±1.6 | 0.137 |
| MOCA | 23.7±1.7 | 24.1±2.0 | 0.006 | 23.8±1.5 | 23.6±1.8 | 0.129 |
| FOME | 13.8±2.2 | 14.0±2.3 | 0.031 | 14.2±2.3 | 14.1±2.5 | 0.171 |
| RVR | 25.7±2.1 | 25.9±2.3 | 0.201 | 26.0±1.9 | 25.8±2.0 | 0.144 |
| WAIS-DS | 6.7±2.1 | 6.9±2.3 | 0.040 | 6.8±2.0 | 6.6±1.9 | 0.158 |
| ADL | 23.0±2.7 | 22.9±2.6 | 0.239 | 23.1±2.8 | 23.0±2.6 | 0.591 |
| NIHSS | 0.72±1.16 | 0.67±1.05 | 0.294 | 0.69±1.15 | 0.63±1.00 | 0.415 |
Of the 84 patients in the CAS group who received CTP
follow-up, 72 (86%) demonstrated improvements in ipsilateral brain
perfusion following the procedure; however, no improvements were
identified in the control group. Table
III shows the close correlations between the change in
perfusion and the change in MMSE (r=0.575) and MOCA (r=0.574), as
well as moderate correlations between the change in perfusion and
the change in WIAS-DS (r=0.464), RVR (r=0.449) and FOME
(r=0.375).
 | Table IIIPearson’s correlation coefficients
between perfusion change and changes in NPE scores. |
Table III
Pearson’s correlation coefficients
between perfusion change and changes in NPE scores.
| MMSE change | MOCA change | FOME change | RVR change | WIAS-DS change |
|---|
| CTP change | 0.575 | 0.574 | 0.375 | 0.449 | 0.464 |
Discussion
Approximately 4.6 million new patients worldwide are
affected by Alzheimer’s disease (AD) every year, which has no
effective treatment (26).
Therefore, it is of great importance to recognize and treat the
subjects at the MCI stage since it is an early stage of dementia
(27) and is associated with an
increased risk for progression to AD (10–15% per year), 10-fold
more than in a normal population (22). Carotid artery stenosis is closely
related to MCI and may be significant in the transition from MCI to
dementia (1). Therefore, treatment
of carotid artery stenosis at the MCI stage may be an important
strategy for preventing and delaying the progression to
dementia.
Mathiesen et al (28) reported that patients without a
history of stroke who have carotid stenosis produce worse scores in
a number of neuropsychological tests compared with those without
carotid stenosis. Therefore, carotid stenosis plays a significant
role in cognitive impairment. In a cohort study of 4,006 patients
with asymptomatic carotid artery stenosis, Johnston et al
(29) discovered that the thicker
the carotid artery intima, the worse the cognitive function
impairment. Additionally, cognitive dysfunction caused by severe
left carotid artery (supplying the dominant cerebral hemisphere)
stenosis is more serious and persistent. Rao (30) demonstrated that carotid stenosis
may lead to frontal lobe damage. Current research suggests that
carotid stenosis leading to cognitive impairment may be a result of
chronic cerebral hypoperfusion, stroke, cerebral white matter
lesions and potential vascular risk factors (5,16).
A multi-center, randomized, double-blind controlled
study confirmed that carotid endarterectomy (CEA) has a positive
effect on severe carotid stenosis (8). With the development of intervention
materials and neuroimaging techniques, particularly the distal
protection device for cerebral embolism, CAS is widely used in
high-risk patients with carotid stenosis (31). The safety and effectiveness of CAS
has been confirmed by clinical studies (9,32).
Italy published the first CAS guide in 2006 and five associations
in the United States also jointly issued a CAS guide in 2007
(33). Additional study has
provided class III evidence that any difference between the effects
of CAS and CEA on cognition at 6 months after revascularization is
small (34), which made it
possible for us to observe the cognitive function of patients with
MCI and carotid artery stenosis by CAS. CEA is not widely applied
in China. Among the 50 public hospitals that are developing the
surgery, only 5 have a mature CEA technology center. As a result,
the majority of patients in China preferentially select CAS, which
is performed extensively. Therefore, the current study was set
based only on the willingness of patients to join the CAS or
control groups. A number of patients in the control group were
finally treated with CAS after 6 months of follow-up.
Our study indicates that CAS increases the
neuropsychological tests scores and/or psychomotor speed in MCI
patients, although patients in the two groups experienced an
ischemic event, stent restenosis (CAS group) and other
complications in the six months of follow-up. Before the procedure,
the MMSE, MOCA, FOME, WAIS-DS and RVR scores of the two groups were
lower than normal. After the procedure, the MMSE, MOCA, FOME and
WAIS-DS scores increased significantly in the CAS group. In
addition, RVR demonstrated an improving trend. The NPE scores of
the control group fell slightly after the 6 month follow-up;
however, the reduction was not statistically significant.
Therefore, this change was regarded as the gradual decline of
cognition in MCI patients.
MMSE was selected as a test for its simple, reliable
and large clinical application, which is sensitive to attention,
repetition and language, but not abstract thinking, judgment,
problem-solving and prediction. MOCA is based on visual-spatial
implementation, naming and delayed memory. FOME focuses on delayed
memory and recognition capability, while WAIS-DS focuses on
evaluating immediate memory and the functioning of graphical
recognition. The results of these two tests improved significantly
following CAS. RVR is aimed at immediate memory and language
fluency. The decline in RVR results was not evident at baseline,
although the results improved slightly following the CAS procedure.
The results of the above tests demonstrated that CAS delays the
cognitive decline in patients with MCI.
To date, there has been no authoritative report on
the incidence of MCI in patients with carotid artery stenosis and
it is unknown which degree of carotid stenosis benefits from CAS.
Particularly for a number of asymptomatic patients whose cognitive
impairment may be subclinical and reversible, timely improved
perfusion may lead to reversal of their cognitive impairment. In
our prospective study, we identified that cerebral perfusion
abnormalities are often observed in patients with severe carotid
stenosis, whose cognitive scores improved more clearly following
the procedure. Of the 84 patients who accepted CTP follow-up in the
CAS group, 72 presented improvements in ipsilateral brain perfusion
following the procedure and there were close correlations between
the improvements in perfusion and improvements in cognitive score.
This suggests that the perfusion improvements caused by vascular
remodeling were the cause of cognitive benefits in patients with
MCI.
In addition, several of the confounding factors
associated with cognitive outcome following CAS may have been
clarified in our study. Firstly, in the 6 month follow-up, we
observed in-stent restenosis in 4 patients (2.8%), ipsilateral
cerebral infarction in 3 patients (2.1%) and ipsilateral TIA in 4
patients (2.8%). Additionally, CAS carries the risk of subclinical
micro-embolism (35–38), without surgical intervention in
treatment group the likelihood of microembolism is smaller, and may
have a negative effect on cognitive performance in the CAS group.
Secondly, there was a natural course of cognitive decline in the 6
month follow-up in MCI patients, Nevertheless, the cognitive scores
of the CAS group improved quite significantly following the
procedure. Therefore, our results may underestimate the effects of
vascular reconstruction on cognitive function improvement.
In the 6 month follow-up, we identified ipsilateral
cerebral infarction in 2 patients (3.1%) and ipsilateral TIA in 3
patients (4.7%) in the control group. An ischemic event may affect
cognitive function in patients to a certain extent, coupled with
the natural decline of cognitive function in patients with MCI. The
cognitive function of the control group declined; however, not
significantly. We must take into account the VRFs, including
hypertension and hyperlipidemia in patients, as well as the
learning effect of completing NCEs twice.
There were no significant differences in baseline
characteristics between the CAS and control groups. The same drug
therapy was used in the two groups, so CAS was the only
intervention. In contrast to previous studies that compared the
preoperative and postoperative state of the same patient, we set up
a control group to exclude the possibility of a learning effect
(39). Therefore, the improvement
in cognitive function in the CAS group is only explained by the
restoration of cerebral perfusion and correction of hemispheric
ischemia.
The current study has several limitations: i) the
groups were selected according to the patients own preference,
after being given a detailed explanation of the requirement for
surgery and the risk of the surgery, rather than by random
allocation; ii) the selection criteria was highly specific to
reduce the effect of other factors, including posterior circulation
and intracranial severe stenosis. Only patients with carotid artery
stenosis and MCI were selected and patients with normal cognitive
or dementia were excluded; iii) although significant improvements
were observed in the patients of the CAS group, there were
variations in individual patients and individual tests. The
limitations of the cognitive tests in the Chinese population should
be in reference to the average educational level or the date of
validation of these cognitive tests in Mandarin. It is necessary to
apply more specific neuropsychological tests to localize specific
cortical functional zones in future studies; iv) the universality
of our results is also relatively limited. It was not possible to
clearly determine the duration of carotid stenosis in the two
groups, particularly in asymptomatic patients. A longer duration of
severe stenosis may potentially affect the reversibility of
cognitive function.
Overall, our prospective study demonstrates
significant improvements in cognition in patients with carotid
stenosis and MCI 6 months after CAS and the improvement of
cognition is closely related to the improvement of cerebral
perfusion. More rigorous randomized controlled experiments and a
longer follow-up duration are required to evaluate the long-term
curative effect of CAS on the improvement of cognitive function in
patients with CAS and MCI, as well as its role in delaying the
progression of MCI to AD.
Abbreviations:
|
CAS
|
carotid artery stenting;
|
|
MCI
|
mild cognitive impairment;
|
|
NPEs
|
neuropsychological examinations;
|
|
VRFs
|
vascular risk factors;
|
|
AD
|
Alzheimer’s disease;
|
|
NIHSS
|
National Institutes of Health Stroke
Scale;
|
|
ADL
|
Activities of Daily Living;
|
|
MMSE
|
Mini-Mental State Examination;
|
|
MOCA
|
Montreal Cognitive Assessment;
|
|
FOME
|
Fuld Object Memory Evaluation;
|
|
RVR
|
rapid verbal retrieval;
|
|
WAIS-DS
|
Wechsler Adult Intelligence
Scale-digital span
|
Acknowledgements
The authors thank Dr Cui Min, Dr Liu
Juan and Dr Li Ling from the Department of Neurology and Center for
Clinical Neuroscience, Daping Hospital, for their work on this
study.
References
|
1
|
Li J, Wang YJ, Zhang M, et al: Vascular
risk factors promote conversion from mild cognitive impairment to
Alzheimer disease. Neurology. 76:1485–1491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silvestrini M, Viticchi G, Falsetti L, et
al: The role of carotid atherosclerosis in Alzheimer’s disease
progression. J Alzheimers Dis. 25:719–726. 2011.
|
|
3
|
Fergenbaum JH, Bruce S, Spence JD, et al:
Carotid atherosclerosis and a reduced likelihood for lowered
cognitive performance in a Canadian First Nations population.
Neuroepidemiology. 33:321–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paulson GW, Kapp J and Cook W: Dementia
associated with bilateral carotid artery disease. Geriatrics.
21:159–166. 1996.
|
|
5
|
Meyer JS, Okayasu H, Tachibana H, et al:
Stable xenon CT CBF measurements in prevalent cerebrovascular
disorders (stroke). Stroke. 15:80–90. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tatemichi TK, Desmond DW, Pronhovnik I, et
al: Dementia associated with bilateral carotid occlusions:
neuropsychological and haemodynamic course after extracranial to
intracranial bypass surgery. J Neurol Neurosurg Psychiatry.
58:633–636. 1995. View Article : Google Scholar
|
|
7
|
Goessens BM, Visseren FL, Kappelle LJ, et
al: Asymptomatic carotid artery stenosis and the risk of new
vascular events in patients with manifest arterial disease. The
SMART Study. Stroke. 38:1470–1475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
North American Symptomatic Carotid
Endarterectomy Trial Collaborators: Beneficial effect of carotid
endarterectomy in symptomatic patients with highgrade stenosis. N
Engl J Med. 325:445–453. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Safian RD, Bresnahan JF, Jaff MR, et al:
Protected carotid stenting in high-risk patients with severe
carotid artery stenosis. J Am Coll Cardiol. 47:2384–2389. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zahn R, Ischinger T, Hochadel M, et al:
Carotid artery stenting in octogenarians: results from the ALKK
Carotid Artery Stent (CAS) Registry. Eur Heart J. 28:370–375. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Rango P, Caso V, Leys D, et al: The
role of carotid artery stenting and carotid endarterectomy in
cognitive performance: a systematic review. Stroke. 39:3116–3127.
2008.PubMed/NCBI
|
|
12
|
Ghogawala Z, Westerveld M and Amin-Hanjani
S: Cognitive outcomes after carotid revascularization: the role of
cerebral emboli and hypoperfusion. Neurosurgery. 62:385–395. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rothwell PM, Eliasziw M, Gutnikov SA, et
al: Analysis of pooled data from the randomised controlled trials
of endarterectomy for symptomatic carotid stenosis. Lancet.
361:107–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brott T, Adams HP Jr, Olinger CP, et al:
Measurements of acute cerebral infarction: a clinical examination
scale. Stroke. 20:864–870. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou H, Deng J, Li J, et al: Study of the
relationship between cigarette smoking, alcohol drinking and
cognitive impairment among elderly people in China. Age Ageing.
32:205–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou DH, Wang JY, Li J, et al: Study on
frequency and predictors of dementia after ischemic stroke: the
Chongqing stroke study. J Neurol. 251:421–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kørner A, Lauritzen L, Abelskov K, et al:
The Geriatric Depression Scale and the Cornell Scale for Depression
in Dementia: a validity study. Nord J Psychiatry. 60:360–364.
2006.
|
|
18
|
Pendlebury ST, Cuthbertson FC, Welch SJ,
et al: Underestimation of cognitive impairment by Mini-Mental State
Examination versus the Montreal Cognitive Assessment in patients
with transient ischemic attack and stroke: a population-based
study. Stroke. 41:1290–1293. 2010. View Article : Google Scholar
|
|
19
|
Fuld PA, Masur DM, Blau AD, et al:
Object-memory evaluation for prospective detection of dementia in
normal functioning elderly: predictive and normative data. J Clin
Exp Neuropsychol. 12:520–528. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M: Prevalence study on dementia and
Alzheimer disease. Zhonghua Yi Xue Za Zhi. 70:424–428. 4301990.(In
Chinese).
|
|
21
|
Welsh KA, Butters N, Hughes JP, et al:
Detection and staging of dementia in Alzheimer’s disease. Use of
the neuropsychological measures developed for the Consortium to
Establish a Registry for Alzheimer’s Disease. Arch Neurol.
49:448–452. 1992.
|
|
22
|
Petersen RC, Smith GE, Waring SC, et al:
Mild cognitive impairment: clinical characterization and outcome.
Arch Neurol. 56:303–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palmer K, Di Iulio F, Varsi AE, et al:
Neuropsychiatric predictors of progression from amnestic-mild
cognitive impairment to Alzheimer’s disease: the role of depression
and apathy. J Alzheimers Dis. 20:175–183. 2010.PubMed/NCBI
|
|
24
|
Van MW, Rennenberg RJ, Schurink GW, et al:
Cerebral hyper-perfusion syndrome. J Lancet Neurol. 4:877–888.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin MS, Chiu MJ, Wu YW, et al:
Neurocognitive improvement after carotid artery stenting in
patients with chronic internal carotid artery occlusion and
cerebral ischemia. Stroke. 42:2850–2854. 2011. View Article : Google Scholar
|
|
26
|
Ferri CP, Prince M, Brayne C, et al:
Global prevalence of dementia: a Delphi consensus study. Lancet.
366:2112–2117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morris JC, Storandt M, Miller JP, et al:
Mild cognitive impairment represents early-stage Alzheimer disease.
Arch Neurol. 58:397–405. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mathiesen EB, Weterloo K, Joakimsen O, et
al: Reduced neuro-psychological test performance in asymptomatic
carotid stenosis: The Tromsø study. Neurology. 62:695–701.
2004.PubMed/NCBI
|
|
29
|
Johnston SC, O’Meara ES, Manolio TA, et
al: Cognitive impairment and decline are associated with carotid
artery disease in patients without clinically evident
cerebrovascular disease. Ann Intern Med. 140:237–247. 2004.
View Article : Google Scholar
|
|
30
|
Rao R: The role of carotid stenosis in
vascular cognitive impairment. J Neurol Sci. 203–204:103–107.
2002.
|
|
31
|
Bates ER, Babb JD, Casey DE, et al:
ACCF/SCAI/SVMB/SCR/ASITN 2007 clinical expert consensus document on
carotid stenting: a report of the American college of cardiology
foundation task force on clinical expert consensus documents. J Am
Coll Cardiol. 49:126–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zahn R, Ischinger T, Hochadel M, et al:
Carotid artery stenting in octogenarians: results from the ALKK
Carotid Artery Stent (CAS) Registry. Eur Heart J. 28:370–375. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cremonesi A, Setacci C, Biqnamini A, et
al: Carotid artery stenting: first consensus document of the
ICCS-SPREAD Joint Committee. Stroke. 37:2400–2409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Altinbas A, van Zandvoort MJ, van den Berg
E, et al: Cognition after carotid endarterectomy or stenting: a
randomized comparison. Neurology. 77:1084–1890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crawley F, Stygall J, Lunn S, et al:
Comparison of microembolism detected by transcranial Doppler and
neuropsychological sequelae of carotid surgery and percutaneous
transluminal angioplasty. Stroke. 31:1329–1334. 2003. View Article : Google Scholar
|
|
36
|
Grunwald IQ, Supprian T, Politi M, et al:
Cognitive changes after carotid artery stenting. Neuroradiology.
48:319–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Braekken SK, Reinvang I, Russell D, et al:
Association between intraoperative cerebral microembolic signals
and postoperative neuropsychological deficit: comparison between
patients with cardiac valve replacement and patients with coronary
artery bypass grafting. J Neurol Neurosurg Psychiatry. 65:573–576.
1998. View Article : Google Scholar
|
|
38
|
Sylivris S, Levi C, Matalanis G, et al:
Pattern and significance of cerebral microemboli during coronary
artery bypass grafting. Ann Thorac Surg. 66:1674–1678. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heyer EJ, Sharma R, Rampersad A, et al: A
controlled prospective study of neuropsychological dysfunction
following carotid endarterectomy. Arch Neurol. 59:217–222. 2002.
View Article : Google Scholar : PubMed/NCBI
|