Introduction
Brain natriuretic peptide (BNP) has been a focus of
cardiac research since it was discovered by Sudoh et al in
1988 (1). The BNP test is used
clinically in the early diagnosis of heart failure (HF), risk
stratification in HF, diagnosis of acute dyspnea, prognosis and
assessment of HF and screening of high-risk groups, as well as risk
stratification in acute coronary syndrome (ACS) and the assessment
of the treatment of HF (2–13). However, at present, opinions
concerning the value of BNP in the early diagnosis of acute
myocardial infarction (AMI) differ (14–19).
The variations in BNP levels and the mechanism of action of BNP at
the early stage of AMI remain unclear, particularly during the
first 4 h following onset. To clearly understand the trend in the
changes of BNP level during the early stage of AMI, particularly
within the first 4 h following onset, animal models were
established in the present study. The changes in BNP levels in the
first 4 h of AMI were determined and compared with the levels of
cardiac troponin I (cTnI).
Materials and methods
Experimental animals
A total of 66 Wistar rats, both male and female,
provided by the Animal Experiment Center of Shangdong University
(Jinan, China), were used in the study. The study was performed in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. The animal use was reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of the Qianfo
Mountain Hospital Affiliated with Shandong University. Body weights
were between 316 and 381 g and the average weight was 337.14±20.55
g. A model of AMI was established in 36 rats (the AMI group), while
30 sham-operated rats were used as the control group.
Ultrastructural observations in the myocardia were performed after
surgery in the two groups. One rat died during surgery in the AMI
group.
Animal models
Thirty-six experimental rats were intraperitoneally
anesthetized with 3.6% chloral hydrate (10 ml/kg) and then bound on
a rat table. The trachea was separated and intubated to connect to
a small animal ventilator supporting a breath rate of 50–60 breaths
per minute and a tidal volume of 4–5 ml per breath. Needle
electrodes connected to an electrocardiography device were inserted
subcutaneously into the limbs and a standard electrocardiogram was
recorded. A left sternal border thoracotomy through the bed of the
second to fourth rib was performed. The pericardium was opened,
exposing the heart and blood vessels on the left ventricular
surface. Using the left main coronary artery as a marker, needles
were inserted 2 mm under the root of the left atrial appendage and
a 3/0 silk thread was used to pass through the myocardial surface.
The needle was withdrawn next to the pulmonary cone. The thread
ring was knotted at both ends of the ligature, tightening the
ligature to cause myocardial infarction. ST-segment elevation in
electrocardiogram represented the successful establishment of AMI
models. Penicillin and streptomycin were administered
postoperatively to prevent infection. One rat died during surgery.
A total of 35 rats successfully underwent the experimental surgery
and 30 rats underwent a sham surgery, which involved the same
procedure as that described above, but without the coronary artery
ligation.
Biochemical indicators
Jugular venous blood (2 ml) was extracted from
experimental (n=35) and control groups (n=30) before and 1, 2, 3
and 4 h after the surgery. A desktop room-temperature centrifuge
(UNIVERSAL Type 320R; Weifang Yuhua Medical Equipment Co., Ltd,
WeiFang, China) was used to centrifuge the blood at 4,500 rpm for
10 min. The serum was separated and stored at −20°C. A
double-antibody sandwich enzyme-linked immunosorbent assay (ELISA)
was used to detect the concentrations of BNP and cTnI. The kit was
purchased from Dalian Pan-State Chemical Technology Development
Co., Ltd, Dalian, China.
AMI size
Rats were anesthetized and sacrificed following the
surgery and analysis. Hearts were removed and had an average weight
of 1.04±0.04 g. The main left coronary artery was ligated. The
aorta was retrograde perfused with 2–3 ml solution containing 0.5%
Evans blue dye. The non-blue-stained ischemic area and the
blue-stained non-ischemic area were separated. After being drawn
through filter paper, the non-blue-stained ischemic myocardium was
frozen at −20°C for 20 min and then underwent horizontal long axis
slicing at a thickness of 1–2 mm. The slices were placed in 1%
triphenyl tetrazolium chloride (TTC) in phosphate buffer solution
(pH 7.4) and incubated at 37°C for 20 min. At this point, the
necrotic zone (NZ) was gray and the non-necrotic zone (NNZ)
scarlet. The NZ and NNZ were separated, drawn through filter paper
and weighed on an AB104-N electronic balance. The infarct size was
defined as the ratio of the weight of the NZ to that of the
ischemic zone (the sum of NZ and NNZ).
Myocardial ultrastructure
Following the surgery, the rat myocardia were fixed
with 3% glutaraldehyde, then fixed with 1% osmium tetroxide and
dehydrated conventionally in an ethanol gradient. Through epoxy
resin embedding and deployment of a hardener, accelerator and
growth agent, ultra-thin sections with a thickness of 70 nm were
cut by ultramicrotomy and stained with uranyl acetate and lead
citrate solution. Changes in the myocardial ultrastructure were
observed with a JEM-1200EX transmission electron microscope (TEM).
The required area was selected for image capture. The myocardia of
8 normal rats were observed with the TEM as the normal control
prior to surgery.
Statistical analysis
SPSS 11.0 software was used to analyze the data.
Data were expressed as the mean ± standard deviation. The paired
t-test was used for comparisons of before and after surgery and
linear correlation analysis was utilized to analyze the correlation
among the variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
Serum BNP concentrations
The serum BNP concentrations were significantly
higher 1 h after the successful establishment of the AMI model than
prior to surgery (P<0.01). The BNP level reached a peak after 2
h and remained significantly higher in the first 4 h (all
P<0.01). The post-surgery serum BNP concentrations of the rats
at 1–4 h were significantly higher than those in the control group
(all P<0.01; Table I).
 | Table IChanges in the serum BNP
concentrations of rats before and after surgery (ϱ/ng/l, mean ±
standard deviation). |
Table I
Changes in the serum BNP
concentrations of rats before and after surgery (ϱ/ng/l, mean ±
standard deviation).
| Group | n | Pre-surgery | Post-surgery
|
|---|
| 1 h | 2 h | 3h | 4 h |
|---|
| AMI | 25 | 53.14±7.49 |
312.33±14.51ab |
520.66±16.85ab |
486.51±15.73ab |
422.11±15.23ab |
| Control | 20 | 53.12±7.46 | 55.33±8.51 | 54.14±7.85 | 52.18±8.34 | 55.37±8.72 |
cTnI serum level
No statistically significant differences were
observed between the serum cTnI concentrations in the AMI group
before and 1–4 h after surgery (P>0.05). The serum cTnI
concentrations of the AMI group before and 1–4 h after surgery were
not significantly different from those of the control group
(P>0.05). In addition, the differences between the serum cTnI
concentrations of the control group before and 1–4 h after surgery
were not statistically significant (P>0.05; Table II).
 | Table IIChanges in the cTnI serum levels of
rats before and after surgery (ϱ/μg/l, mean ± standard
deviation). |
Table II
Changes in the cTnI serum levels of
rats before and after surgery (ϱ/μg/l, mean ± standard
deviation).
| Group | n | Pre-surgery | Post-surgery
|
|---|
| 1 h | 2 h | 3 h | 4 h |
|---|
| AMI | 25 | 0.43±0.06 | 0.51±0.08 | 0.45±0.08 | 0.52±0.09 | 0.61±0.08 |
| Control | 20 | 0.43±0.05 | 0.47±0.09 | 0.46±0.09 | 0.51±0.07 | 0.58±0.07 |
Correlation analysis
The serum BNP concentrations showed a significant
positive correlation with the infarct size in the AMI group at 1,
2, 3 and 4 h after surgery (r=0.34, P<0.05; r=0.72, P<0.05;
r=0.57, P<0.05; and r=0.48, P<0.05, respectively). The serum
cTnI concentrations of the AMI group at 1, 2, 4 and 4 h after
surgery were not significantly correlated with the myocardial
infarct range (r=–0.07, P>0.05; r=–0.05, P>0.05; r=–0.09,
P>0.05; and r=–0.05, P>0.05, respectively).
Myocardial ultrastructure
The cardiac muscle fibers of the rats showed a clear
regular structure when the myocardium was viewed under a microscope
prior to surgery (Fig. 1A). At 4 h
after the establishment of the AMI model, the myocardial
mitochondria of the rats varied in size and shape and exhibited
hyperplasia and muscle fracture (Fig.
1B). The cardiac muscle fibers of the rats in the control group
prior to and following surgery were normal, with a clear regular
structure and rows of mitochondria among the sarcomeres (Fig. 1C and D).
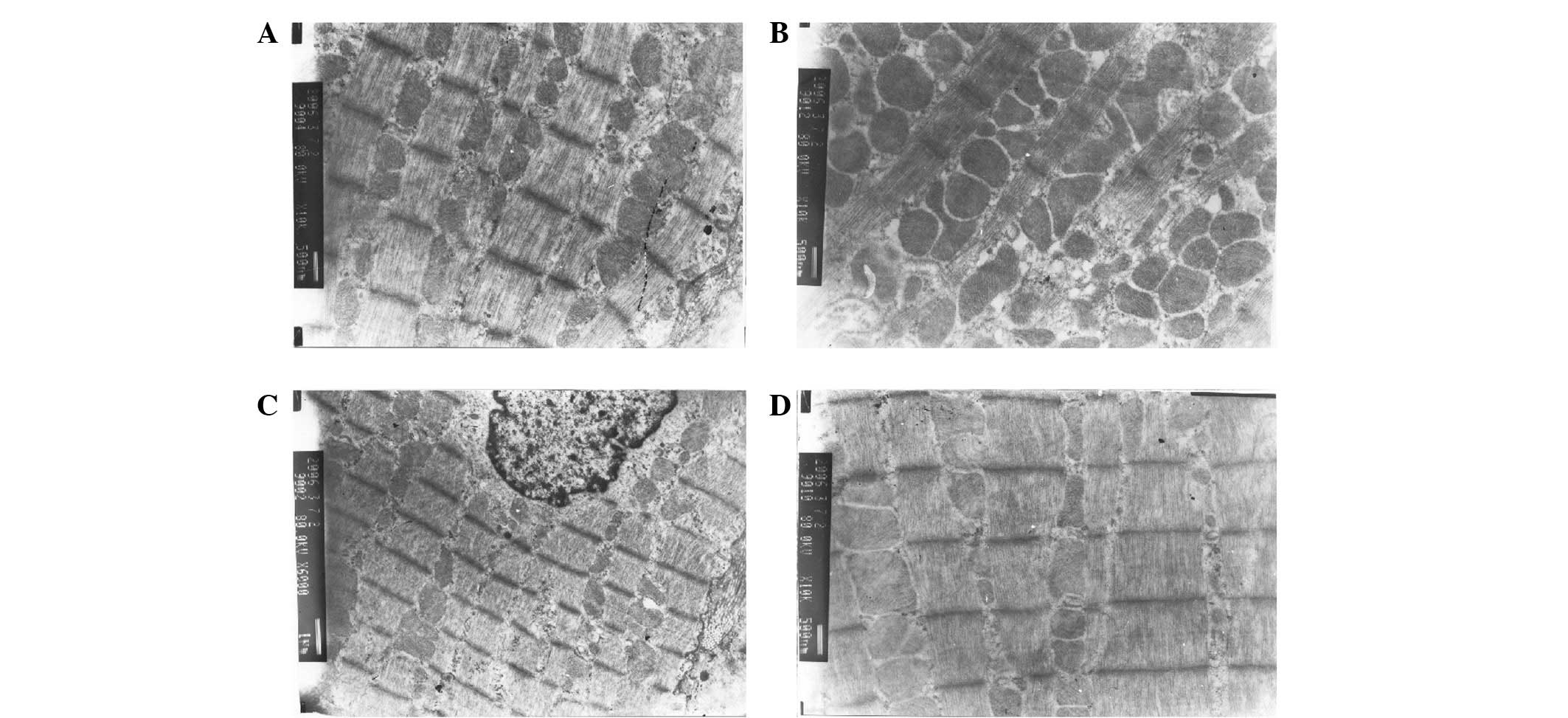 | Figure 1Myocardial ultrastructure. (A) Normal
myocardial fibers of rats prior to surgery (TEM, x10,000). (B)
Variations in the size and shape, hyperplasia and muscle fracture
of the mitochondria of rats following AMI (TEM, x10,000). (C)
Normal myocardial fiber prior to surgery in the control group (TEM,
x6,000). (D) Normal myocardial fiber following surgery in the
control group with clear regular structure and rows of mitochondria
among the sarcomeres (TEM, x10,000). TEM, transmission electron
microscopy; AMI, acute myocardial infarction. |
Discussion
The rapid and accurate diagnosis in the early stage
of AMI, particularly within 4 h of the onset, is significant for
timely processing by clinicians and the prognosis of patients. The
increase in the levels of the cardiac marker cTnI is only
significant 4 h after the onset of AMI. Therefore, within 4 h of
the onset of AMI, the diagnostic value of cTnI is limited (20,21).
Studies have identified that BNP levels increase significantly at
the early stage of non-ST elevation myocardial infarction (NSTEMI),
which may be useful for the early diagnosis of AMI. BNP may be a
complementary indicator for the early diagnosis of AMI (14). However, there has been no
systematic review of animal studies of AMI to demonstrate this.
BNP is a member of the natriuretic peptide family,
identified following the first description of atrial natriuretic
peptide. BNP was named as such following its discovery in porcine
brains by Sudoh et al (1)
in 1988. BNP is a cardiac hormone predominantly synthesized in and
secreted from the ventricles and has the effects of promoting
natriuresis, diuresis and a marked dilation of blood vessels, as
well as acting against the vasoconstriction of the
renin-angiotensin-aldosterone system. The natriuretic peptide
system is activated when cardiac dysfunction occurs. Ventricular
overload contributes to its release. The BNP concentration is
useful for to determining heart function and assessing the
prognosis of patients with HF.
Following the study by Bassan et al (14), clinicians gained a new
understanding of BNP. Since the previous knowledge of BNP had been
limited to the risk stratification of HF prognoses, the diagnostic
value of BNP in acute myocardial ischemia at the early stage had
not been investigated. The study by Bassan et al
demonstrated that BNP may be used as an early indicator of NSTEMI.
Among chest pain patients without ST-segment elevation, the
detection of BNP may help to filter out cases of NSTEMI. BNP levels
significantly increased 2 h after NSTEMI while those of creatine
kinase-MB (CK-MB) and cTnI did not. This study also revealed that
the increase in BNP levels occurred earlier than the increases in
CK-MB and cTnI, which aided the identification of patients in the
early stage (chest pain within 4 h) of AMI without ST-segment
elevation. For patients with chest pain without ST-segment
elevation who were not in the diagnostic window for the indicators
of myocardial necrosis, CK-MB and cTnI (4 h), the significant
increase in BNP levels suggested the possibility of the onset of
AMI. These patients required closer and continued monitoring of the
changes in CK-MB or cTnI.
The study by Bassan et al was performed on
NSTEMI patients. There has been no systematic animal study to
determine whether the same or similar conclusions may be drawn
under strict experimental conditions. The results of the present
study showed that serum BNP concentrations were significantly
increased 1 h after the successful establishment of the AMI model
(compared with those in the control group prior to and following
surgery, all P<0.01). The BNP levels reached a peak after 2 h,
which was 9-fold higher than the normal value prior to surgery and
persisted at a high level for 4 h after surgery. This was due to
the accelerated secretion of BNP, which was mainly concentrated in
the fringes of the border areas between the infarction and
non-infarction regions. The mechanical tension of the ventricle
wall in this area was the strongest.
Changes in the tension of the ventricular wall local
to the infarction may be reflected by the BNP level. Since the
tension is caused by the size of the infarction, morphological
changes in the left ventricle, myocardial mechanical stress and
other factors, the acceleration of ventricular wall tension may
rapidly stimulate BNP gene expression, causing a large amount of
synthesized BNP to be secreted into the blood. The study by Ogawa
et al (22) showed that the
ventricle was the key location for synthesizing and secreting BNP.
The change in ventricular wall tension may stimulate the ventricle
to synthesize and secrete BNP. Regional left ventricular diastolic
and/or systolic dysfunction is a sign of myocardial acute or
persistent ischemia. When acute coronary artery occlusion and
sublethal myocardial ischemia occur, the ventricle may release BNP.
Therefore, plasma BNP level measurements may predict the onset of
AMI. The results of the present study demonstrated that BNP levels
at the early stage of AMI (within 4 h) showed significant increases
prior to notable changes in cTnI being exhibited. The BNP levels
reached a peak at 2 h and then decreased. From 1 to 4 h, the serum
BNP concentrations showed a significant positive correlation with
the infarct size of the AMI. This was due to the ventricular muscle
cells producing and secreting more BNP. The secretion of BNP was
basically regulated by the increase in ventricular wall tension and
left ventricular extension. The increase in ventricular wall
tension rapidly stimulated BNP gene expression and a large amount
of synthesized BNP was secreted into the blood. The ventricular
wall tension in areas bordering the infarction and non-infarction
regions was the strongest. The secretion of BNP was concentrated in
this area and may exactly reflect the change of ventricular wall
tension in local infarctions. The ventricular wall tension is
affected by the size of the infarction, morphological changes in
the left ventricle and other factors, so the determination of serum
BNP concentrations in rats after AMI may predict the size of AMI.
The present study showed that the BNP serum level in rats 2 h after
surgery had the most marked correlation with AMI size (r=0.72,
P<0.05). This was due to the myocardium showing significant
necrosis 2 h after the establishment of the AMI model. The first
indication was diastolic dysfunction, which stimulated ventricular
synthesis and BNP release. There was a significant correlation
between the BNP level and AMI size at every time point between 1
and 4 h after surgery. Therefore, measuring plasma BNP levels may
predict the infarct size of AMI. According to the results observed
under a TEM, the establishment of the AMI model in rats was
successful. Compared with the data from before the surgery, the
myocardial fibers of rats were broken 4 h after surgery,
euchromatin in the nucleus was significantly reduced, mitochondria
varied in size and shape, hyperplasia occurred and an obvious notch
was present in the nucleus. These observations were due to the
effects of the AMI.
The results of the present study demonstrate that
BNP levels, which increase earlier than cTnI levels in the early
stage of AMI, may be used as a blood marker for the early diagnosis
of AMI and to predict infarct size. cTnI is a specific indicator of
AMI, but is not particularly sensitive. No significant increase in
the serum cTnI level was observed 4 h after the induction of AMI in
rats; similar observations have been made previously for CK-MB.
This clearly limits the diagnostic value of cTnI and CK-MB in the
early phase, 4 h after AMI. The detection of BNP levels at this
time is of value. Excluding other factors, BNP may be used as an
early indicator for the rapid diagnosis of AMI.
References
|
1
|
Sudoh T, Kangawa K, Minamino N and Matsuo
H: A new natriuretic peptide in porcine brain. Nature. 332:78–81.
1988. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Lamos JA, McGuire DK and Drazner MH:
B-type natriuretic peptide in cardiovascular disease. Lancet.
362:316–322. 2003.PubMed/NCBI
|
|
3
|
Maisel AS, Krishnaswamy P, Nowak RM, et
al: Rapid measurement of B-type natriuretic peptide in the
emergency diagnosis of heart failure. N Engl J Med. 347:161–167.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCullough PA, Nowak RM, McCord J, et al:
B-type natriuretic peptide and clinical judgment in emergency
diagnosis of heart failure: analysis from Breathing Not Properly
(BNP) Multinational Study. Circulation. 106:416–422. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maisel A: B-type natriuretic peptide
levels: diagnostic and prognostic incongestive heart failure:
what's next? Circulation. 105:2328–2331. 2002.
|
|
6
|
Mueller C, Scholer A, Laule-Kilian K, et
al: Use of B-type natriuretic peptide in the evaluation and
management of acute dyspnea. N Engl J Med. 350:647–654. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morrow DA, de Lemos JA, Sabatine MS, et
al: Evaluation of B-type natriuretic peptide for risk assessment in
unstable angina/non-ST-elevation myocardial infarction: B-type
natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll
Cardiol. 41:1264–1272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jernberg T, Stridsberg M, Venge P and
Lindahl B: N-terminal pro brain natriuretic peptide on admission
for early risk stratification of patients with chest pain and no
ST-segment elevation. J Am Coll Cardiol. 40:437–445. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Lemos JA, Morrow DA, Bentley JH, et al:
The prognostic value of B-type natriuretic peptide in patients with
acute coronary syndromes. N Engl J Med. 345:1014–1021. 2001.
|
|
10
|
Bettencourt P, Azevedo A, Pimenta J,
Friões F, Ferreira S and Ferreira A: N-terminal-pro-brain
natriuretic peptide predicts outcome after hospital discharge in
heart failure patients. Circulation. 110:2168–74. 2004. View Article : Google Scholar
|
|
11
|
Hunt SA, Abraham WT, Chin MH, et al: 2009
focused update incorporated into the ACC/AHA 2005 guidelines for
the diagnosis and management of heart failure in adults: a report
of the American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines: developed in
collaboration with the International Society for Heart and Lung
Transplantation. Circulation. 119:e391–e479. 2009.
|
|
12
|
Cleland JG, McMurray JJ, Kjekshus J, et
al: Plasma concentration of amino-terminal pro-brain natriuretic
peptide in chronic heart failure: prediction of cardiovascular
events and interaction with the effects of rosuvastatin: a report
from CORONA (Controlled Rosuvastatin Multinational Trial in Heart
Failure). J Am Coll Cardiol. 54:1850–1859. 2009.
|
|
13
|
Anderson JL, Adams CD, Antman EM, et al:
ACC/AHA 2007 guidelines for the management of patients with
unstable angina/non ST-elevation myocardial infarction: a report of
the American College of Cardiology/American Heart Association Task
Force on Practice Guidelines (Writing Committee to Revise the 2002
Guidelines for the Management of Patients With Unstable Angina/Non
ST-Elevation Myocardial Infarction): developed in collaboration
with the American College of Emergency Physicians, the Society for
Cardiovascular Angiography and Interventions, and the Society of
Thoracic Surgeons: endorsed by the American Association of
Cardiovascular and Pulmonary Rehabilitation and the Society for
Academic Emergency Medicine. Circulation. 116:e148–e304. 2007.
|
|
14
|
Bassan R, Potsch A, Maisel A, et al:
B-type natriuretic peptide: a novel early blood marker of acute
myocardial infarction in patients with chest pain and no ST-segment
elevation. Eur Heart J. 26:234–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morita E, Yasue H, Yoshimura M, et al:
Increased plasma levels of brain natriuretic peptide in patients
with acute myocardial infarction. Circulation. 88:82–91. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haaf P, Reichlin T, Corson N, et al:
B-type natriuretic peptide in the early diagnosis and risk
stratification of acute chest pain. Am J Med. 124:444–452. 2011.
View Article : Google Scholar
|
|
17
|
Maisel A, Mueller C, Adams K Jr, et al:
State of the art: using natriuretic peptide levels in clinical
practice. Eur J Heart Fail. 10:824–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sabatine MS, Morrow DA, de Lemos JA, et
al: Acute changes in circulating natriuretic peptide levels in
relation to myocardial ischemia. J Am Coll Cardiol. 44:1988–1995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Staub D, Nusbaumer C, Zellweger MJ, et al:
Use of B-type natriuretic peptide in the detection of myocardial
ischemia. Am Heart J. 151:1223–1230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Melanson SE, Morrow DA and Jarolim P:
Earlier detection of myocardial injury in a preliminary evaluation
using a new troponin I assay with improved sensitivity. Am J Clin
Pathol. 128:282–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reichlin T, Hochholzer W, Bassetti S, et
al: Early diagnosis of myocardial infarction with sensitive cardiac
troponin assays. N Engl J Med. 361:858–867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogawa Y, Nakao K, Mukoyama M, et al:
Natriuretic peptides as cardiac hormone in normotensive and
spontaneously hypertensive rats: the ventricle is a major site of
synthesis and secretion of brain natriuretic peptide. Circ Res.
69:491–500. 1991. View Article : Google Scholar
|















