Introduction
Pilomatricoma is a benign adnexal neoplasm with
follicular differentiation similar to matrical cells of the hair
bulb, which occurs in middle-aged to older dogs (2–7 years of age)
with normal gender distribution (1–5).
Pilomatricoma manifests as an asymptomatic dermal or subcutaneous
mass with alopecia of overlying skin, usually situated on the
scalp, posterior area of the neck, buttocks and on the upper
extremities (6,7). Breeds with continuous hair growth
(Kerry Blue Terriers, Poodles, Bedlington Terriers, Bichon Frisés
and Schnauzers) have higher susceptibility due to the greater
mitotic activity of their hair follicles (7). Although benign pilomatricoma is a
relatively frequent adnexal tumor (3% of all epithelial skin
tumors) the incidence of its malignant counterpart [matrical
carcinoma or malignant pilomatricoma (MP)] is extremely low
(7–9). To the best of our knowledge, only
nine reports of MP have been reported thus far in dogs (10–17),
most of which presented metastasis in bone (10,12–16).
The localization and the clinical features of MP are similar to
that of its benign counterpart, with the exception of its tendency
to recur following incomplete excision and to metastasize to the
underlying bone through contiguity or to distant sites (7).
Case report
An eleven year-old male mongrel dog was referred
with a history of a left forelimb lameness, treated with
non-steroidal anti-inflammatory drugs, without improvement. On
physical examination, a painful zone near the left elbow was
observed, without any abnormality demonstrated by radiography. An
ulcerated mass on the neck was also detected. The mass was 4 cm in
diameter, well circumscribed, firm and not painful on palpation.
Cytological examination of samples obtained by fine needle
aspiration showed small cohesive epithelial aggregates composed of
basaloid cells, a small amount of amorphous keratinized material
and a few ghost cells. Due to these findings, an initial diagnosis
of a follicular tumor with matrical differentiation was made. The
mass was excised under general anesthesia, fixed in 10% formalin
and routinely processed for histopathological examination.
Microscopic examination revealed an epithelial neoplasia localized
in the dermis and subcutaneous tissue, without connection to the
overlying epidermis. The tumor was characterized by irregular
cystic lobules, within which neoplastic cells were arranged in a
circular configuration, with nucleated basaloid-type cells on the
periphery and pink amorphous keratin with enucleated shadow cells
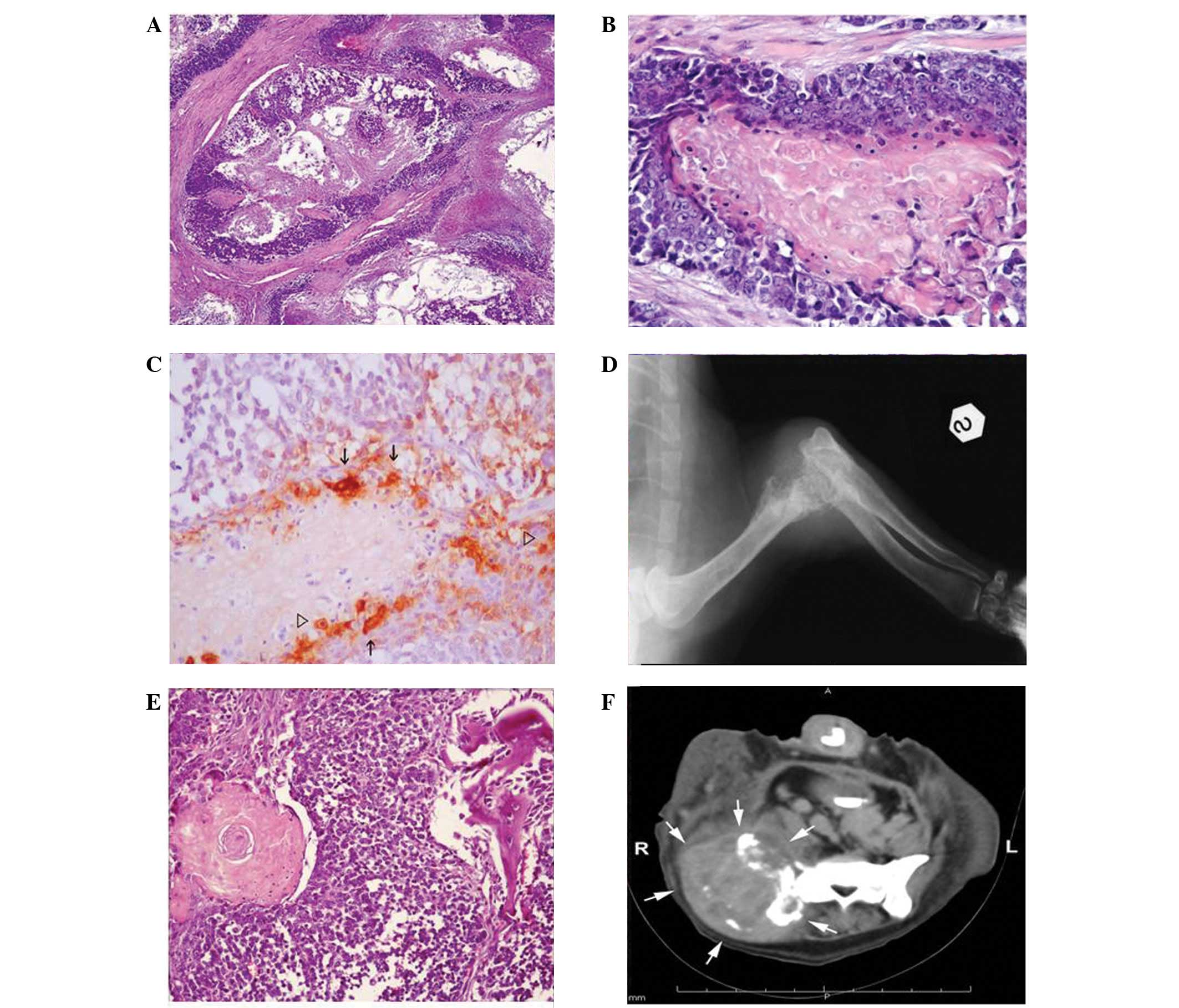
in the centre (Fig. 1A). There was
an abrupt transition between the basaloid cells and the central
core. The basaloid cells had large, vesicular, hyperchromatic
nuclei with prominent nucleoli and a moderate amount of clear
cytoplasm and the centrally located cells showed well-delineated
cell borders and a central unstained area corresponding to the lost
nucleus (Fig. 1B). Scattered
throughout the basaloid cell layer, 3–4 mitotic figures were
observed at high power field (×400 magnification), some of which
were atypical. In certain areas inflammatory cells, including
mainly lymphocytes, plasma cells, macrophages and foreign body
giant cells, were observed. The histological features were
consistent with an MP.
To better define the matrical differentiation of
this tumor, immunohistochemistry was performed by the
streptavidin-biotin peroxidase complex method using a commercially
available antibody against β catenin (monoclonal mouse anti-human,
clone E-5, sc-7963; Santa Cruz Biotechnology, Heidelberg, Germany).
The reaction of β catenin in the neoplastic tissue was observed in
the cytoplasm of certain centrally located well-differentiated
keratinocytes and in the nuclei of some of the basaloid cells. In
the overlying epidermis that served as a control, β catenin was
shown to be expressed in the keratinocytes in a perimembranous
pattern (Fig. 1C).
After one month the dog presented with a severe
lameness in the same left limb. Radiography of the elbow showed a
severe permeative osteolysis with a long transitional zone of the
distal epiphysis of the left humerus (Fig. 1D). A bone biopsy was performed and
a neoplastic lesion similar to those described for the primary
cutaneous tumor was observed. Due to these staging results,
amputation was performed. A nodular bone mass was observed on the
excised bone. The entire bone mass, which was 4 cm in diameter,
white and friable, was fixed in 10% formalin for histopathology.
Upon microscopic examination, performed following the routine
procedure for decalcification, a neoplasia similar to the type
identified in the primary skin mass was observed. It was confined
to the bone with associated areas of bone lysis, mineralization,
chondroid and osseous metaplasia (Fig.
1E). Compared with the primary tumor, there were larger areas
of necrosis and a greater proportion of basaloid cells, which
showed more severe atypical features. Immunohistochemical findings
using the β catenin antibody showed the presence of scattered cells
with cytoplasmic positivity and nuclear staining in the majority of
the basaloid cells. All the results derived from clinical,
surgical, histological and immunohistochemical investigations
strongly supported the diagnosis of a metastatic MP.
The first five-month follow-up was excellent but,
one month later, the dog presented again due to hind limb lameness
and skin lesions. On clinical evaluation, multiple palpable masses
were appreciable on the skin of the right forehead, the skin of the
left mandible, right humerus, left ribs (from third to seventh),
right ileus, right femur, left knee and both tarsi. All the lesions
were CT scanned and were characterized by a dishomogeneous pattern
with mineralized spots, periosteal interrupted proliferations and
moth-eaten osteolysis (Fig. 1F).
No lung or abdominal metastasis was appreciable by thoracic
radiography and abdominal ultrasonography, respectively.
Due to the extremely poor prognosis, euthanasia but
not necropsy was permitted by the owner.
Discussion
The histopathological features of pilomatricoma are
characterized by irregularly shaped, lobulated islands of a dual
cell population of basaloid cells and shadow or ghost cells, which
represent keratinized immature hair cells. Within the lobules, an
abrupt keratinization from basaloid to shadow cells is
characteristic of this neoplasia (3,5).
Histologically, the criteria for malignancy in the
differential diagnosis between MP and its benign counterpart are
the presence of an encapsulated asymmetric ulcerated tumor growth,
increased mitotic figures with nuclear atypical features,
infiltration of the adjacent skin and lymphatic invasion at the
periphery of the mass (3,7,8).
Since almost all the above histological findings
were observed in the present case, the diagnosis of MP was made.
The malignant behavior of the tumor was confirmed subsequently by
the detection of multiple bone metastases. In addition,
immunohistochemical reactivity to β catenin confirmed the diagnosis
of MP.
β catenin is a 92-kDa-sized cytoplasmic protein,
involved in intercellular adhesion and the Wnt-signaling pathway.
The cellular localization of β catenin is determined by its
phosphorylation state. At the cell surface, as a subunit of the
cadherin complex, it interacts with E cadherin, linking it to the
actin cytoskeleton to create the cell-cell adherens junctions. When
this protein is free in the cytosol, it is constitutively
phosphorylated and directed to the nucleus for destruction
(18). Deregulation of the Wnt/β
catenin pathway, attributable to abnormalities of CTNNB1, the gene
encoding β catenin, has been recognized to prevent phosphorylation
of β catenin, resulting in its accumulation in the nucleus. At the
nucleus, β catenin interacts with Tcf/Lef transcription factors to
provide a stimulus for cell proliferation and differentiation, and
most likely for neoplastic transformation (19). Numerous human studies have
demonstrated that mutation of CTNNB1 is a frequent cause of Wnt
signaling pathway activation in pilomatricoma (20,21).
In skin tumors the variability of membrane,
cytoplasmic and nuclear staining of β catenin is great, however, in
pilomatricoma and its malignant counterpart an intense and diffuse
nuclear pattern in the proliferating matrix (basaloid) cells is the
main finding in the majority of the literature (22).
In the present case we observed a predominant
nuclear staining of β catenin in basaloid cells, while a few
transitional cells showed a prevalent membrane-associated
reactivity.
In conclusion, this study documents a case of canine
MP and raises awareness of the potential aggressiveness of matrical
tumors. In addition, the immunohistochemical observations suggest
that β catenin is involved in the pathogenesis of this canine
neoplasm and may be a useful diagnostic marker for MP in dogs, as
previously demonstrated in humans.
References
|
1
|
Stannard AA and Pulley LT: Tumours of the
skin and soft tissues. Tumors in Domestic Animals. Moulton JE: 2nd
edition. University of California Press; Los Angeles: pp.
511978
|
|
2
|
Gould E, Kurzon R, Kowalczyk AP and
Saldana M: Pilomatrix carcinoma with pulmonary metastasis. Report
of a case. Cancer. 54:370–372. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walders E and Gross TL: Neoplastic disease
of the skin. Veterinary Dermatopathology: A Macroscopic and
Microscopic Evaluation of Canine and Feline Skin Disease. Gross TL,
Ihrke PJ and Walder EJ: 2nd edition. Mosby; St. Louis: pp. 365–367.
1992
|
|
4
|
Niedermeyer HP, Peris K and Höfler H:
Pilomatrix carcinoma with multiple visceral metastases. Report of a
case. Cancer. 77:1311–1314. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKee P: Essential Skin Pathology. 2nd
edition. Mosby, International Ltd; London: 1999
|
|
6
|
Abramo F, Pratesi F, Cantile C, Sozzi S
and Poli A: Survey of canine and feline follicular tumours and
tumour-like lesions in central Italy. J Small Anim Pract.
40:479–481. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldschmidt MH and Hendrick MJ: Tumors of
the skin and soft tissues. Tumors in Domestic Animals. Meuten DJ:
4th edition. Iowa State Press; Ames: pp. 61–63. 2002
|
|
8
|
Goldschmidt MH and Shofer FS: Skin Tumours
of the Dog and Cat. 1st edition. Pergamon Press; Oxford: 1992
|
|
9
|
Pakhrin B, Kang MS, Bae IH, et al:
Retrospective study of canine cutaneous tumors in Korea. J Vet Sci.
8:229–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sells DM and Conroy JD: Malignant
epithelial neoplasia with hair follicle differentiation in dogs.
Malignant pilomatrixoma. J Comp Pathol. 86:121–129. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldschmidt MH, Thrall DE, Jeglum KA,
Everett JI and Wood MG: Malignant pilomatricoma in a dog. J Cutan
Pathol. 8:375–381. 1981. View Article : Google Scholar
|
|
12
|
Johnson RP, Johnson JA, Groom SC and
Burgess L: Malignant pilomatrixoma in an old english sheepdog. Can
Vet J. 24:392–394. 1983.PubMed/NCBI
|
|
13
|
Rodriguez F, Herraez P, Rodriguez E,
Gomez-Villamandos JC and Espinosa de los Monteros A: Metastatic
pilomatrixoma associated with neurological signs in a dog. Vet Rec.
137:247–248. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jackson K, Boger L, Goldschmidt M and
Walton RM: Malignant pilomatricoma in a soft-coated Wheaten
Terrier. Vet Clin Pathol. 39:236–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carroll EE, Fossey SL, Mangus LM, et al:
Malignant pilomatricoma in 3 dogs. Vet Pathol. 47:937–943. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Ham L, van Bree H, Maenhout T, et al:
Metastatic pilomatrixoma presenting as paraplegia in a dog. J Small
Anim Pract. 32:27–30. 1991.
|
|
17
|
Huzella L, Ide A, Steinbach TJ, Blanchard
TW, Lipscomb TP and Schulman FY: Osteosarcoma in malignant
pilomatricoma. Vet Pathol. 42:7002005.
|
|
18
|
Morin PJ, Sparks AB, Korinek V, et al:
Activation of beta-catenin-Tcf signaling in colon cancer by
mutations in beta-catenin or APC. Science. 275:1787–1790. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barth AI, Näthke IS and Nelson WJ:
Cadherins, catenins and APC protein: interplay between cytoskeletal
complexes and signaling pathways. Curr Opin Cell Biol. 9:683–690.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan EF, Gat U, McNiff JM and Fuchs E: A
common human skin tumour is caused by activating mutations in
beta-catenin. Nat Genet. 21:410–413. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia J, Urabe K, Moroi Y, et al:
beta-Catenin mutation and its nuclear localization are confirmed to
be frequent causes of Wnt signaling pathway activation in
pilomatricomas. J Dermatol Sci. 41:67–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreno-Bueno G, Gamallo C, Pérez-Gallego
L, Contreras F and Palacios J: beta-catenin expression in
pilomatrixomas. Relationship with beta-catenin gene mutations and
comparison with beta-catenin expression in normal hair follicles.
Br J Dermatol. 145:576–581. 2001. View Article : Google Scholar : PubMed/NCBI
|