Introduction
Carotid artery stenosis is one of the most common
neurological diseases. Depending on the severity of this disease,
patients show a number of brain and/or ocular ischemic symptoms and
signs (1). Considering its high
occurrence rate in neurology and its correlation with risk factors
such as gender, hypertension and smoking, ocular ischemic diseases
caused by carotid artery stenosis are generally recognized as a
neurological disease (2,3). Complex and diverse clinical
manifestations of the disease may, however, lead to a missed
diagnosis or misdiagnosis. Some patients may then develop amaurosis
fugax, diplopia, decreased vision or permanent blindness. Doctors
must therefore analyze conditions thoroughly to offer timely and
effective treatments.
Some scholars (1)
specifically defined ‘ocular ischemic syndrome’ as a series of
brain and eye clinical syndromes caused by chronic severe carotid
artery obstruction or stenosis resulting in brain and eye
insufficiency. However, in this study, a generalized concept was
applied and all ocular ischemic diseases related with carotid
artery obstruction or stenosis were analyzed, including ocular
ischemic syndrome (according to definition), retinal central/branch
vein occlusion, external ophthalmoplegia, ischemic optic neuropathy
and neovascular glaucoma. By retrospective analysis of patients
with carotid artery stenosis who were diagnosed at the First
Affiliated Hospital of Xinxiang Medical University in recent years,
the related etiological factors, clinical presentations and
treatment effects were investigated.
Subjects and methods
Subjects
The clinical data were gathered from 182 patients
with carotid artery stenosis who were diagnosed in the
ophthalmology, neurology, neurosurgery or intervention departments
of the First Affiliated Hospital of Xinxiang Medical University
(Weihui, China) from January 2002 to November 2009. Among them, 107
were males and 75 were females aged between 24 and 87 years (mean,
65.24±8.65 years). All patients were diagnosed using carotid color
Doppler or digital subtraction angiography (DSA) to exclude the
possibility of various primary eye diseases, such as primary
glaucoma, iridocyclitis, retinal vein occlusion, high myopia,
retinal pigment degeneration, retinochoroiditis, diabetic
retinopathy or congenital fundus anomaly. This study was conducted
in accordance with the declaration of Helsinki, and with the
approval of the Ethics Committee of the First Affiliated Hospital
of Xinxiang Medical University. Written informed consent was
obtained from all participants.
Patients’ general information
The clinical data of the patients were gathered for
retrospective analysis. The data included general characteristics
of patients, body and eye medical histories (including amaurosis
fugax and eye pain), past medical histories (including
hypertension, diabetes, hyperlipidemia and coronary heart disease),
personal histories (including smoking and drinking), the department
where the patient was first treated, detailed eye symptoms and
treatment procedures. The 182 patients were divided into two
groups; 78 cases with ocular ischemic symptoms were classified as
group A and 104 cases without ocular ischemic symptoms were
classified as group B.
The 78 cases in group A were treated with drugs
(including antihypertensives, antihyperglycemic agents,
antihyperlipidemic agents or anticoagulants in a total of 19
cases), carotid artery stenting (26 cases) or carotid
endarterectomy (33 cases). Certain patients with ocular ischemic
symptoms also received eye treatments concomitantly (such as
retinal laser photocoagulation, drugs to lower intraocular pressure
or cyclocryotherapy).
Evaluation criteria
The position and degree of the carotid artery
stenosis were judged according to the results of the angiography.
The status of the ophthalmic artery hemodynamics was analyzed using
color Doppler flow imaging (Philips HD7). The detection frequency
was 10 MHz and various ophthalmic artery hemodynamic indices were
measured, including peak systolic velocity (Psv), end diastolic
velocity (Edv), mean glow velocity (Vm), resistive index (RI) and
pulsatility index (PI). Each index was measured in triplicate and
the mean value was used for further analysis.
The effects of different treatments on group A
patients were evaluated according to the following scale: i)
Significantly effective; the patient felt that eye condition
improved markedly. Vision of patients with decreased visual acuity
was improved at least 2 lines after treatment, or ophthalmic artery
hemodynamic indices were obviously improved; ii) Improved; the
patient felt that eye status remained stable or had mild
improvement. Vision of patients with decreased visual acuity was
improved 1 line or remained stable after treatment, or ophthalmic
artery hemodynamic indices showed some improvement; iii) Invalid;
the patient felt symptoms were worse or vision decreased or
ophthalmic artery hemodynamic indices were notably decreased; iv)
Uncertain; the patient had occasional ocular ischemic symptoms or
had no ophthalmic artery hemodynamic indices.
Statistical analysis
Statistical data were presented as means ± SD or a
percentage. The data were analyzed using SPSS 14.0 software (SPSS
Inc., Chicago, IL, USA) for the t-test and χ2 test to
calculate whether the two groups were statistically different. To
avoid confounding and interaction between disease-related factors,
one-way ANOVA and unconditional logistic regression were performed
to analyze the correlation between various factors and onsets of
the two groups of patients. P<0.05 was considered to indicate a
statistically significant result.
Results
Comparison of patient age and gender
Among 182 patients, 19 were aged <50, 35 were
aged 51 to 60, 68 were aged 61 to 70, 45 were aged 71 to 80, and 15
were aged >80. The average age of group A (64.41±9.45) was a
little higher than that of group B (62.12±11.32) but there was no
significant statistical difference (t=0.754, P>0.05).
The position and degree of carotid artery
stenosis
Carotid artery stenosis of both group A and B
patients was mainly located in the common carotid artery
bifurcation and internal carotid artery inlet. In group A, 75.64%
(59) of cases had an over half diameter reduction and the degree of
stenosis was 43–100% (mean, 69.13±7.46). The degree of stenosis in
group B was 34–100% (mean, 48.34±9.23). There was a significant
difference between the degree of the two groups (t=0.754,
P>0.05).
Risk factors associated with carotid
artery stenosis
Group A consisted of 43 males and 35 females,
including 46 patients with hypertension, 41 with hyperlipidemia and
35 smokers; group B consisted of 59 males and 45 females, including
53 patients with hypertension, 48 with hyperlipidemia and 40
smokers. According to the results of one-way ANOVA and
unconditional logistic regression, the onset of carotid artery
stenosis was closely associated with gender (male), hypertension,
hyperlipidemia and smoking (χ2=4.562, 5.151, 4.471 and
4.463, respectively; P<0.05).
Distribution of first-visit patients
Among the 78 patients with ocular ischemic symptoms
(group A), 39 were first seen in the Neurology department (20 of
them transferred to interventional treatment later) and 31 were
first seen in Neurosurgery (4 of them transferred to interventional
treatment later). Only 8 patients were first seen in Ophthalmology
(2 of them transferred to Neurosurgery and 2 patients transferred
to interventional treatment later).
Incidence of ocular ischemic
diseases
In addition to investigating ophthalmic medical
records (including retinal hemorrhage, edema, visual field defects
and increased intraocular pressure), discharged patients were
followed up. Patients of group A recalled several symptoms, such as
amaurosis fugax, eye swelling and periorbital pains (ischemia of
ocular anterior segment) or diplopia. The frequencies of ocular
ischemic symptoms are shown in Table
I.
 | Table IThe ocular ischemic symptoms and their
frequency in group A. |
Table I
The ocular ischemic symptoms and their
frequency in group A.
| Symptoms and
signs | No. of cases | Percentage of
cases |
|---|
| Amaurosis fugax | 37 | 47.44 |
| Eye and periorbital
pains | 28 | 35.90 |
| Diplopia | 11 | 14.10 |
| Hypopsia (visual
field defects) | 26 | 33.33 |
| Retinal hemorrhage,
edema | 19 | 24.36 |
| Increased intraocular
pressure | 3 | 3.85 |
Composition of ocular ischemic
diseases
After classifying cases according to final
diagnoses, 31 patients in group A had ocular ischemic diseases. The
composition is shown in Table
II.
 | Table IIComposition of ocular ischemic
diseases in group A (n=31). |
Table II
Composition of ocular ischemic
diseases in group A (n=31).
| Diseases | No. of cases | Constituent ratio
(%) |
|---|
| Ischemic optic
neuropathy | 10 | 32.26 |
| Ocular ischemia
syndrome | 7 | 22.58 |
| Retinal
central/branch vein occlusion | 7 | 22.58 |
| External
ophthalmoplegia | 4 | 12.90 |
| Neovascular
glaucoma | 3 | 9.68 |
Ophthalmology treatments
In the 8 patients who were first seen in
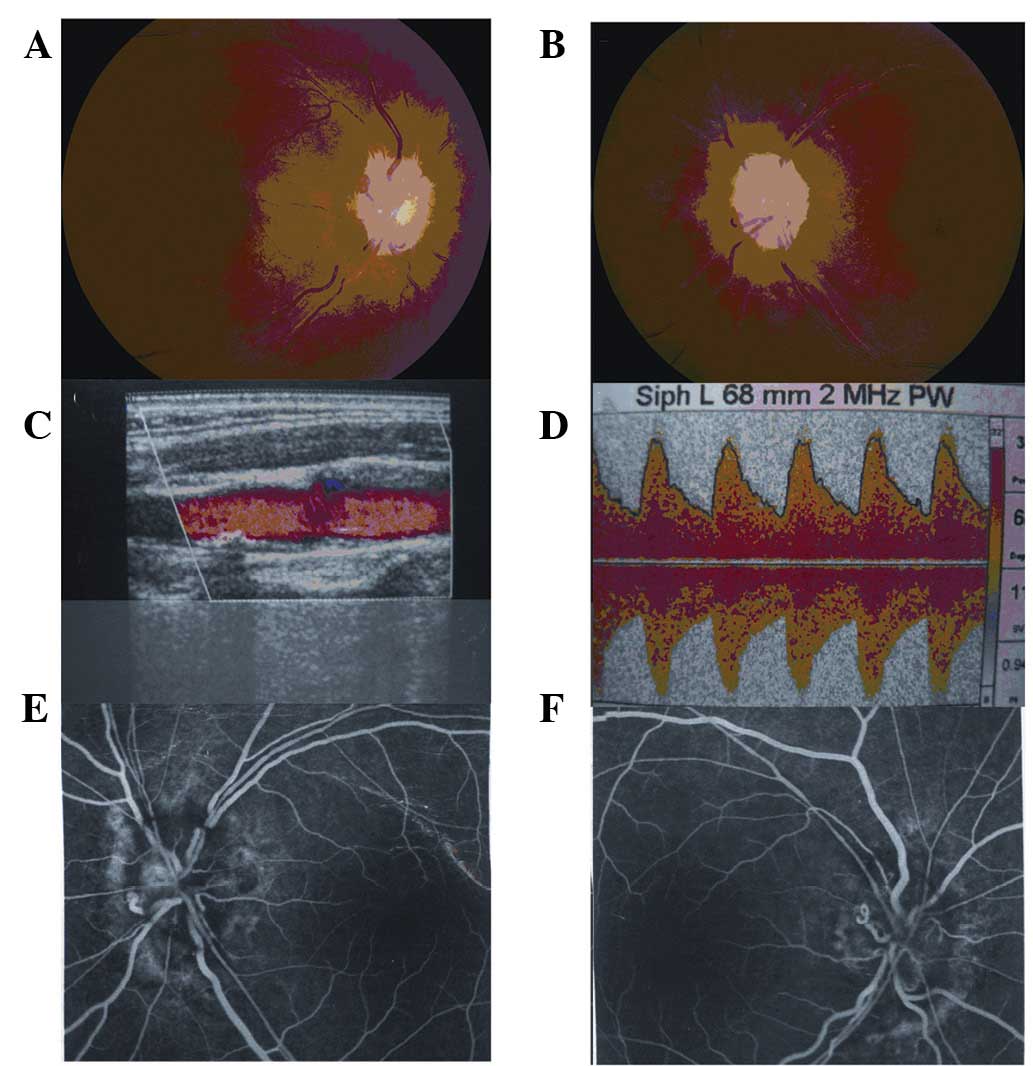
Ophthalmology, 3 patients with ischemic optic neuropathy (Fig. 1) and 3 patients with retinal vein
occlusion had been given several treatments, such as drugs to lower
intraocular pressure, hypodermic injection with anisodine on the
temporal side or circulation-improving medications. Two patients
with neovascular glaucoma had been treated with retinal laser
photocoagulation and cyclocryotherapy, respectively. The other 56
patients of group A had no eye symptoms (such as amaurosis fugax)
or only had transient symptoms (such as eye swelling, periorbital
pains, diplopia) so they were not diagnosed or misdiagnosed and not
administered specific treatment.
Ophthalmic artery hemodynamics
indices
In the 78 patients of group A, the ophthalmic artery
hemodynamics index of 34 patients was examined with color Doppler
flow imaging-before and 3 months after treatment. The results
suggested that all indices were significantly improved (P<0.01)
after treatment with carotid artery stenting (13 cases) and carotid
endarterectomy (11 cases). The indices were also improved after
medical treatment (10 cases) but the difference was not
statistically significant (P>0.05) (Tables III, IV and V).
 | Table IIIComparison of ophthalmic artery
hemodynamic indices before and after medical treatment (mean ± SD,
n=10). |
Table III
Comparison of ophthalmic artery
hemodynamic indices before and after medical treatment (mean ± SD,
n=10).
| Index | Before treatment | After treatment | D-value | P-value |
|---|
| Psv (cm/sec) | 18.74±5.17 | 27.54±6.87 | 8.80±5.58 | 0.067 |
| Edv (cm/sec) | 13.31±4.23 | 18.36±5.21 | 5.05±4.94 | 0.053 |
| Vm (cm/sec) | 14.57±5.03 | 20.17±6.07 | 5.60±6.16 | 0.057 |
| RI | 0.8±0.14 | 0.43±0.11 | 0.41±0.21 | 0.058 |
| PI | 1.48±0.47 | 0.81±0.29 | 0.67±0.38 | 0.063 |
 | Table IVComparison of ophthalmic artery
hemodynamic indices before and after surgical treatment (mean ± SD,
n=11). |
Table IV
Comparison of ophthalmic artery
hemodynamic indices before and after surgical treatment (mean ± SD,
n=11).
| Index | Before treatment | After treatment | D-value | P-value |
|---|
| Psv (cm/sec) | 9.12±2.28 | 28.58±3.63 | 19.46±4.01 | <0.01 |
| Edv (cm/sec) | 7.03±1.35 | 20.83±3.12 | 13.80±2.42 | <0.01 |
| Vm (cm/sec) | 8.09±1.85 | 25.83±3.56 | 17.74±3.31 | <0.01 |
| RI | 1.17±0.06 | 0.33±0.09 | 0.84±0.03 | <0.01 |
| PI | 1.69±0.29 | 0.61±0.13 | 1.08±0.32 | <0.01 |
 | Table VComparison of ophthalmic artery
hemodynamic indices before and after interventional treatment (mean
± SD, n=13). |
Table V
Comparison of ophthalmic artery
hemodynamic indices before and after interventional treatment (mean
± SD, n=13).
| Index | Before treatment | After treatment | D-value | P-value |
|---|
| Psv (cm/sec) | 8.32±2.28 | 24.91±3.76 | 16.59±3.91 | <0.01 |
| Edv (cm/sec) | 7.17±1.35 | 20.72±2.92 | 13.55±2.24 | <0.01 |
| Vm (cm/sec) | 8.76±1.85 | 25.30±3.73 | 16.56±2.93 | <0.01 |
| RI | 1.28±0.06 | 0.52±0.14 | 0.76±0.11 | <0.01 |
| PI | 1.58±0.29 | 0.61±0.23 | 0.97±0.36 | <0.01 |
Treatment effects
Following the comprehensive analyses of patient
information, the results of treatment effects are summarized in
Table VI. The total effective
rates of surgical and interventional treatment were higher than
those observed with medical treatment (t=2.725, t=3.137,
P<0.01).
 | Table VIComparison of effects of three
different treatment methods. |
Table VI
Comparison of effects of three
different treatment methods.
| Treatment | Significantly
effective | Improved | Invalid | Uncertain | Total effective
rate(%) |
|---|
| Medical | 3 | 5 | 8 | 3 | 42.11 |
| Interventional | 12 | 9 | 3 | 2 | 80.77 |
| Surgical | 17 | 11 | 3 | 2 | 84.85 |
Discussion
Ocular ischemic diseases are a series of symptoms
and signs, such as ocular anterior or posterior segment ischemia,
that result from insufficient blood supply from the ophthalmic
artery for a number of reasons (including carotid stenosis or
occlusion). Ocular ischemic diseases include the following five
categories: i) Ocular ischemic (hypoperfusion) syndrome. Carotid
arterial atherosclerosis is the main cause, and Raynaud’s disease
(also known as acral artery spasm caused by vasospastic disorder)
is another possible cause. ii) Obstruction of the central retinal
artery. iii) Retinal vein occlusion (ischemic type). The lumen is
narrowed while the vein passes through the sieve plate area. iv)
Ischemic optic neuropathy. v) Carotid cavernous fistula. Ocular
ischemic diseases in the acute phase show amaurosis fugax, retinal
central/branch vein occlusion, eye swelling or periorbital pains
(ischemia of ocular anterior segment). As the disease develops,
ocular ischemic syndrome and hypoperfusion retinopathy ultimately
develop into neovascular glaucoma in the chronic phase, leading to
permanent blindness (4–6).
This analysis of general information revealed that
patients with ocular ischemic diseases caused by carotid artery
stenosis were mostly elderly (mean age, 64.37±9.70). One-way ANOVA
and unconditional logistic regression analyses also suggested that
the onset of carotid artery stenosis was closely related with
gender (male), hypertension, hyperlipidemia and smoking. These
conclusions agreed with a previous epidemiological survey (7).
Angiography analysis showed that 75.64% (59 cases)
of patients with ocular ischemic symptoms in group A had an over
half diameter reduction following treatment. The degree of stenosis
(mean, 69.13±7.46) was significantly higher than group B (mean,
48.34±9.23). This difference implied that ocular ischemic symptoms
were one of the important signs of severe carotid artery stenosis.
The majority of the 78 patients in group A were first seen in the
Neurology or Neurosurgery departments. Only 8 cases (10.26%) were
first diagnosed in Ophthalmology. Another 14 cases (17.95%) were
seen in Ophthalmology following consultations in Neurology or
Neurosurgery. The other 56 patients (71.79%) were not diagnosed or
misdiagnosed, as eye symptoms (such as amaurosis fugax) were absent
or only had transient symptoms (such as eye swelling, periorbital
pains or diplopia). Some patients developed neovascular glaucoma
and permanent blindness due to lack of timely treatment.
Conversely, chief complaints including amaurosis fugax,
conjunctival swelling, periorbital pains and diplopia were often
misdiagnosed as asthenopia, conjunctivitis, supraorbital neuralgia
and ophthalmoplegia by ophthalmologists, leading to delayed
treatments.
The analyses of ocular ischemic disease composition
suggested that in patients with carotid artery stenosis associated
with ocular ischemic symptoms, ischemic optic neuropathy (32.26%)
ranked first, followed by ocular ischemia syndrome (22.58%) and
retinal central/branch vein occlusion (22.58%). External
ophthalmoplegia (12.90%) and neovascular glaucoma (9.68%) ranked
fourth and fifth respectively. The data provided advantageous
indications for investigating risk factors of ophthalmic clinical
diseases. If unexplained clinical situations, such as decreased
vision, retinal central/branch vein occlusion, ocular ischemic
diseases, external ophthalmoplegia or neovascular glaucoma occur,
doctors should be alert to the possibility of carotid artery
stenosis.
The three main treatment methods for carotid artery
stenosis are medical, surgical (carotid endarterectomy) and
interventional (carotid artery stenting) treatments. Therapists
often provide treatments according to the degree of stenosis, age
and the overall condition. Havelius et al(8) reported that scotopic vision and
photosensitivity of patients improved significantly after carotid
endarterectomy. Wolintz et al(9) suggested that surgical treatments were
helpful for improving blood flow to the brain and eye but not for
eyesight.
Most researchers (10) considered that clinical applications
of carotid endarterectomy and carotid artery stenting had been used
in many cases over many years, and its stable effects had been
confirmed. In addition, patients treated with carotid
endarterectomy or carotid artery stenting are usually provided
adjuvant medical treatment, such as aspirin for anticoagulation.
This comprehensive surgical treatment improves ophthalmic artery
hemodynamics more effectively after carotid endarterectomy and
carotid artery stenting compared with absolute medical treatment.
However, there have been no large-scale clinical experiments to
judge the curative effects of carotid endarterectomy or
interventional treatment for eyesight improvement in China or
abroad as yet (11).
This study further indicated that total effective
rates of surgical and interventional treatment were higher than
absolute medical treatment. Consequently, besides the necessary
ophthalmic treatments, surgical and interventional treatments
should also be actively applied to patients with ocular ischemic
symptoms caused by carotid artery stenosis. This will help treat
the primary disease and promote eye blood circulation in a timely
and effective manner. In addition, the curative effects of nearly
1/5 patients were found to be invalid or uncertain after surgical
or interventional treatments. This is because vision is often
improved in patients with mild to moderate ocular ischemic disease
but not in diseases of long duration, neovascularization disease or
secondary fundus hemorrhage. Fundus lesions and visual impairment
of patients with severe ocular ischemic syndromes may be
irreversible, even if the ocular blood supply and flow are improved
effectively. Diagnosis and treatment in a timely and correct manner
is the best way to treat declining eyesight caused by carotid
artery stenosis.
References
|
1.
|
Wang YL, Zhao L, Huang YX, Feng X and Li
MM: Clinical characteristics of ocular ischemic syndromes. Zhonghua
Yan Ke Za Zhi. 45:1080–1083. 2009.(In Chinese).
|
|
2.
|
Zheng Y, Hua Y, Ling C, et al: Correlation
analysis between risk factors of carotid artery stenosis and
ischemic stroke. Chin J Ultrasound Diagnosis. 5:4–6. 2004.
|
|
3.
|
Zhao HQ, Pan XD, Wang JH, et al: Common
causes of carotid artery stenosis. Foreign Med Sci (Section of
Cranial vascular disease). 12:4972004.
|
|
4.
|
Zhang HR: Ocular changes caused by carotid
artery obstruction or stenosis. Eye Encyclopedia (middle volume).
Li FM: People’s Medical Publishing House; Beijing: pp. 2306–2308.
1995
|
|
5.
|
Takaki Y, Nagata M, Shinoda K, et al:
Severe acute ocular ischemia associated with spontaneous internal
carotid artery dissection. Int Ophthalmol. 28:447–449. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Telman G, Kouperberg E, Nitecki S, et al:
Cerebral hemodynamics in symptomatic and asymptomatic patients with
severe unilateral carotid stenosis before and after carotid
endarterectomy. Eur J Vasc Endovasc Surg. 32:375–378. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chen CS and Miller NR: Ocular ischemic
syndrome: review of clinical presentations, etiology,
investigation, and management. Compr Ophthalmol Update. 8:17–28.
2007.PubMed/NCBI
|
|
8.
|
Havelius U, Bergqvist D, Hindfelt B and
Krakau T: Improved dark adaptation after carotid endarterectomy.
Evidence of a long-term Ischemic penumbra Neurology. 49:1360–1364.
1997.PubMed/NCBI
|
|
9.
|
Wolintz RJ: Carotid endarterectomy for
ophthalmic manifestations: is it ever indicated? J Neuroophthalmol.
25:299–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Faries PL, Chaer RA, Patel S, Lin SC,
DeRubertis B and Kent KC: Current management of extracranial
carotid artery disease. Vasc Endovascular Surg. 40:165–175. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Malhotra R and Gregory-Evans K: Management
of ocular ischemic syndrome. Br J Ophthalmol. 84:1428–1431. 2000.
View Article : Google Scholar
|