Contents
Introduction
Regeneration processes in human myocardium
Characteristics of CSCs
Properties and proliferation of CSCs in the
myocardium of patients with heart disease
Potency of myocardial regeneration in patients with
heart disease
Origin of the c-kit+
CD34−CD45− cells and Ki-67+
cardiomyocytes in the myocardium of patients with heart disease
Origin of Ki-67+ cardiomyocytes and
c-kit+ CD34−CD45− cells in
patients with aortic stenosis
Conclusions
Introduction
Cell therapy in cardiology is the process of
transplantation of sufficient cells into a particular myocardial
area, ensuring the survival and integration of the transplanted
cells. Thus, cells for cell therapy should be multipotent and
non-immunogenic, without oncogenic potency. Several types of stem
cells have been discovered, including embryonic stem cells (ESCs),
hematopoietic stem cells, mesenchymal stem cells (MSCs), bone
marrow stem cells (BMSCs) and cardiac stem cells (CSCs). However, a
suitable stem cell type for cell therapy in cardiology has not yet
been identified.
Previously, it was assumed that the loss of
cardiomyocytes was irreversible, a hypothesis based on the
generally accepted doctrine that cardiomyocytes are subject to
terminal differentiation after birth and no longer participate in
the cell cycle (1). However,
myocardial regeneration in the human heart and the discovery of
CSCs have led to a new paradigm in cell therapy.
Thus, this review discusses the distribution,
properties and proliferation of CSCs in the myocardium and the
potency of myocardial regeneration in patients with heart disease.
In addition, the regeneration processes in the human myocardium are
also discussed.
Regeneration processes in human
myocardium
Previously, it was assumed that the loss of
cardiomyocytes is irreversible and that hypertrophy of remaining
cardiomyocytes is the only compensation for loss of cells in the
heart. These assumptions were based on the generally accepted
doctrine that cardiomyocytes were subject to terminal
differentiation after birth and no longer participated in the cell
cycle (1). However, evidence of
myocardial regeneration in the human heart has led to a new
paradigm. Consequently, the heart may not be a terminally
differentiated organ and may have a higher regenerative capacity
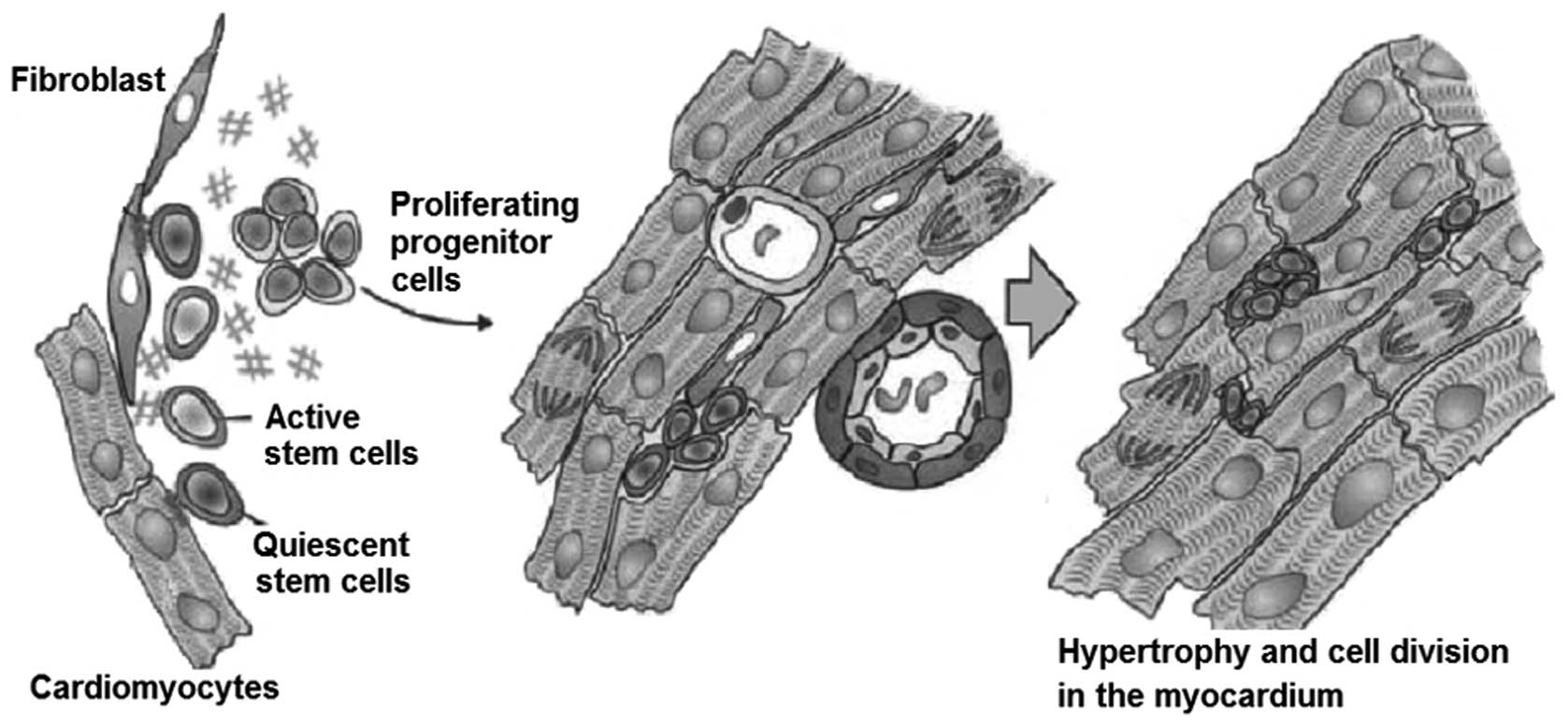
than previously assumed (Fig. 1)
(2).
One study demonstrated that cardiomyocytes possess
regenerative potential in the adult heart, as well as in animal
hearts (3). Anversa et al
first provided evidence of regenerative processes in the human
heart following cardiac transplantation (4). The transplanted heart clearly
contained immigrant cardiomyocyte cells with surface markers. The
origin of these newly-formed cells are the progenitor cells of the
primary heart, in addition to circulating progenitor cells from the
untreated atrial remnants of the receiver (5). Further research revealed the
existence of CSCs in the myocardium of rats, dogs and humans. In
particular, the authors identified a cell population, which were
the marker molecules of adult stem cells. However, these markers
were not expressed by hematopoietic cells. The cell population was
described as CSCs (4).
Characteristics of CSCs
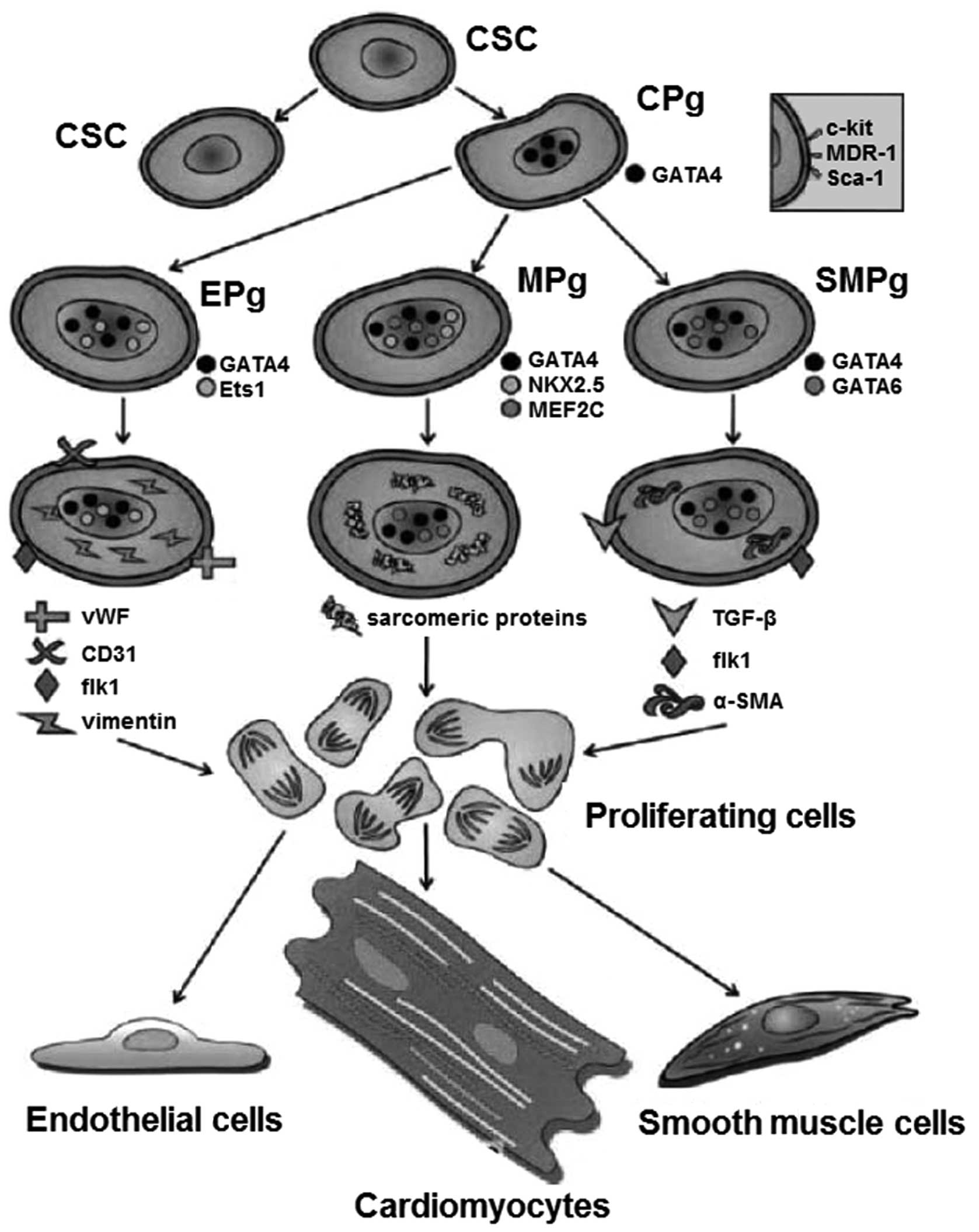
Rodent models of myocardial infarction have revealed
that CSCs have a potential for proliferation and myocardial
regeneration (7). In animal
studies, these cells are differentiated into cardiac cell types,
including cardiomyocytes, endothelial cells and smooth muscle cells
(Fig. 2) (4). This basic hypothesis was strengthened
by the results of other research groups, that described other cell
populations with the characteristics of adult CSCs and the methods
for the isolation and expansion of CSCs (5,7–13).
Therefore, it is important to meet the prerequisites for the
application of this cell population in cell therapy. The surface
markers of CSCs, including stem cell growth factor receptor
(c-kit), a transporter protein (multidrug resistance protein 1;
MDR-1) and a stem cell antigen (Sca-1) have been detected (4). Furthermore, the expression of cardiac
transcription factors, including the cardiac myosin and cytoplasmic
proteins have also been detected in CSCs. Hematopoietic or bone
marrow cell markers were not identified in CSCs (12). Further research is required to
clarify whether similar cell populations also exist in human
tissues and whether they play a role in pathology. Due to their
local resistance, CSCs are the optimal choice for cell replacement
therapy. Among all the cell populations, they are the most similar
to cardiomyocytes and easily integrate into the myocardium. One
study demonstrated that CSCs are able to differentiate into
cardiomyocytes and vascular cells, which is a prerequisite for a
viable myocardium. Furthermore, CSCs are easily isolated and
duplicated in cell culture (5).
This fact predisposes the cell population of CSCs for cell therapy.
A limited factor may be the extremely small number of CSCs
available. To date, it remains unclear how CSCs may be multiplied
to produce sufficient numbers.
Properties and proliferation of CSCs in the
myocardium of patients with heart disease
Studies of the myocardium of various animal species,
including mice (9), rats (6), dogs (14) and pigs (15), revealed the presence of a
c-kit+ CD34−CD45− cell population,
which are similar to CSCs in the human myocardium of patients with
heart disease (4).
c-kit is the receptor of stem cell growth factor and
a marker for the detection of CSCs, since c-kit+ plays
an important role in regeneration processes of the human heart and
has the ability to regenerate myocardial cells (16,17).
Anversa et al demonstrated that c-kit+ cardiac
cells are clonogenic multipotent cells with the ability to
self-renew and differentiate into at least three different
cardiogenic cell lines, including myocytes, smooth muscle cells and
endothelial cells (18). An animal
model demonstrated that c-kit+ cardiac cells are able to
regenerate functional myocardium in vivo, which was not
produced through cell fusion (5).
These cells also succeeded in the differentiation of three
different cardiogenic cells in in vitro beating myocytes
(9). Thus, c-kit+
cardiac cells demonstrated the potential for regeneration of the
various components of the myocardium (5,10,12).
In addition to c-kit, Anversa et al identified the
population of CSCs by the two surface markers MDR-1 and Sca-1
(5). Urbanek et al also
examined three markers, c-kit, Sca-1 and MDR-1, on CSCs. The
authors demonstrated that ∼60% of CSCs expressed all three markers
and 80% of CSCs were c-kit+(19). Thus, the population of CSCs that
are c-kit+ is large. The absolute numbers of the total
CSC population may be identified using c-kit as a marker. The
potential and exact properties of these cells were investigated
further in vivo and in vitro(20,21).
It is known that c-kit, as a marker of hematopoietic
and endothelial stem cells and mast cells, may also be expressed in
the myocardium. Other studies identified that heart cells with
surface markers of stem cells did not express the hematopoietic or
endothelial markers (12,13,22).
The number of c-kit+
CD34−CD45− cells has been determined. The
number of c-kit+ CD34−CD45− cells
in patients with dilated cardiomyopathy, heart failure and aortic
stenosis was 0.19–0.21 cells/mm2(13,23).
The average number of c-kit+ MDR-1-Isl-1
CD45+ cells was 2.7±1.3 in human myocardium biopsies
(17). Beltrami et al
reported one stem cell per 10,000 cardiomyocytes in rats (5) and CSCs were 0.56 per 10,000
cardiomyocytes in the myocardium of dogs (14). Further studies identified a range
of CSCs per 8,000–80,000 cardiomyocytes in mice, rats, dogs and
humans (4). Although all the
studies used c-kit as a stem cell marker, a number of studies used
MDR-1 or Sca-1 (5,16). Moreover, not all CD34+
and CD45+ cells were excluded (5,17).
However, CSCs are relatively rare. It is generally assumed that
adult stem cells are rare, regardless of tissue (24). Thus, hematopoietic
c-kit+ CD34+ stem cells, which were
discovered ∼40 years ago and therefore extremely well studied, were
estimated at 1:10,000–1:15,000 (24).
Potency of myocardial regeneration in
patients with heart disease
Studies have identified that myocardial regeneration
occurs not only in acute pathological conditions, including
following acute myocardial infarction, but also in chronically
damaged hearts (13,19). It was observed that patients with
heart failure or idiopathic dilated cardiomyopathy had
significantly more c-kit+
CD34−CD45− cells than control subjects
without cardiac disease (23).
Several studies demonstrated the activation and proliferation of
CSCs in damaged heart tissue (5,13,19,23).
The occurrence of regenerative processes in pathologically altered
myocardium suggests an increased number of CSCs, as well as an
increased proliferative activity in the myocardium (7,10).
It is interesting that the number of other CSC populations,
including the number of Sca-1+ cells in the myocardium
increases when the adult heart is subjected to a load (25). The increased expression of Sca-1 in
cells of the hypertrophic myocardium leads to an increased demand
for Sca-1+ cells (25).
Studies have revealed cardiac regeneration processes
in pathologically altered myocardium; however, the regeneration
process is not clinically apparent. The small number of
c-kit+ CD34−CD45− CSCs, with an
average of 1.3±1.3 CSCs per 40,000 cardiomyocytes, may not be
sufficient; therefore, the functionally effective regeneration
processes to compensate for serious damage in the myocardium remain
uncertain.
Studies have shown that the number of CSCs in
patients with chronic ischemic heart damage is lower than those
with acute myocardial infarction (19). The formation of new cardiomyocytes
is weakened by prolonged and end-stage heart failure decompensation
(3,26). However, an increased number of
Ki-67+ cardiomyocytes was observed in terminal heart
failure patients who received a heart transplant. Therefore,
regeneration processes may be secondary to myocardial apoptosis.
Urbanek et al observed an increase in apoptotic processes of
CSCs from 0.3 in healthy to 9.6% in chronically damaged myocardium.
The authors identified that heart failure patients have a higher
number of p53-positive senescent CSCs with short telomeres. p53 and
telomere shortening are markers of cellular aging processes
(27). p53 induces growth arrest
and cellular senescence via cyclin-dependent kinase (CDK)4 and
CDK6, which block the retinoblastoma protein in its active
hypophosphorylated state (28).
This loss of functionally competent CSCs in chronic ischemic damage
is the progressive loss of function in terminal heart failure
(19). It is of note that
c-kit+ CD34−CD45− cells could be
detected in the myocardium of heart failure patients and healthy
individuals (23). An increase of
c-kit+ CD34−CD45− cells was
observed in the damaged heart. However, 59% of the
c-kit+ CD34−CD45−positive cells
cells were p16INK4a-positive in the damaged heart, but
only 14% of the c-kit+ CD34−CD45−
cells expressed p16INK4a in the normal myocardium of
control patients (23). The
c-kit+ cells cells significantly increased in the
myocardium of patients with heart failure (23).
An interesting aspect is the consequence of a sudden
interruption of blood supply in all organs, regardless of whether
an institution possesses proliferation capacity. The kidneys are
known to have cells that are able to re-enter the cell cycle and
actively proliferate (29).
However, a heart attack results in cell death, tissue loss and scar
formation in the ischemic region. Thus, the self-renewal potential
of the heart is due to cell regeneration from stem cells. Slowly
progressive lesions are compensated by this pool of cells before
they enter into clinical appearance (13,30).
Origin of the c-kit+
CD34−CD45− cells and Ki-67+
cardiomyocytes in the myocardium of patients with heart
disease
A significantly increased number of
c-kit+ CD34−CD45− cells was
observed in the myocardium of 19 heart failure patients compared
with seven healthy control subjects (23). A ten-fold increase in the number of
dividing cardiomyocytes in heart failure was observed in another
study (19). If we define the
dividing cardiomyocytes by the presence of the marker Ki-67, there
is an increase of at least four times in patients with heart
failure.
Based on the increased detection of
c-kit+ CD34−CD45−CSCs and
Ki-67+ cardiomyocytes with terminal heart failure, the
question arises as to the origin of these cells. The fundamental
problem regarding adult stem cells is the lack of definition.
Unlike embryonic stem cells, which are defined by their origin from
the inner cell mass of the blastocyst, there is no exact definition
for adult stem cells. The real origin of adult stem cells,
independent of tissue is unknown (5). Thus, the origin of the increased
number of c-kit+ CD34−CD45−
cardiac cells and Ki-67+ cardiomyocytes with terminal
heart failure was interpreted in several ways: i) activation of
CSCs, which increases the total number and proliferative activity
of the myocardium (5,13,14,19);
ii) immigration of non-CSCs; iii) lack of differentiation of
progenitor cells under pathological conditions and thus
accumulation of juvenile cardiomyocytes; iv) dedifferentiation and
expression of fetal and embryonic molecules of damaged adult
cardiomyocytes.
In relation to ii), the detection of
CD34−CD45−cells in the myocardium of heart
failure patients may involve immigrant endothelial or hematopoietic
progenitor cells lagging behind their migration into the myocardium
using their endothelial and hematopoietic markers (31).
Origin of Ki-67+ cardiomyocytes
and c-kit+ CD34−CD45−cells in
patients with aortic stenosis
To analyze the cellular levels in the left ventricle
of the heart, a large number of samples should be collected to
obtain statistically reliable results. Approximately half the total
number of c-kit+ CD34−CD45− or
Ki-67+ cardiomyocytes are present in the left ventricle
of patients with aortic stenosis. The myocardium of the left
ventricle is involved in the pathogenesis of this disease and if
these cells were identified here, the regeneration processes would
be expected, particularly since the rate of newly formed myocytes
increases with an increase in wall stress (7,10).
The reason for this discrepancy may be in the evaluation of a given
area that is exposed, particularly in aortic stenosis of the left
ventricle, to chronic pressure load, resulting in the development
of concentric hypertrophy (30).
Consequently, larger and fewer cells may have been included in the
analysis. Nevertheless, more c-kit+ Lin− and
Ki-67+ cells were detected in samples from the outflow
tract of 36 patients with relevant aortic valve stenosis and valve
replacement compared with 12 control subjects who succumbed to a
non-cardiac cause (13). An
increase in Ki-67+ cardiomyocytes was also reported
compared with c-kit+ CD34−CD45−
cells. The growth and differentiation of stem cells into mature
cardiac myocytes in the hypertrophied myocardium of patients with
chronic aortic valve was associated with the fact that patients
with aortic stenosis have good ventricular function for a number of
years before decompensation becomes visible (32). It is known that patients with
aortic stenosis typically remain asymptomatic for a long period
(30).
These patients also had a good pump function [left
ventricular ejection fraction (LVEF), 53.2±13.2%]. This suggests
that small, gradual restriction of ventricular function may be
balanced in contrast to fulminant ischemia. This regeneration
process may contribute to hemodynamic changes and remain stable
over long periods.
Conclusions
The heart has long been regarded as a terminally
differentiated organ. However, there are numerous indicators of
potential regeneration of the myocardium. Previously, CSCs were
discovered in the human heart and have been attributed to a
particular role. Cell therapy in cardiology is a new method for the
treatment of patients with heart disease. However, a suitable stem
cell type has not yet been identified for cell therapy in
cardiology. This review discusses the distribution, properties and
proliferation of CSCs in the myocardium. The potency of myocardial
regeneration and the regeneration processes in patients with heart
disease were also discussed.
References
|
1.
|
Nakamura T and Schneider MD: The way to a
human’s heart is through the stomach: visceral endoderm-like cells
drive human embryonic stem cells to a cardiac fate. Circulation.
107:2638–2639. 2003.
|
|
2.
|
Kajstura J, Leri A, Castaldo C, et al:
Myocyte growth in the failing heart. Surg Clin North Am.
84:161–177. 2004. View Article : Google Scholar
|
|
3.
|
Beltrami AP, Urbanek K, Kajstura J, et al:
Evidence that human cardiac myocytes divide after myocardial
infarction. N Engl J Med. 344:1750–1757. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Anversa P, Kajstura J, Leri A, et al: Life
and death of cardiac stem cells: a paradigm shift in cardiac
biology. Circulation. 113:1451–1463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Beltrami AP, Barlucchi L, Torella D, et
al: Adult cardiac stem cells are multipotent and support myocardial
regeneration. Cell. 114:763–776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Laugwitz KL, Moretti A, Lam J, et al:
Postnatal isl1+ cardio-blasts enter fully differentiated
cardiomyocyte lineages. Nature. 433:647–653. 2005.
|
|
7.
|
Anversa P, Leri A, Kajstura J, et al:
Myocyte growth and cardiac repair. J Mol Cell Cardiol. 34:91–105.
2002. View Article : Google Scholar
|
|
8.
|
Hierlihy AM, Seale P, Lobe CG, et al: The
post-natal heart contains a myocardial stem cell population. FEBS
Lett. 530:239–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Matsuura K, Nagai T, Nishigaki N, et al:
Adult cardiac Sca-1-positive cells differentiate into beating
cardiomyocytes. J Biol Chem. 279:11384–11391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nadal-Ginard B, Kajstura J, Leri A, et al:
Myocyte death, growth, and regeneration in cardiac hypertrophy and
failure. Circ Res. 92:139–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Oh H, Bradfute SB, Gallardo TD, et al:
Cardiac progenitor cells from adult myocardium: homing,
differentiation, and fusion after infarction. Proc Natl Acad Sci
USA. 100:12313–12318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Quaini F, Urbanek K, Beltrami AP, et al:
Chimerism of the transplanted heart. N Engl J Med. 346:5–15. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Urbanek K, Quaini F, Tasca G, et al:
Intense myocyte formation from cardiac stem cells in human cardiac
hypertrophy. Proc Natl Acad Sci USA. 100:10440–10445. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Leri A, Kajstura J and Anversa P: Myocyte
proliferation and ventricular remodeling. J Card Fail. 8:S518–S525.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Smith RR, Barile L, Cho HC, et al:
Regenerative potential of cardiosphere-derived cells expanded from
percutaneous endomyocardial biopsy specimens. Circulation.
115:896–908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Linke A, Muller P, Nurzynska D, et al:
Stem cells in the dog heart are self-renewing, clonogenic, and
multipotent and regenerate infarcted myocardium, improving cardiac
function. Proc Natl Acad Sci USA. 102:8966–8971. 2005. View Article : Google Scholar
|
|
17.
|
Pouly J, Bruneval P, Mandet C, et al:
Cardiac stem cells in the real world. J Thorac Cardiovasc Surg.
135:673–678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Dawn B, Stein AB, Urbanek K, et al:
Cardiac stem cells delivered intravascularly traverse the vessel
barrier, regenerate infarcted myocardium, and improve cardiac
function. Proc Natl Acad Sci USA. 102:3766–3771. 2005. View Article : Google Scholar
|
|
19.
|
Urbanek K, Torella D, Sheikh F, et al:
Myocardial regeneration by activation of multipotent cardiac stem
cells in ischemic heart failure. Proc Natl Acad Sci USA.
102:8692–8697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wagers AJ, Christensen JL and Weissman IL:
Cell fate determination from stem cells. Gene Ther. 9:606–612.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tsuura Y, Hiraki H, Watanabe K, et al:
Preferential localization of c-kit product in tissue mast cells,
basal cells of skin, epithelial cells of breast, small cell lung
carcinoma and seminoma/dysgerminoma in human: immunohistochemical
study on formalin-fixed, paraffin-embedded tissues. Virchows Arch.
424:135–141. 1994. View Article : Google Scholar
|
|
22.
|
Messina E, De Angelis L, Frati G, et al:
Isolation and expansion of adult cardiac stem cells from human and
murine heart. Circ Res. 95:911–921. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Chimenti C, Kajstura J, Torella D, et al:
Senescence and death of primitive cells and myocytes lead to
premature cardiac aging and heart failure. Circ Res. 93:604–613.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Weissman IL: Stem cells: units of
development, units of regeneration, and units in evolution. Cell.
100:157–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Rosenblatt-Velin N, Lepore MG, Cartoni C,
et al: FGF-2 controls the differentiation of resident cardiac
precursors into functional cardiomyocytes. J Clin Invest.
115:1724–1733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kajstura J, Leri A, Finato N, et al:
Myocyte proliferation in end-stage cardiac failure in humans. Proc
Natl Acad Sci USA. 95:8801–8805. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
McConnell BB, Starborg M, Brookes S, et
al: Inhibitors of cyclin-dependent kinases induce features of
replicative senescence in early passage human diploid fibroblasts.
Curr Biol. 8:351–354. 1998. View Article : Google Scholar
|
|
28.
|
Hara E, Smith R, Parry D, et al:
Regulation of p16CDKN2 expression and its implications for cell
immortalization and senescence. Mol Cell Biol. 16:859–867.
1996.PubMed/NCBI
|
|
29.
|
Kim K, Lee KM, Han DJ, et al: Adult stem
cell-like tubular cells reside in the corticomedullary junction of
the kidney. Int J Clin Exp Pathol. 1:232–241. 2008.PubMed/NCBI
|
|
30.
|
Daniel WG, Baumgartner H, Gohlke-Barwolf
C, et al: Aortic stenosis. Clin Res Cardiol. 95:620–641. 2006.(In
German).
|
|
31.
|
Nadin BM, Goodell MA and Hirschi KK:
Phenotype and hematopoietic potential of side population cells
throughout embryonic development. Blood. 102:2436–2443. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Carabello BA and Crawford FA Jr: Valvular
heart disease. N Engl J Med. 337:32–41. 1997. View Article : Google Scholar
|