Introduction
Type 2 diabetes mellitus (T2DM) has become a
significant worldwide health problem. More than 300 million people
have diabetes, representing 6% of the global adult population, with
seven million more people developing the disease each year
(1). T2DM may lead to premature
death in both children and adults, and have devastating
complications, including amputations, kidney and heart disease.
T2DM results from diverse environmental factors, such as viral
infection, obesity, chemical poisoning and genetic variants
(2). Even mutations in a single
gene, such as calpain-10 (CAPN10), sulfonylurea receptor (ABCC8) or
the glucagon receptor (GCGR), may result in this disease. Increased
morbidity and mortality in T2DM mainly result from long-term
microvascular and macrovascular complications. At present, disease
progression of T2DM may be prevented by maintaining a healthy
lifestyle and medical management. Moreover, the treatment of T2DM
requires a combination of drugs, surgery, and dietary and activity
modifications, to improve glycemic control and reduce long-term
complications (3). Therefore, an
effective treatment for T2DM is urgently required.
Of the current treatments for T2DM, Roux-en-Y
gastric bypass (RYGB) surgery is considered to be an effective
long-term treatment (4–6). The post-RYGB change in
gastrointestinal anatomy is reported to be associated with
increased postprandial secretion of incretins, peptides secreted by
the gut that may improve glucose tolerance and decrease insulin
resistance (IR) (7). However, RYGB
surgery involves gastric restriction and bypassing the duodenum. By
contrast, ileal interposition (IT) surgery has the advantages of no
mechanical restriction of meal size, no loss of absorptive surface
and no bypass of the foregut (8).
The mechanisms by which IT surgery controls plasma glucose are not
clear.
WNT signaling is critical for β-cell proliferation
and insulin secretion (9).
Transcription factor 7-like 2 (TCF7L2), also known as TCF-4, is a
transcription factor that functions as a component of the Wnt
signaling pathway. This gene is expressed in several tissues,
including the gut and the pancreas, and a variant of the protein is
linked to an increased risk of developing T2DM. Shu et al
reported that the reduced levels of TCF7L2 gene expression in T2DM
correlated with the down-regulation of receptors for glucagon-like
peptide 1 (GLP-1R) and glucose-dependent insulinotropic polypeptide
(GIP-R) expression, and impaired β-cell function (10).
In the current study, we used Goto-Kakizaki (GK)
rats, a genetic model of T2DM, to investigate whether IT surgery
improves glucose tolerance through upregulating the expression of
TCF7L2. Our findings have identified a critical gene mediating the
treatment of T2DM following IT surgery and thus provides a
theoretical basis for the clinical treatment of T2DM.
Materials and methods
Animals
Eight-week-old male GK rats were purchased from
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The GK
rats were randomly assigned to the GK-IT and GK-Sham groups (n=6
for each). Six age-matched Wistar (WS) rats (Shanghai SLAC
Laboratory Animal Co., Ltd.) were allocated to the WS-Sham group.
All rats were housed in individual cages for ≥7 days prior to
surgery. The rats were kept in a climate-controlled room with 12 h
light/dark cycle and received a standard chow diet and water ad
libitum. All animal experimental procedures were approved by
the Capital Medical University Institutional Animal Investigation
Committee.
Surgery and post-surgical care
IT surgery was performed as described by Culnan
et al(11). In the GK-IT
group, rats fasted overnight were anesthetized with an
intraperitoneal injection of 300 mg/kg chloral hydrate. The ileum
was divided 5 cm from the cecum and again at 15 cm, isolating a 10
cm segment of neurovascularly intact ileum on a mesenteric pedicle.
The ileum was then anastomosed with interrupted 5-0 silk. The
jejunum was then divided 5 cm from the ligament of Treitz and the
ileal segment was anastomosed in an isoperistaltic direction with
5-0 interrupted silk sutures. The abdomen was closed
thereafter.
Sham surgeries were performed on the GK-Sham and
WS-Sham groups. Sham-surgery animals were anesthetized in the same
manner as the IT group. Sham surgeries were performed by making
transections in the same locations as in the IT-operated animals,
but the bowel segments were reattached by anastomosis to their
original position.
All animals were housed individually following the
surgery. A liquid diet (10% glucose) was administered for the first
2 days, and the rats were then returned to regular chow.
Body weight and food intake
The body weight and food consumption of the rats
were recorded at 7 days pre-surgery and 7, 14, 21 and 28 days
post-surgery. The rate of food intake was calculated using the
following equation: Food intake rate = daily food consumption
(g)/rat body weight (kg).
Measurement of fasting plasma
glucose
The fasting plasma glucose levels of the rats were
measured 7 days prior to surgery and 28 days following surgery
using a blood glucose meter after 12 h of fasting.
ELISA
At day 7 pre-surgery and day 28 post-surgery, 0.5 ml
blood was collected from the tail vein at 0 or 30 min following
glucose gavage (1 g/kg body weight) after 12 h of fasting. Serum
was isolated by centrifugation and stored at −80°C until analysis
of the insulin concentrations using the ELISA kit (IBL
International GmbH, Hamburg, Germany) according to the
manufacturer’s instructions.
IR analysis
The homeostasis model assessment (HOMA) was used to
assess IR from fasting plasma glucose and plasma insulin levels as
follows: HOMA-IR = fasting plasma glucose (mmol/l) x fasting
insulin (mU/ml)/22.5.
Tissue collection
All rats were sacrificed 28 days post-surgery. The
rats were anesthetized with an intraperitoneal injection of chloral
hydrate (300 mg/kg) and pancreatic tissue was collected and stored
at −80°C for further analysis.
RNA extraction and quantitative
RT-PCR
Total RNA was isolated from pancreatic tissue with
TRIzol (Invitrogen Life Technologies, Grand Island, NY, USA; Cat #
15596026), and the first strand of cDNA was synthesized using
M-MLV-RTase (Promega Corporation, Madison, WI, USA) according to
the manufacturer’s instructions. The resulting cDNA was used for
PCR using the SYBR-Green Master PCR mix (Applied Biosystems,
Carlsbad, CA, USA) in triplicate. All quantitations were normalized
to the endogenous β-actin control. Primers for qRT-PCR were as
follows: TCF7L2, forward: GCCTCTCATCACGTACAGCA and reverse:
GGATGGGGGATTTGTCCTAC; β-actin, forward: CACCACCATGTACCCTGGCA and
reverse: GCTGTCACCTTCACCGTTCC. PCR and data collection were
performed on the TP800 qPCR System (Takara Bio, Inc., Otsu, Japan).
The relative quantitation value for the TCF7L2 gene was expressed
as 2−(Ct−Cc), where Ct and Cc are the mean threshold
cycles of the calibrator and target, respectively, after
normalizing to β-actin.
Western blotting
Total protein was obtained from the pancreatic
tissues. Cell lysates (30 μg protein) were resolved on 10%
SDS-PAGE and transferred to a polyvinylidene difluoride membrane
(Millipore, Bedford, MA, USA). Protein was probed for rabbit
anti-TCF7L2 (Abcam, Inc., Cambridge, MA, USA) or rabbit anti-actin
(Saier Biotechnology Co., Ltd., Tianjing, China) antibodies,
followed by incubation with an HRP-conjugated secondary antibody
(Saier Biotechnology Co., Ltd.) and visualized using a Western
Lightning® Plus enhanced chemiluminescence substrate
(ECL; PerkinElmer Inc., Waltham, MA, USA). The density of the bands
was analyzed using LabWorks™ 4.0 (UVP, Upland, CA, USA).
Statistical analysis
The data were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA) and presented as the means ± SD.
Statistically significant differences were determined using one-way
analysis of variance (ANOVA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Effects of IT on rat body weight and food
intake
Following surgery, all rats survived the 28-day
experiment. No significant differences were observed in body weight
and food intake per 1 kg body weight among the three groups
pre-surgery. However, two weeks post-surgery, the body weight and
food consumption of rats in the GK-IT group were significantly
lower than those in the other two groups (P<0.05; Tables I and II).
 | Table IRat body weight (g) 1 week prior to
and at each of the 4 weeks after surgery in each group. |
Table I
Rat body weight (g) 1 week prior to
and at each of the 4 weeks after surgery in each group.
| Time point | GK-Sham | GK-IT | WS-Sham |
|---|
| 1 week
pre-surgery | 242.33±2.73 | 240.83±3.31 | 240.67±4.32 |
| 1 week
post-surgery | 233.50±3.08 | 232.83±3.66 | 231.50±6.41 |
| 2 weeks
post-surgery | 239.33±2.50 |
222.50±3.39a,b | 236.67±5.89 |
| 3 weeks
post-surgery | 247.50±4.97 |
234.33±3.61a,b | 249.00±2.10 |
| 4 weeks
post-surgery | 255.33±3.08 |
239.83±4.99a,b | 267.67±3.08 |
 | Table IIFood intake rate (g/kg/d) 1 week prior
to and at each of the 4 weeks after surgery in each group. |
Table II
Food intake rate (g/kg/d) 1 week prior
to and at each of the 4 weeks after surgery in each group.
| Time point | GK-Sham | GK-IT | WS-Sham |
|---|
| 1 week
pre-surgery | 66.78±1.23 | 67.02±2.27 | 67.50±2.34 |
| 1 week
post-surgery | 52.94±2.29 | 52.30±1.46 | 54.07±1.15 |
| 2 weeks
post-surgery | 59.61±1.49 |
40.08±3.53a,b | 58.78±5.17 |
| 3 weeks
post-surgery | 65.09±2.63 |
46.08±2.55a,b | 64.05±3.08 |
| 4 weeks
post-surgery | 73.86±2.51 |
51.98±2.70a,b | 77.78±2.64 |
Fasting plasma glucose
The fasting plasma glucose levels of the GK-IT and
GK-Sham groups were higher than that of the WS-Sham group
pre-surgery. The fasting plasma glucose in the GK-IT group after
surgery showed a significant reduction when compared with the
GK-Sham group (P<0.05), and had no apparent difference compared
with the WS-Sham group at 4 weeks post-surgery (Table III).
 | Table IIIFasting plasma glucose levels (mg/dl)
pre-surgery and 4 weeks post-surgery in each group. |
Table III
Fasting plasma glucose levels (mg/dl)
pre-surgery and 4 weeks post-surgery in each group.
| Group | Pre-surgery | 4 weeks
post-surgery |
|---|
| GK-IT | 123.84±7.02a | 95.40±6.48b |
| GK-Sham | 119.34±4.86a | 130.86±9.54 |
| WS-Sham | 92.34±4.32 | 88.74±6.84b |
Insulin levels
There were no significant differences in fasting
plasma insulin levels among all groups pre- and post-surgery.
However, 30 min after the intragastric administration of glucose,
the insulin levels in the GK-IT and GK-Sham groups were
significantly lower than that of the WS-Sham group pre-surgery
(Table IV, P<0.05). There was a
significant increase in the postgavage insulin levels in the GK-IT
group compared with the GK-Sham group after surgery (P<0.05),
although the postsurgical insulin level of the GK-IT group remained
lower than that of the WS-Sham group (P<0.05). In the GK-IT
group, the postgavage insulin levels were significantly higher than
the corresponding pre-surgical levels (P<0.05).
 | Table IVPlasma insulin levels (ng/ml)
pregavage and 30 min postgavage in each group. |
Table IV
Plasma insulin levels (ng/ml)
pregavage and 30 min postgavage in each group.
| Pre-surgery
| 4 weeks post-surgery
|
|---|
| Group | Pregavage | Postgavage | Pregavage | Postgavage |
|---|
| GK-IT | 1.18±0.09 | 2.84±0.07a | 1.12±0.07 |
4.26±0.15a,b,c |
| GK-Sham | 1.19±0.08 | 2.81±0.08a | 1.19±0.08 | 2.90±0.12a |
| WS-Sham | 1.09±0.03 | 5.80±0.24 | 1.14±0.11 | 5.62±0.28 |
IT improves IR
To investigate whether IT improves IR in GK rats,
the HOMA-IR value was examined. As shown in Table V, HOMA-IR values in the GK-IT and
GK-Sham groups were significantly higher than those in the WS-Sham
group pre-surgery (P<0.05). There was no significant difference
in the HOMA-IR values between the GK-IT and WS-Sham groups on day
28 post-surgery, which were both less than that of the GK-Sham
group (P<0.05). Post-surgery values of HOMA-IR were higher than
pre-surgical values in the GK-Sham group (P<0.05). These results
showed that IT improves IR in GK rats.
 | Table VHOMA-IR changes before and after
surgery in each group. |
Table V
HOMA-IR changes before and after
surgery in each group.
| Group | Pre-surgery | 4 weeks
post-surgery |
|---|
| GK-IT | 7.61±0.20a |
5.58±0.12b,c |
| GK-Sham | 7.43±0.58a | 8.09±0.25c |
| WS-Sham | 5.28±0.18 | 5.28±0.18b |
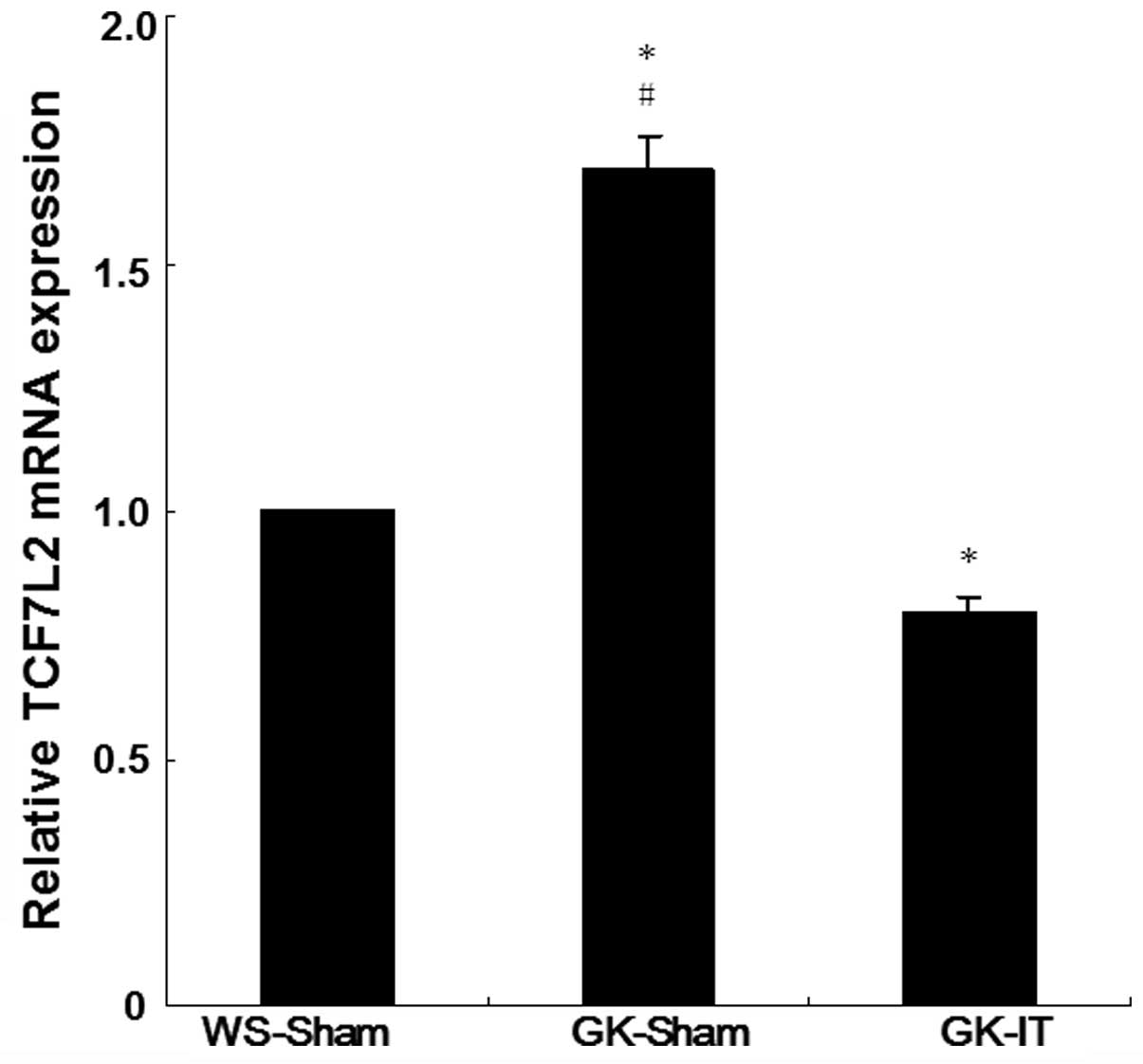
IT surgery upregulates the expression of
TCF7L2
To determine the effect of IT on TCF7L2 expression,
we first investigated the expression of TCF7L2 mRNA in pancreatic
tissue 28 days post-surgery using qRT-PCR. The TCF7L2 mRNA levels
in the GK-Sham group were 1.69-fold higher than those of the
WS-Sham group, with a statistically significant difference
(Fig. 1, P<0.05), suggesting
that the expression level of TCF7L2 mRNA was higher in diabetic
rats than in normal rats. Following IT surgery, the relative
expression levels of TCF7L2 mRNA in GK-IT rats were 20% lower than
those of the WS-Sham group, a significant difference (P<0.05).
These results indicate that IT surgery decreases the level of
TCF7L2 mRNA in the diabetic rats.
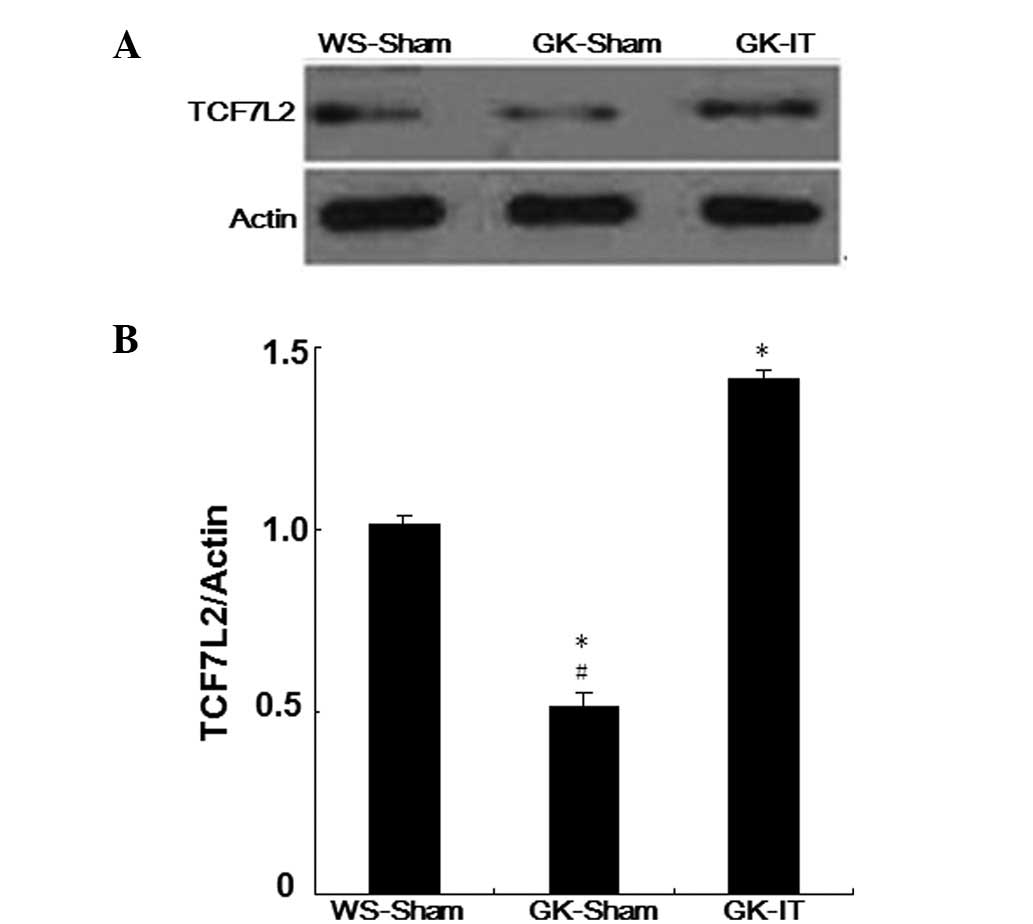
The expression of TCF7L2 protein was analyzed by
western immunoblotting. Notably, an increased TCF7L2 protein level
was observed in the pancreatic tissues of GK rats following IT
surgery (P<0.05 vs. the WS-Sham and. GK-Sham groups; Fig 2). Moreover, the levels of TCF7L2
protein expression in the GK-Sham group were significantly lower
than those of the WS-Sham group after surgery (P<0.05; Fig. 2). These findings demonstrate that
IT surgery is able to increase the levels of TCF7L2 protein in
diabetic rats.
Discussion
In T2DM, deficiency of insulin production and a
decrease in β-cell mass may be attributed to a complex interplay
between genetic predisposition and environmental factors. Many
studies that have focused on the influence of IT on T2DM rats have
discovered that following IT, the rats did not regain weight
post-surgery at the same rate as rats that underwent a sham
surgical procedure (12–14). Moreover, rats with IT have been
able to maintain weight loss and reduce food intake for as long as
6 months after surgery (8).
Reduced calorie intake following IT surgery may be one cause of the
significantly decreased body weight in the GK-IT group. We
discovered that concentrations of fasting plasma glucose and
HOMA-IR dropped to normal levels in the GK-IT group following
surgery. Although the energy intake and body weight in the GK-IT
group post-surgery was increased, the decreased plasma glucose
levels did not reverse. The result indicated that energy intake and
body weight were not direct reasons for the reduced glucose
levels.
In our study, postgavage insulin levels in the GK-IT
group increased significantly and there were no differences in
fasting plasma insulin levels among the three groups pre-surgery.
Previous studies have demonstrated that IT surgery improves islet
structure and increases pancreatic insulin content (15,16).
The increased insulin levels in the GK group after IT are
suggestive of the promotion of β-cell surival and function, since
pancreatic β-cells are the only source of insulin production.
The WNT signaling pathway is known to be associated
with developmental processes such as embryogenesis, pancreatic
islet proliferation and tumorigenesis (17,18).
TCF7L2 plays an important role in the downstream signals of the WNT
pathway (19). Common genetic
variations in the gene that encodes TCF7L2 reveal a strong
association of this protein with T2DM (20,21),
and a number of studies on T2DM have focused on this transcription
factor. It has been shown that, in rodent and human islets, TCF7L2
is essential for normal β-cell survival and secretory functions
(22). Shu et al reported
that silencing the formation of all TCF7L2 isoforms through siRNAs
affects the capacity for insulin secretion in rodents (10). In vitro, TCF7L2 depletion in
islets has been reported to reduce proliferation, induce β-cell
apoptosis and reduce glucose-stimulated insulin secretion (22). A recent study has confirmed that
the selective deletion of TCF7L2 in the mouse pancreas impairs
insulin release and glucose homeostasis (23). In turn, the overexpression of
TCF7L2 protects β-cells from apoptosis induced by chronically
elevated glucose and cytokines.
In our study, the levels of TCF7L2 mRNA in the GK-IT
group were markedly lower than those in the GK-Sham group at 28
days post-surgery, while the protein levels of TCF7L2 were higher.
It has been suggested that IT may result in an increase in the
expression of TCF7L2 in GK rats. Our results are consistent with
those of Shu et al(10),
and showed that TCF7L2 mRNA and protein levels are reciprocally
changed in the islets of rodents with T2DM. The mechanism
underlying the difference in regulation of transcription and
translation remains unclear. It has been speculated that
post-transcriptional regulation of TCF7L2, not the changes in mRNA
levels, may be involved in TCF7L2-regulated β-cell function and
survival. Therefore, increased TCF7L2 mRNA expression in diabetes
may be a consequence of impaired β-cell function owing to a
deficiency of TCF7L2 protein. We considered TCF7L2 to be key in the
downregulation of plasma glucose after IT.
In conclusion, our study illustrated that IT
efficiently down-regulates plasma glucose levels, improves IR, and
increases postgavage insulin levels in T2DM. This type of surgery
improves TCF7L2 protein expression in pancreatic tissues.
Therefore, upregulation of TCF7L2 may be a potential mechanism by
which changes are mediated following IT. Further studies are
required to detect whether increased TCF7L2 protein levels
correlate with upregulation of the GIP receptor and the GLP-1
receptor, which are important for pancreas and β-cell survival,
after IT in T2DM.
Acknowledgements
This study was supported by the
Beijing Medicine Research and Development Fund (Grant No.
2009-3104).
References
|
1.
|
King H, Aubert RE and Herman WH: Global
burden of diabetes, 1995–2025: prevalence, numerical estimates, and
projections. Diabetes Care. 21:1414–1431. 1998.
|
|
2.
|
Stumvoll M, Goldstein BJ and van Haeften
TW: Type 2 diabetes: principles of pathogenesis and therapy.
Lancet. 365:1333–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zimmet P, Alberti KG and Shaw J: Global
and societal implications of the diabetes epidemic. Nature.
414:782–787. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schauer PR, Burguera B, Ikramuddin S, et
al: Effect of laparoscopic Roux-en Y gastric bypass on type 2
diabetes mellitus. Ann Surg. 238:467–484. 2003.PubMed/NCBI
|
|
5.
|
Rubino F, Gagner M, Gentileschi P, et al:
The early effect of the Roux-en-Y gastric bypass on hormones
involved in body weight regulation and glucose metabolism. Ann
Surg. 240:236–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gill RS, Sharma AM, Al-Adra DP, Birch DW
and Karmali S: The impact of bariatric surgery in patients with
type-2 diabetes mellitus. Curr Diabetes Rev. 7:185–189. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Baggio LL and Drucker DJ: Biology of
incretins: GLP-1 and GIP. Gastroenterology. 132:2131–2157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chen DC, Stern JS and Atkinson RL: Effects
of ileal transposition on food intake, dietary preference, and
weight gain in Zucker obese rats. Am J Physiol. 258:R269–273.
1990.PubMed/NCBI
|
|
9.
|
Rulifson IC, Karnik SK, Heiser PW, et al:
Wnt signaling regulates pancreatic beta cell proliferation. Proc
Natl Acad Sci USA. 104:6247–6252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shu L, Matveyenko AV, Kerr-Conte J, Cho
JH, McIntosh CH and Maedler K: Decreased TCF7L2 protein levels in
type 2 diabetes mellitus correlate with downregulation of GIP- and
GLP-1 receptors and impaired beta-cell function. Hum Mol Genet.
18:2388–2399. 2009. View Article : Google Scholar
|
|
11.
|
Culnan DM, Albaugh V, Sun M, Lynch CJ,
Lang CH and Cooney RN: Ileal interposition improves glucose
tolerance and insulin sensitivity in the obese Zucker rat. Am J
Physiol Gastrointest Liver Physiol. 299:G751–G760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Strader AD, Vahl TP, Jandacek RJ, Woods
SC, D’Alessio DA and Seeley RJ: Weight loss through ileal
transposition is accompanied by increased ileal hormone secretion
and synthesis in rats. Am J Physiol Endocrinol Metab.
288:E447–E453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wang TT, Hu SY, Gao HD, et al: Ileal
transposition controls diabetes as well as modified duodenal
jejunal bypass with better lipid lowering in a nonobese rat model
of type II diabetes by increasing GLP-1. Ann Surg. 247:968–975.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chelikani PK, Shah IH, Taqi E, Sigalet DL
and Koopmans HH: Comparison of the effects of Roux-en-Y gastric
bypass and ileal transposition surgeries on food intake, body
weight, and circulating peptide YY concentrations in rats. Obes
Surg. 20:1281–1288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Patriti A, Aisa MC, Annetti C, et al: How
the hindgut can cure type 2 diabetes. Ileal transposition improves
glucose metabolism and beta-cell function in Goto-Kakizaki rats
through an enhanced Proglucagon gene expression and L-cell number.
Surgery. 142:74–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cummings BP, Strader AD, Stanhope KL, et
al: Ileal interposition surgery improves glucose and lipid
metabolism and delays diabetes onset in the UCD-T2DM rat.
Gastroenterology. 138:2437–2446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Welters HJ and Kulkarni RN: Wnt signaling:
relevance to beta-cell biology and diabetes. Trends Endocrinol
Metab. 19:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Angus-Hill ML, Elbert KM, Hidalgo J and
Capecchi MR: T-cell factor 4 functions as a tumor suppressor whose
disruption modulates colon cell proliferation and tumorigenesis.
Proc Natl Acad Sci USA. 108:4914–4919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Jin T and Liu L: The Wnt signaling pathway
effector TCF7L2 and type 2 diabetes mellitus. Mol Endocrinol.
22:2383–2392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Grant SF, Thorleifsson G, Reynisdottir I,
et al: Variant of transcription factor 7-like 2 (TCF7L2) gene
confers risk of type 2 diabetes. Nat Genet. 38:320–323. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lyssenko V, Lupi R, Marchetti P, et al:
Mechanisms by which common variants in the TCF7L2 gene increase
risk of type 2 diabetes. J Clin Invest. 117:2155–2163. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shu L, Sauter NS, Schulthess FT,
Matveyenko AV, Oberholzer J and Maedler K: Transcription factor
7-like 2 regulates beta-cell survival and function in human
pancreatic islets. Diabetes. 57:645–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
da Silva Xavier G, Mondragon A, Sun G,
Chen L, McGinty JA, French PM and Rutter GA: Abnormal glucose
tolerance and insulin secretion in pancreas-specific Tcf7l2-null
mice. Diabetologia. 55:2667–2676. 2012.PubMed/NCBI
|