Introduction
Asthma is a common global public health concern,
affecting ~300 million people worldwide (1). It is a complex disease, which is the
result of interactions between genetic and environmental factors
(2). Investigation of the
correlation between genetic variants and asthma risk has identified
numerous genes conferring susceptibility to asthma (3); among these, the uteroglobulin-related
protein 1 (UGRP1) gene has been extensively studied.
The gene encoding UGRP1, a secreted protein, was
first identified by Niimi et al (4). The human UGRP1 gene is located on
chromosome 5q31–32, a region containing a number of candidate genes
that may play a role in asthma and other allergic diseases. These
genes encode proinflammatory cytokines, such as interleukin-3, −4,
−5, −9 and −13 (5). As a result of
the similarity in the amino acid sequences of UGRP1 and Clara cell
protein (CC16), which exhibits several immunomodulatory and
anti-inflammatory effects, it is possible that UGRP1 may possess
similar functions (6).
Furthermore, UGRP1 mRNA is predominantly expressed in the lung,
with a high level of expression in the epithelial cells of the lung
airway (4). These observations
suggest that the UGRP1 gene may be important in the pathogenesis of
asthma.
Previous studies have investigated the association
between the -112G/A polymorphisms of the UGRP1 gene and asthma
risk. However, while certain studies have described a significant
association (4,7), no such association was observed in
other studies (8–12). Since a single study may lack the
robust power to provide a reliable conclusion, in the present
study, a comprehensive search of the literature and a meta-analysis
was performed to examine whether UGRP1 gene polymorphisms
contribute to asthma susceptibility.
Materials and methods
Publication search
Pubmed, BIOSIS Previews and EBSCOhost were
comprehensively searched, with the last search updated on March 12,
2013. The Medical Subject Heading terms and/or text words utilized
were ‘asthma’ or ‘bronchial hyperreactivity’ or ‘respiratory
hypersensitivity’ or ‘bronchial asthma’, in combination with
‘polymorphism*’ or ‘variant*’ or ‘genetic’ or ‘mutant*’ and in
combination with ‘SCGB3A2’ (secretoglobin, family 3A, member 2) or
‘UGRP1’ or ‘Clara cell secretory protein-related protein’. No
publication language restrictions were imposed. All the searched
studies were retrieved, and their references were checked for other
relevant publications. The search strategy for the study is shown
in Table I.
 | Table ISearch strategies. |
Table I
Search strategies.
| Database | Time span | Search
strategies |
|---|
| Pubmed | 1966-March 12,
2013 | ((“SCGB3A2”[MH] →
“SCGB3A2 protein, human[supplementary concept]”) OR(“SCG3A2”[ALL]
OR “Uteroglobin-related protein1”[ALL] OR “Clara cell secretory
protein-related protein”[ALL] OR “UGRP1”[ALL])) AND
((“polymorphism, genetic”[MH] OR “polymorphism, single
nucleotide”[MH] OR (“polymorphism*”[ALL] OR “variant*”[ALL] OR
“genetic*”[ALL] OR “mutant*” [ALL])) AND (“asthma”[MH] OR
“asthma”[ALL] OR “bronchial hyperreactivity”[ALL] OR “respiratory
hypersensitivity” [ALL] OR “bronchial asthma*”[ALL]) AND
“humans”[MH] |
| EBSCOhost | 1997-March 8,
2013 | (asthma[subject
terms] OR asthma[all text] OR “bronchial asthma”[all text] OR
“bronchial hyperreactivity”[all text] OR “respiratory
hypersensitivity”[all text]) AND (polymorphism[subject terms] OR
polymorphism[all text] OR “genetic polymorphism”[all text] OR
“single nucleotide polymorphism”[all text] OR variant*[all text] OR
mutant*[all text]) AND (SCG3A2[all text] OR “Uteroglobin-related
protein1”[all text] OR “Clara cell secretory protein-related
protein” [all text]) |
| BIOSIS Previews | 1950-March 12,
2013 | #1. Topic=(UGRP1) OR
Topic=(SCGB3A2) OR Topic=(Uteroglobin-related protein1) OR
Topic=(Clara cell secretory protein-related protein)
#2. Topic=(polymorphism*) OR Topic=(variant*) OR Topic=(mutant*) OR
Topic=(genetic*)
#3. Topic=(asthma) OR Topic=(bronchial asthma) OR Topic=(bronchial
hyperreactivity) OR Topic=(respiratory hypersensitivity)
#4. #1 AND #2 AND #3 |
Inclusion and exclusion criteria
Human studies were included if they met the
following criteria: i) Evaluation of the -112G/A polymorphism of
the UGRP1 gene and asthma risk; ii) using a case-control design;
and iii) genotype distributions in comparison groups were available
for estimating an odds ratio (OR) with 95% confidence interval
(CI). Studies were excluded if one of the following existed: i) Not
relevant to UGRP1 gene polymorphisms or asthma risk; ii) design
based on family or sibling pairs; and iii) reviews or abstracts.
When the same patient population was included in several
publications, only the most complete study was included in the
meta-analysis. If the original data for the genotype frequencies
were unavailable in the relevant studies, an email was sent to the
corresponding author for additional data.
Data extraction
Two investigators (Xie and Wu) independently
reviewed the full manuscripts of the eligible studies, and data
were extracted independently into a predesigned data collection
form. The accuracy of the data was verified by comparing the
collection forms from each investigator. Disagreements were
resolved by discussion or by a third author (Cheng) assessing the
articles. The following information was collected from each study:
First author’s name, year of publication, original country,
ethnicity, sample size, asthma definition, genotyping method,
atopic status and genotype numbers in the cases and controls.
Quality score evaluation
The quality score evaluation was performed in
accordance with a previous study (13). Briefly, the following variables
were assessed: Representativeness of cases and controls,
ascertainment of asthma and controls, genotyping examination,
Hardy-Weinberg equilibrium (HWE), association assessment and
response rate. The quality score had a maximum of 15 points. The
higher the study scored, the better the quality was. Studies with
quality scores <4 were excluded (14). All studies included in our
meta-analysis were of a high quality (Table II).
 | Table IICharacteristics of the seven
case-control studies included in the meta-analysis. |
Table II
Characteristics of the seven
case-control studies included in the meta-analysis.
| | | | | | Genotype
AA/GA/GG | | | | | |
|---|
| | | | | |
| | | | | |
|---|
| First author | Year | Country | Ethnicity | Age group | Atopic status | Case, n | Control, n | Asthma
definition | Atopic
definition | Genotyping
method | HWE | Quality scores |
|---|
| Batra J | 2005 | India | Asian | Adults | Atopic | 4/40/121 | 2/40/118 | ATS diagnosis
criteria | History | Sequencing | Yes | 9 |
| Heinzmann A | 2003 | Germany | Caucasian | Children | NA | 5/38/139 | 7/63/169 | Asthmatic symptoms,
medication and bronchial hyperreactivity | SPT, specific and
total IgE | PCR-RFLP | Yes | 10 |
| Inoue K | 2008 | Japan | Asian | Adults | Mixedb
77.3% | 12/53/76 | 5/32/66 | Symptoms, FEV1 or
PEFR, the absence of any other pulmonary diseases | Antigen-specific IgE
or a non-specific IgE | RT-PCR | Yes | 12 |
| Jian Z | 2003 | Japan | Asian | Children
Mixeda | NA | 11/42/78 | 15/109/224 | NA | NA | PCR-RFLP | Yes | 11 |
| Niimi T | 2002 | Japan | Asian | Adults | Mixedb
67.9% | 3/31/50 | 2/13/70 | Symptoms, FEV1 or
PEFR, airway hyperresponsiveness | NA | Sequencing | Yes | 10 |
| Rigoli L | 2007 | Italy | Caucasian | Children
Mixeda | Atopic | 9/30/74 | 10/67/153 | Signs or symptoms
were present | i) SPT, ii) specific
IgE, iii) total IgE | PCR-RFLP | Yes | 10 |
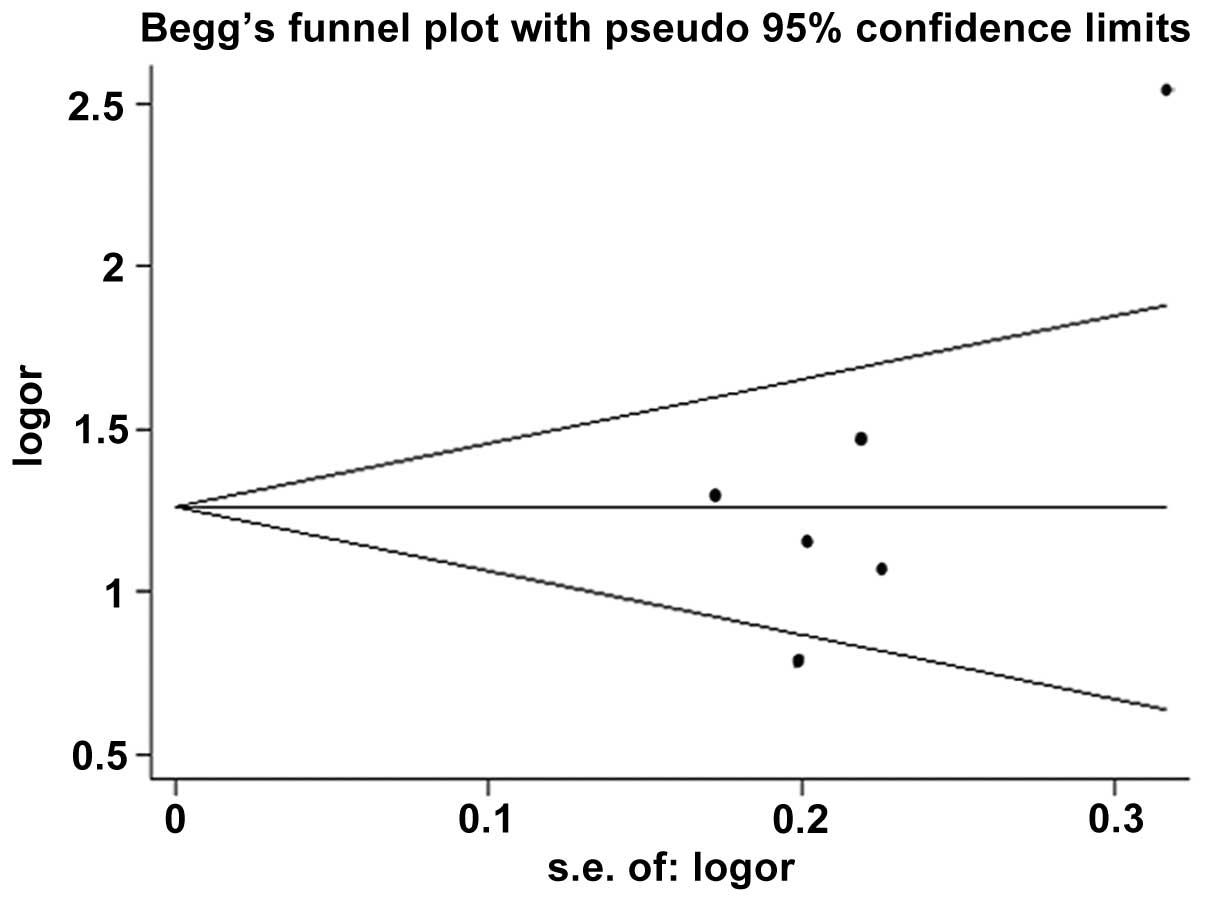
Publication bias
The publication bias of the studies was assessed
using Begg’s funnel plots, and P<0.05 was considered to indicate
a statistically significant difference. Since this method required
a range of studies with varying sizes and subjective judgments
(14), publication bias was also
evaluated using Egger’s linear regression test.
Statistical analysis
Departures from the HWE in the control groups were
assessed using the χ2 test. The meta-analyses were
performed using the following models: i) Allelic (A versus G); ii)
additive (AA versus GG); iii) recessive (AA/AG versus GG); and iv)
dominant (AA versus AG/GG). Subgroup analyses were conducted
according to ethnicity, age and atopic status. The heterogeneity
between the studies was assessed using the χ2 test,
based on the Cochrane Q-test. In addition, I2 was used
to examine the heterogeneity among the included studies. P>0.10
for the Q-test indicated a lack of heterogeneity among the studies.
The pooled OR estimate of each study was then calculated using the
fixed effects model. Otherwise, the random effects model was
used.
All statistical tests were performed using Review
Manager software (version 5.2; The Nordic Cochrane Center,
Copenhagen, Denmark) and STATA 11.0 software (Stata Corp., College
Station, TX, USA). P<0.05 was considered to indicate a
statistically significant difference, with the exception of
heterogeneity tests where a level of 0.10 was used.
Results
Studies included in the
meta-analysis
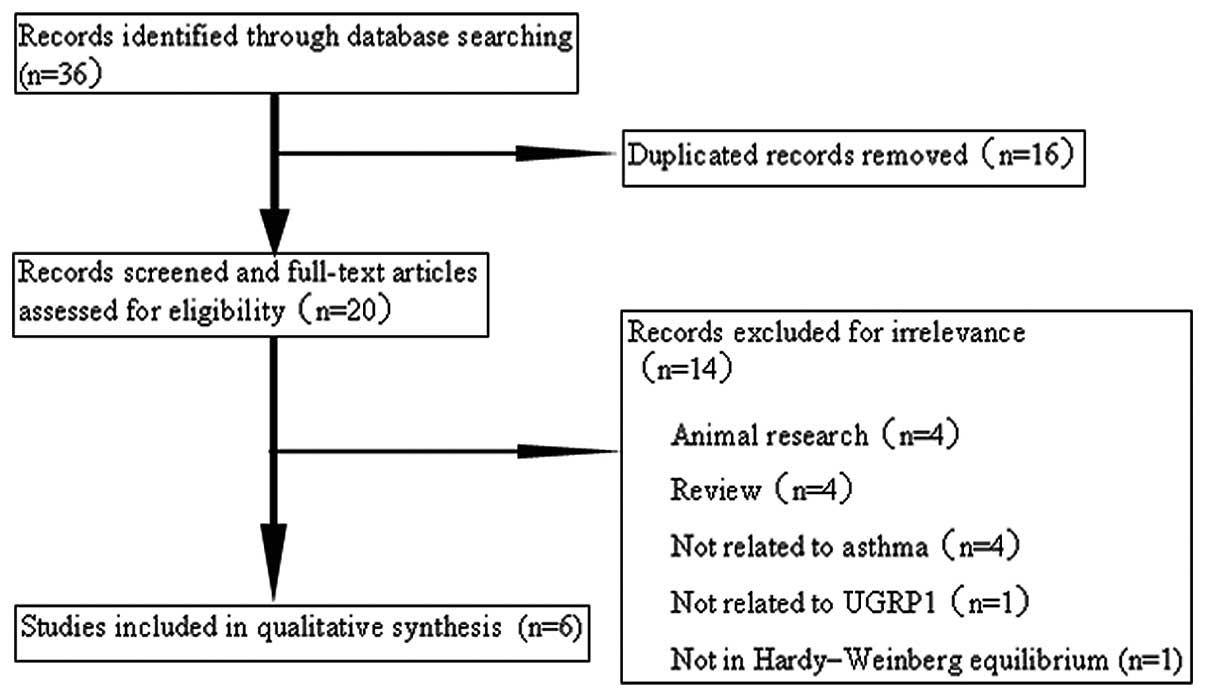
Fig. 1 outlines the
selection process. Briefly, a total of 36 articles were identified
in the initial search. Having reviewed the titles, abstracts and
full-texts, and removed the duplications, six relevant articles
were included in the meta-analysis. These eligible case-control
studies contained 816 cases and 1,165 controls. Two studies
investigated a Caucasian population and four investigated an Asian
population. Three studies were performed with adults and three with
children. Two studies included only patients with atopic asthma,
two studies included patients with atopic asthma and those with
non-atopic asthma (data for these patients were able to be
separately extracted) and two studies did not offer detailed
information with regard to atopic status. The characteristics of
each study included in the meta-analysis are presented in Table II.
Meta-analysis of the UGRP1 gene -112G/A
polymorphism and asthma
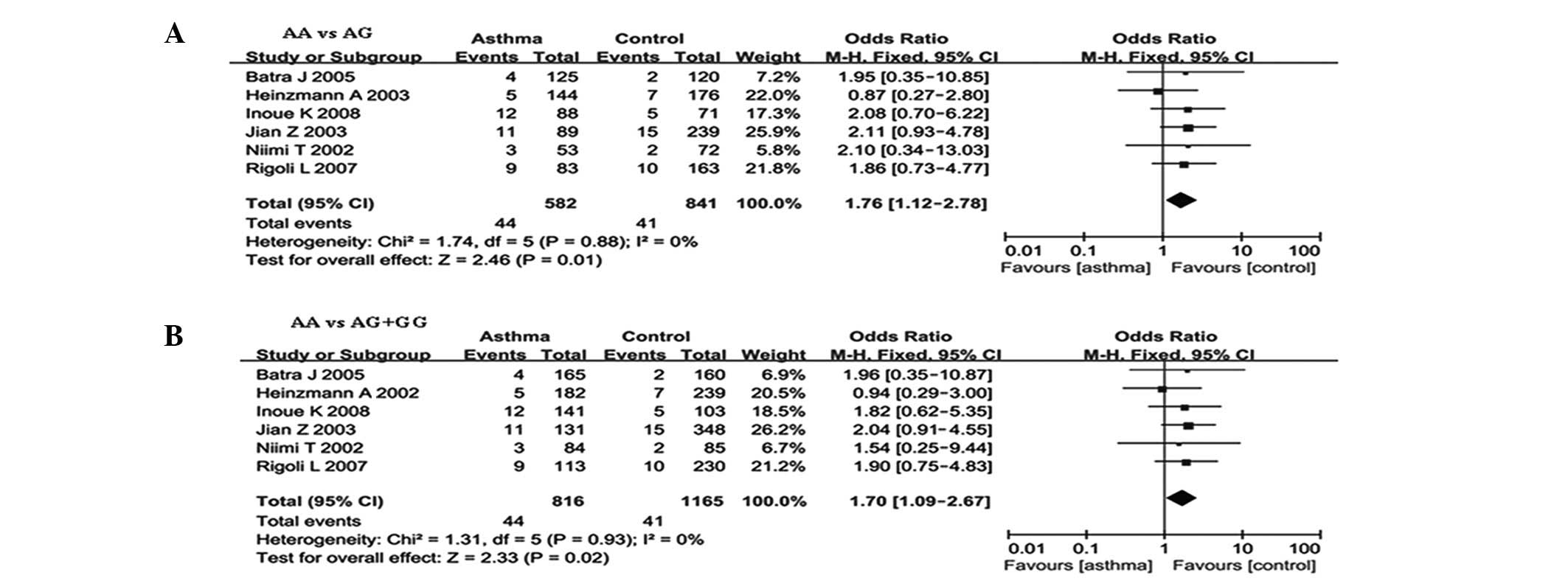
The meta-analysis results are shown in Table III. The combined results of all
the studies showed that there were significant associations between
the UGRP1-112G/A polymorphism and asthma risk in the genetic model
of AA versus GG (OR, 1.76; 95% CI, 1.12–2.78; P=0.01) and in the
genetic model of AA versus GA/GG (OR, 1.70; 95% CI, 1.09–2.67;
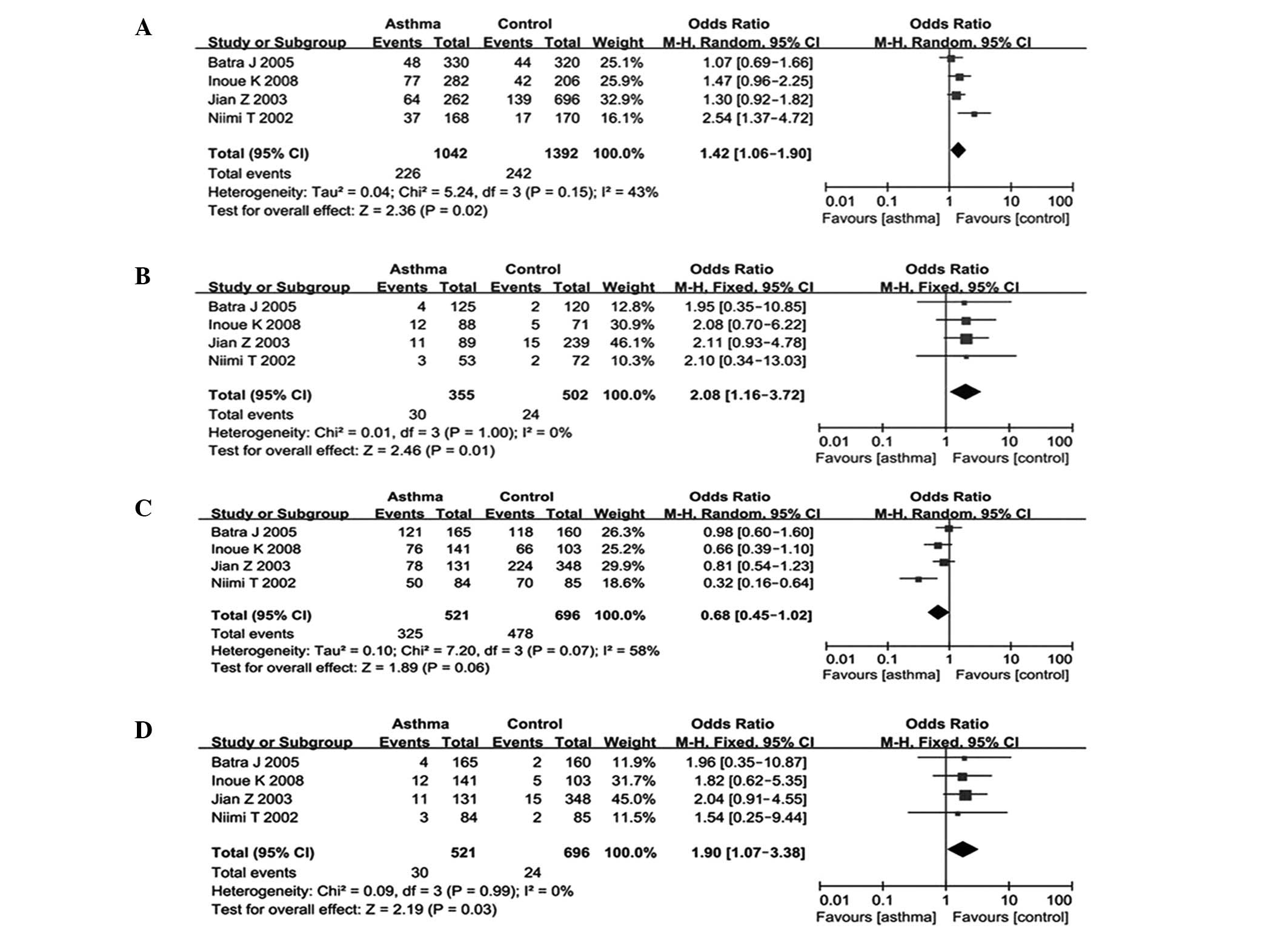
P=0.02) (Table III and Fig. 2). In the subgroup analysis by
ethnicity, significant associations were observed among Asians in
the genetic model of A versus G (OR, 1.42; 95% CI, 1.06–1.90;
P=0.02), AA versus GG (OR, 2.08; 95% CI, 1.16–3.72; P=0.01) and AA
versus GA/GG (OR, 1.90; 95% CI, 1.07–3.38; P=0.03); however, these
associations were not observed in Caucasian populations (Table III and Fig. 3). The subgroup analysis by atopic
status showed associations for A versus G (OR, 1.84; 95% CI,
1.08–3.13; P=0.02) and AA/GA versus GG (OR, 0.47; 95% CI,
0.23–0.97; P=0.04) in the mixed atopic group (Table III). Subgroup analysis was also
performed by age, but no associations were found.
 | Table IIIResults of the pooled and subgroup
analyses of the included studies for the association between the
UGRP1 -112G/A polymorphism and asthma risk. |
Table III
Results of the pooled and subgroup
analyses of the included studies for the association between the
UGRP1 -112G/A polymorphism and asthma risk.
| | | A vs. G | AA vs. GG | AA/GA vs. GG | AA vs. GA/GG |
|---|
| | |
|
|
|
|
|---|
| Variables | n | Cases/controls,
n | OR (95% CI) | PEff | OR (95% CI) |
PEff | OR (95% CI) |
PEff | OR (95% CI) |
PEff |
|---|
| Overall | 6 | 816/1,165 | 1.24
(0.95–1.61) | 0.11 | 1.76
(1.12–2.78) | 0.01 | 0.81
(0.59–1.13) | 0.22 | 1.70
(1.09–2.67) | 0.02 |
| Subgroup by
ethnicity |
| Asian | 4 | 521/696 | 1.42
(1.06–1.90) | 0.02 | 2.08
(1.16–3.72) | 0.01 | 0.68
(0.45–1.02) | 0.06 | 1.90
(1.07–3.38) | 0.03 |
| Caucasian | 2 | 295/469 | 0.95
(0.66–1.39) | 0.81 | 1.40
(0.68–2.92) | 0.41 | 1.14
(0.82–1.59) | 0.42 | 1.43
(0.69–2.94) | 0.33 |
| Subgroup by
atopy |
| Atopic | 2 | 278/390 | 1.12
(0.83–1.50) | 0.47 | 1.88
(0.82–4.30) | 0.13 | 0.97
(0.69–1.36) | 0.84 | 1.92
(0.85–4.35) | 0.12 |
| Mixed | 2 | 225/188 | 1.84
(1.08–3.13) | 0.02 | 2.09
(0.82–5.34) | 0.12 | 0.47
(0.23–0.97) | 0.04 | 1.75
(0.69–4.40) | 0.24 |
| NA | 2 | 313/587 | 1.02
(0.63–1.66) | 0.93 | 1.54
(0.79–3.00) | 0.21 | 1.04
(0.64–1.69) | 0.88 | 1.55
(0.80–3.01) | 0.19 |
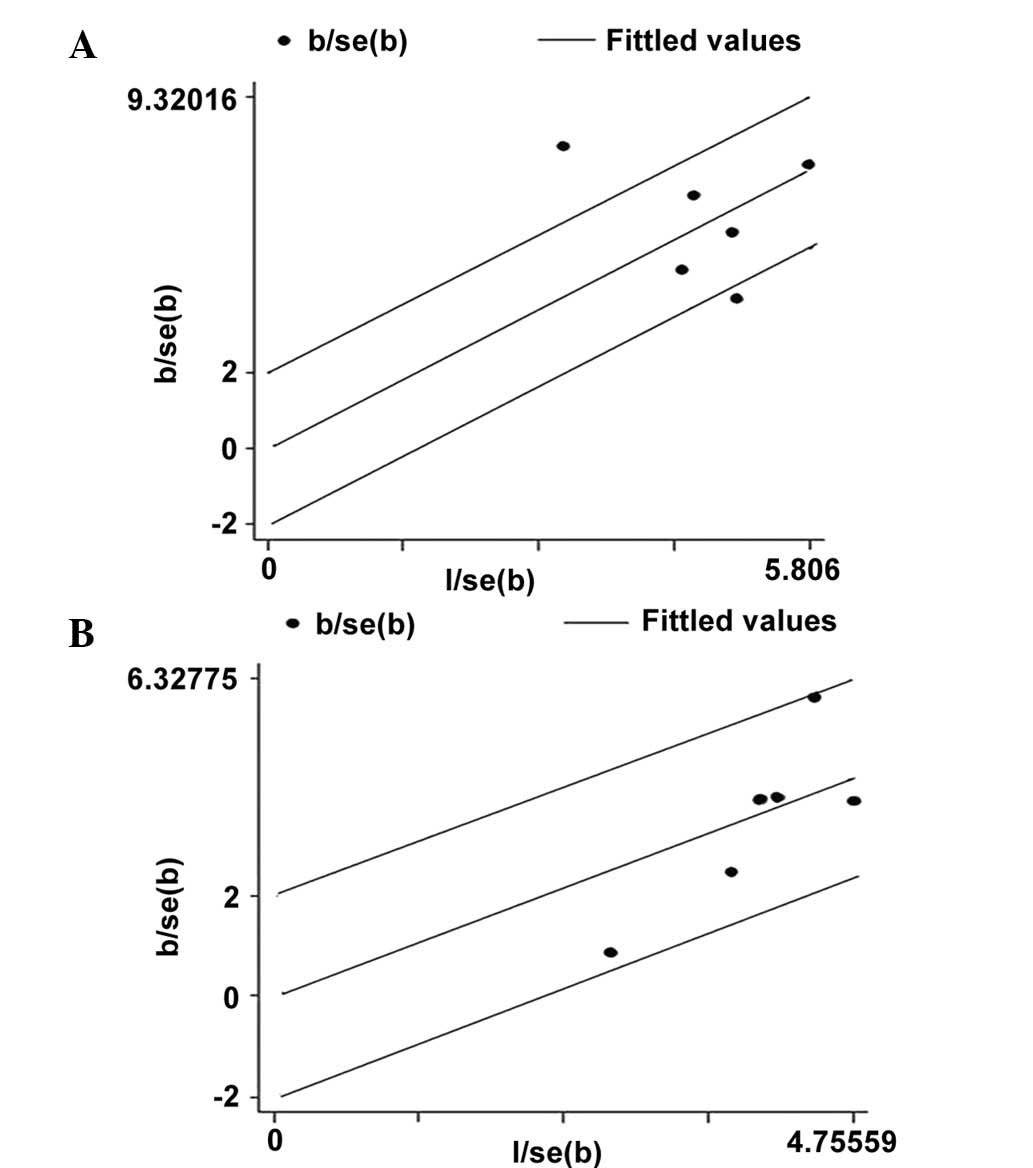
Heterogeneity analysis
No heterogeneity was observed in the additive and
dominant models, however, marked heterogeneity existed in the
allelic and recessive models. Therefore, Galbraith plots were used
to graphically evaluate the source of the heterogeneity. For the
allelic model, one study (5) was
hypothesized to be the main contributor to the heterogeneity
(Fig. 4).
Publication bias
Begg’s funnel plots were used to investigate the
potential publication bias of the studies. The funnel plot
demonstrated evidence of asymmetry for the allelic model (Fig. 5). No evidence of publication bias
was observed in other comparison models using Begg’s funnel plots.
In addition, no publication bias was revealed among the studies
when using Egger’s regression test (P=0.109, 0.794, 0.153 and 0.482
for the allelic, additive, recessive and dominant models,
respectively).
Discussion
Asthma is a complex pulmonary disorder that is
caused by numerous genetic and environmental factors and is the
result of genetic and environmental interaction. The hallmarks of
asthma are airway inflammation, remodeling and hyperresponsiveness
(2,15). The UGRP1 gene was identified by
Niimi et al (4), and is
located in a chromosomal region harboring a number of genes
involved in allergic diseases. UGRP1 is similar to CC16 with regard
to its amino acid sequence and site of tissue-specific expression
(4). As demonstrated by in
vivo and in vitro studies (6,16),
CC16 functions as an anti-inflammatory agent. Similarly, UGRP1 has
been suggested to exhibit anti-inflammatory functions. It has been
shown that UGRP1 is associated with an increased risk for Graves’
disease (17,18). However, there have been confounding
results with regard to the correlation between UGRP1 gene
polymorphisms and asthma risk (5,7,9,19).
Therefore, in the present study, a comprehensive meta-analysis was
conducted to examine the association between the UGRP1 gene -112G/A
polymorphism and asthma risk.
The present meta-analysis of six articles, including
816 patients with asthma and 1,165 controls, investigated the
association between the UGRP1-112G/A polymorphism and asthma risk.
Overall, the pooled results revealed significant associations
between the UGRP1-112G/A polymorphism and asthma risk in the
genetic models of AA versus GG and AA versus GA/GG. To conduct a
more comprehensive study of the correlation between the
UGRP1-112G/A polymorphism and asthma, subgroup analyses were
performed. In the subgroup analysis by ethnicity, significant
associations with asthma were revealed among Asians in the allelic,
additive and dominant genetic models. The allelic model suggested
that carriers of the A allele, including the AA and AG genotypes,
were at a 1.42-fold higher risk of asthma than G allele carriers.
The additive model demonstrated that the AA genotype increased the
risk by 108%, indicating that individuals with the homozygous AA
genotype were likely to have a higher risk of asthma than those
with a GG genotype. Furthermore, the dominant model indicated that
individuals with a homozygous AA genotype were likely to have
higher risk of asthma than those with AG and GG genotypes
(OR=1.90). However, these associations were not observed in the
Caucasian subgroup. The ethnic differences may have been due to
chance, since studies with small sample sizes are likely to have a
low statistical power to detect slight effects. In the
stratification by atopy, associations were observed for the allelic
and recessive models in the mixed group. No significant
associations were revealed for the age stratification.
In the present meta-analysis, heterogeneity existed
in the allelic and recessive models. This issue may have affected
the interpretation of the results. In order to explore the source
of heterogeneity, Galbraith plots were produced for all of the
studies. As shown in Fig. 4, it is
possible that one study (5) may
have been the main source of heterogeneity. The funnel plots were
symmetrical, except the allelic genetic model, and it was indicated
that there was no significant publication bias among the selected
studies using Egger’s test. Despite this, the meta-analysis results
of the present study should be interpreted with caution, due to the
following limitations: i) The number of studies included in the
meta-analysis was small, with only two studies performed with a
Caucasian population; ii) heterogeneity may have affected the
meta-analysis; iii) although Egger’s regression test was performed,
publication bias may still have affected the analysis, as studies
with negative results may not have been published; and iv) the
meta-analysis was not able to assess gene-gene and gene-environment
interactions.
To the best of our knowledge, this is the first
meta-analysis conducted to explore the association between the
UGRP1-112G/A polymorphism and asthma risk. All studies included in
this meta-analysis were of a high quality, which was shown by the
quality score assessment results in Table II.
In conclusion, this meta-analysis indicated that the
-112G/A polymorphism of the UGRP1 gene may be involved in asthma,
particularly in Asian populations. In the future, more
well-designed, high-quality studies are required to assess the role
of the UGRP1-112G/A polymorphisms in the pathogenesis of
asthma.
Acknowledgements
The authors thank Dr Fook T Chew (Department of
Biological Sciences, National University of Singapore, Singapore),
Dr E. Noguchi (Department of Medical Genetics, Institute of Basic
Medical Sciences, University of Tsukuba, Ibaraki, Japan), Dr
Mitsuru Munakata (Department of Pulmonary Medicine, School of
Medicine, Fukushima Medical University, Fukushima, Japan) and Dr
Shioko Kimura (Laboratory of Metabolism, National Cancer Institute,
National Institutes of Health, Bethesda, MD, USA) for providing
relevant information. This study was supported by a research grant
from the Natural Science Foundation of Guangdong Province (no.
2012010009036).
References
|
1
|
Masoli M, Fabian D, Holt S and Beasley R;
Global Initiative for Asthma (GINA) Program. The global burden of
asthma: executive summary of the GINA Dissemination Committee
report. Allergy. 59:469–478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mukherjee AB and Zhang Z: Allergic asthma:
influence of genetic and environmental factors. J Biol Chem.
286:32883–32889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vercelli D: Discovering susceptibility
genes for asthma and allergy. Nat Rev Immunol. 8:169–182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niimi T, Keck-Waggoner CL, Popescu NC,
Zhou Y, Levitt RC and Kimura S: UGRP1, a uteroglobin/Clara cell
secretory protein-related protein, is a novel lung-enriched
downstream target gene for the T/EBP/NKX2.1 homeodomain
transcription factor. Mol Endocrinol. 15:2021–2036. 2001.
View Article : Google Scholar
|
|
5
|
Niimi T, Munakata M, Keck-Waggoner CL, et
al: A polymorphism in the human UGRP1 gene promoter that regulates
transcription is associated with an increased risk of asthma. Am J
Hum Genet. 70:718–725. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh G and Katyal SL: Clara cells and
Clara cell 10 kD protein (CC10). Am J Respir Cell Mol Biol.
17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue K, Wang X, Saito J, et al: Plasma
UGRP1 levels associate with promoter G-112A polymorphism and the
severity of asthma. Allergol Int. 57:57–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heinzmann A, Dietrich H and Deichmann KA:
Association of uteroglobulin-related protein 1 with bronchial
asthma. Int Arch Allergy Immunol. 131:291–295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jian Z, Nakayama J, Noguchi E, Shibasaki M
and Arinami T: No evidence for association between the -112G/A
polymorphism of UGRP1 and childhood atopic asthma. Clin Exp
Allergy. 33:902–904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rigoli L, Di Bella C, Procopio V, et al:
Uteroglobin-related protein 1 gene -112G/a polymorphism and atopic
asthma in Sicilian children. Allergy Asthma Proc. 28:667–670. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Batra J, Niphadkar PV, Sharma SK and Ghosh
B: Uteroglobin-related protein 1(UGRP1) gene polymorphisms and
atopic asthma in the Indian population. Int Arch Allergy Immunol.
136:1–6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andiappan AK, Yeo WS, Parate PN, et al:
Variation in Uteroglobin-Related Protein 1 (UGRP1) gene is
associated with allergic rhinitis in Singapore Chinese. BMC Med
Genet. 12:392011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thakkinstian A, McEvoy M, Minelli C, et
al: Systematic review and meta-analysis of the association between
{beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J
Epidemiol. 162:201–211. 2005.
|
|
14
|
Hyun MH, Lee CH, Kang MH, Park BK and Lee
YH: Interleukin-10 promoter gene polymorphisms and susceptibility
to asthma: a meta-analysis. PLoS One. 8:e537582013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koppelman GH: Gene by environment
interaction in asthma. Curr Allergy Asthma Rep. 6:103–111. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arsalane K, Broeckaert F, Knoops B, Wiedig
M, Toubeau G and Bernard A: Clara cell specific protein (CC16)
expression after acute lung inflammation induced by intratracheal
lipopolysaccharide administration. Am J Respir Crit Care Med.
161:1624–1630. 2000. View Article : Google Scholar
|
|
17
|
Chistiakov DA, Voronova NV, Turakulov RI
and Savost’Anov KV: The -112G>A polymorphism of the
secretoglobin 3A2 (SCGB3A2) gene encoding uteroglobin-related
protein 1 (UGRP1) increases risk for the development of Graves’
disease in subsets of patients with elevated levels of
immunoglobulin E. J Appl Genet. 52:201–207. 2011.
|
|
18
|
Song HD, Liang J, Shi JY, et al:
Functional SNPs in the SCGB3A2 promoter are associated with
susceptibility to Graves’ disease. Hum Mol Genet. 18:1156–1170.
2009.PubMed/NCBI
|
|
19
|
de Burbure C, Pignatti P, Corradi M, et
al: Uteroglobin-related protein 1 and clara cell protein in induced
sputum of patients with asthma and rhinitis. Chest. 131:172–179.
2007.PubMed/NCBI
|