Introduction
Cognitive dysfunction is a symptom characterized by
dysfunction in intellectual performance and learning (1,2).
However, its pathogenesis has yet to be fully elucidated.
Increasing evidence has shown that infection of the central nervous
system is associated with the pathogenesis of cognitive
dysfunction.
Lipopolysaccharide (LPS) is a cell wall component of
Gram-negative bacteria and induces neuronal death, inhibits
neurogenesis and impairs synaptic plasticity and memory (3–6).
Previous studies have indicated that peripheral administration of
LPS causes functional impairments in the brain (7,8).
LPS-induced peripheral infection activates the immune system, which
conveys a message to the brain causing the production of
inflammatory cytokines. Excessive expression of pro-inflammatory
cytokines in the brain may cause behavioral deficits (9,10).
Regulating the inflammatory response in the brain following a
peripheral infection may be important in protection against
behavioral disorders (7).
Moreover, an LPS-induced inflammatory response is characterized by
an increased expression of pro-inflammatory cytokines, which
include interleukin (IL)-1β and tumor necrosis factor-α (TNF-α).
Chronic activation of pro-inflammatory cytokines has been indicated
to be a pivotal factor in the development of cognitive impairment
(11–14). Although large studies have raised
the possibility that pro-inflammatory cytokines are implicated in
cognitive impairments induced by the peripheral administration of
LPS, other studies have not found that cognitive deficits are
improved following antibiotic treatment (15,16).
In addition, a possible mechanism by which the
peripheral inflammatory response may affect cognitive function is
via interference with the expression of amyloid-β (Aβ) and
brain-derived neurotrophic factor (BDNF) (17,18).
The aim of the present study was to investigate the behavioral
performance of rats receiving intraperitoneal injections of LPS and
to determine the expression levels of Aβ, BDNF and pro-inflammatory
cytokines in the hippocampus.
Materials and methods
Animals and drugs
In total, 30 male Wistar rats weighing 180–220 g
were purchased from the Shanghai Animal Center (Shanghai, China).
The rats were housed five per cage with access to food and water
ad libitum and were maintained on a 12-h light/dark cycle
(lights on at 07:00 a.m.). Rats were randomly divided into three
groups (n=10 each) and were intraperitoneally administered saline
or LPS (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 250 μg/kg
for 3 or 7 days consecutively. The experimental procedures were
approved by the Institutional Animal Ethics Committee of Soochow
University (Changzhou, China).
Morris water maze
Following intraperitoneal injections of LPS for 3 or
7 days, the Morris maze test was conducted to measure the cognitive
function of the rats. As previously described (19), the water maze model was performed
in a circular tank (diameter, 1 m) filled with water. A platform
was submerged below the surface of the water in the center of the
target quadrant. The swimming paths of the rats were recorded by a
video camera and analyzed by Videomot software (Huaibei Zhenghua
Biologic Apparatus Facilities Co., Ltd., Huaibei, China). Rats were
placed in the maze from four random points of the tank and were
allowed to search for the platform for 60 sec. However, if this was
not achieved, the rat was gently placed on the platform and left
for 10 sec. The latency to the platform and the proportion of time
spent in the target quadrant were recorded.
Determination of IL-1β, IL-6 and TNF-α
expression levels
Following the behavioral test, rats were immediately
sacrificed by decapitation and the hippocampi were harvested. BDNF,
IL-1β, IL-6 and TNF-α expression levels in the hippocampus were
measured using a sandwich-ELISA with anti-BDNF, IL-1β, IL-6 and
TNF-α antibodies, according to the manufacturer’s instructions
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The
hippocampi were homogenized in phosphate buffer solution with 1 mM
phenylmethylsulfonyl fluoride and 1 mM ethylene
glycol-O,O’-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid.
Microtiter plates (96-well; flat-bottom) were coated for 24 h with
the samples and diluted 1:2 in sample diluent. The standard curve
ranged between 7.8 and 500 pg/ml. Plates were washed three times
with sample diluent and then monoclonal rabbit antibodies, that
were diluted 1:200 in sample diluent, were added to each well. The
plate was then incubated for 2 h at room temperature. After
washing, peroxidase-conjugated anti-rabbit antibodies (1:2,000)
were added to each well and the plate was incubated at room
temperature for 1 h. Following the addition of streptavidin-enzyme,
substrate and stop solution, the levels of BDNF, IL-1β, IL-6 and
TNF-α were determined by absorbance at 450 nm. The standard curve
demonstrated a direct relationship between optical density and
BDNF, IL-1β, IL-6 and TNF-α concentration. Total protein was
measured by the Lowry method, using bovine serum albumin as a
standard.
Determination of Aβ expression
levels
Total RNA was isolated from frozen muscle biopsy
tissues using TRIzol reagent (Tiangen Biotech Co., Ltd., Beijing,
China), according to the manufacturer’s instructions. The
concentration of total RNA was measured by spectrophotometry and
reverse-transcribed with an RT-PCR kit (Tiangen Biotech Co., Ltd.).
Quantitative PCR was performed using a SYBR Green I kit (Tiangen
Biotech Co., Ltd.). Primer sequences were as follows: Aβ forward,
5′-CCAGCCAATACCGAAAATGA-3′ and reverse, 5′-TGATGTTTGTCAGCCCAGAA-3′;
and β-actin forward, 5′-CCTGTGCTGCTCACCGAGGC-3′ and reverse,
5′-GACCCCGTCTCTCCGGAGTCCATC-3′. PCR conditions were 50°C for 2 min,
95°C for 10 min and 40 cycles at 95°C for 15 sec and 60°C for 60
sec.
Statistical analysis
Data are expressed as mean ± SD. Statistical
analyses were performed by one-way analysis of variance and post
hoc analyses were performed using Fisher’s least significant
difference tests. Statistical analyses were conducted using
Statistical Product for Social Sciences (SPSS), version 17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Behavioral performance in the Morris
water maze
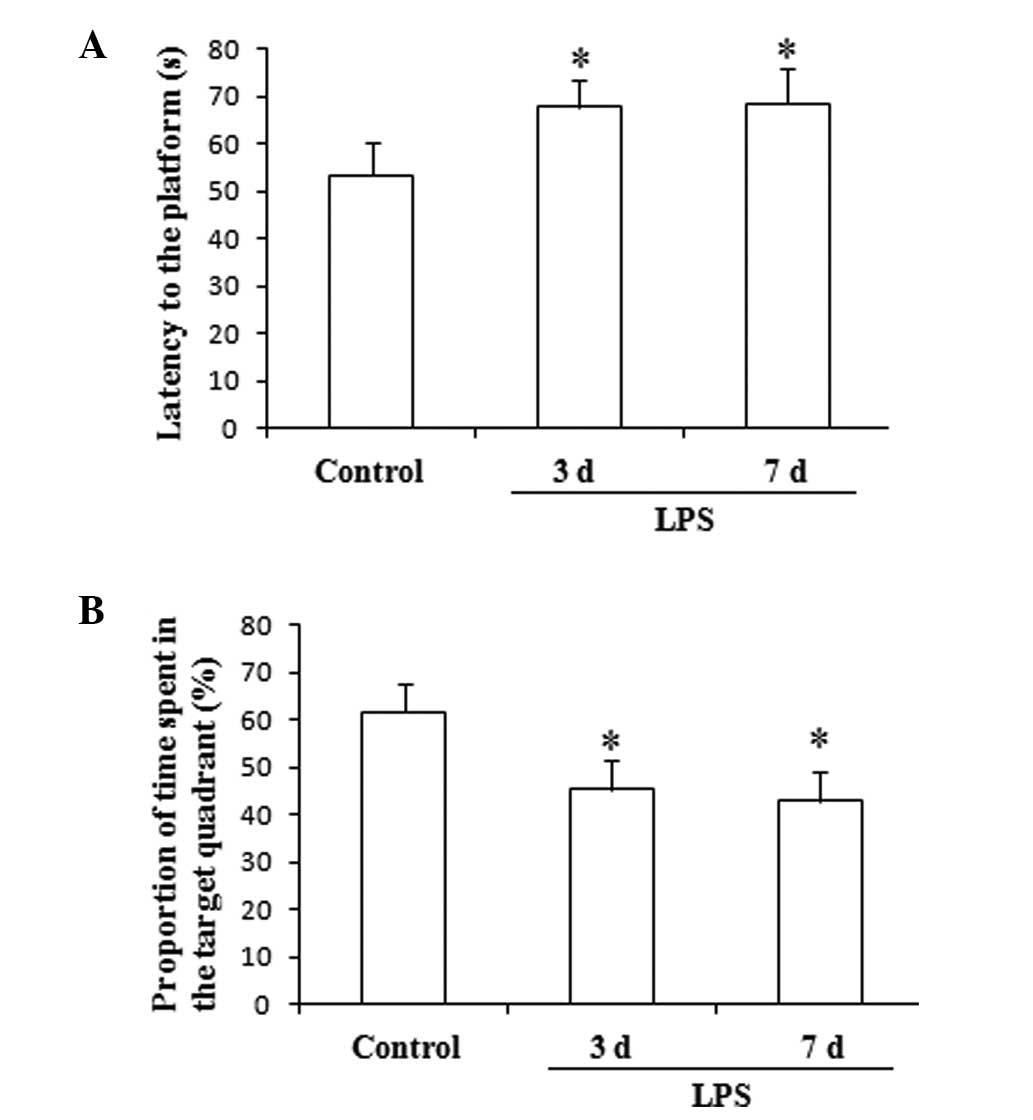
The results of this test, which are presented in
Fig. 1, indicate that the
administration of LPS for 3 and 7 days significantly increased the
latency to the platform and decreased the proportion of time spent
in the target quadrant, compared with the control group
(F(2,27), 11.75; P<0.05; Fig. 1).
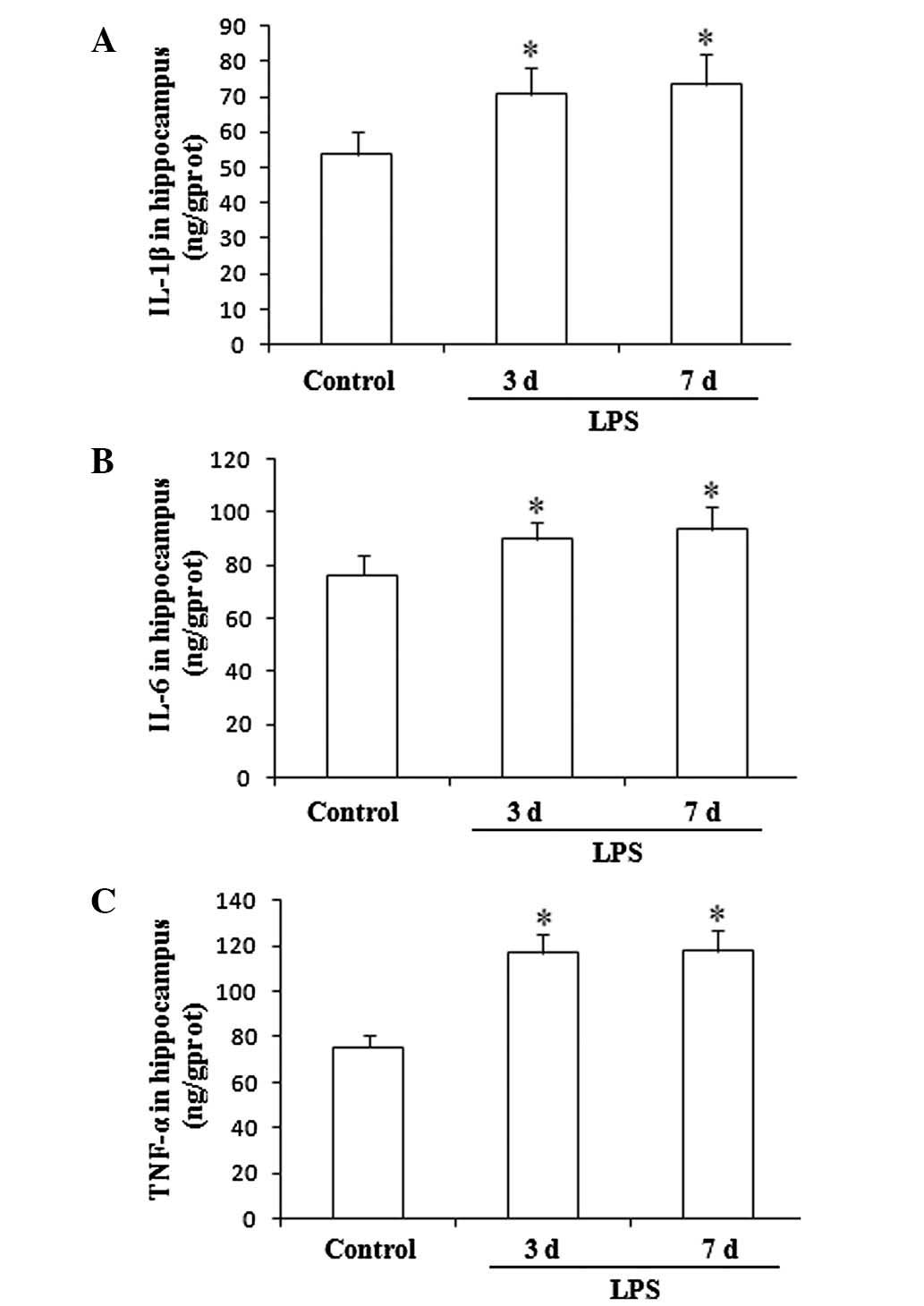
Expression levels of pro-inflammatory
cytokines in the hippocampus
The hippocampal expression levels of IL-1β, IL-6 and
TNF-α showed significant increases in the rats undergoing LPS
administration for 3 and 7 consecutive days, compared with the
levels in the control group. (F(2,27), 26.21, P<0.01;
Fig. 2).
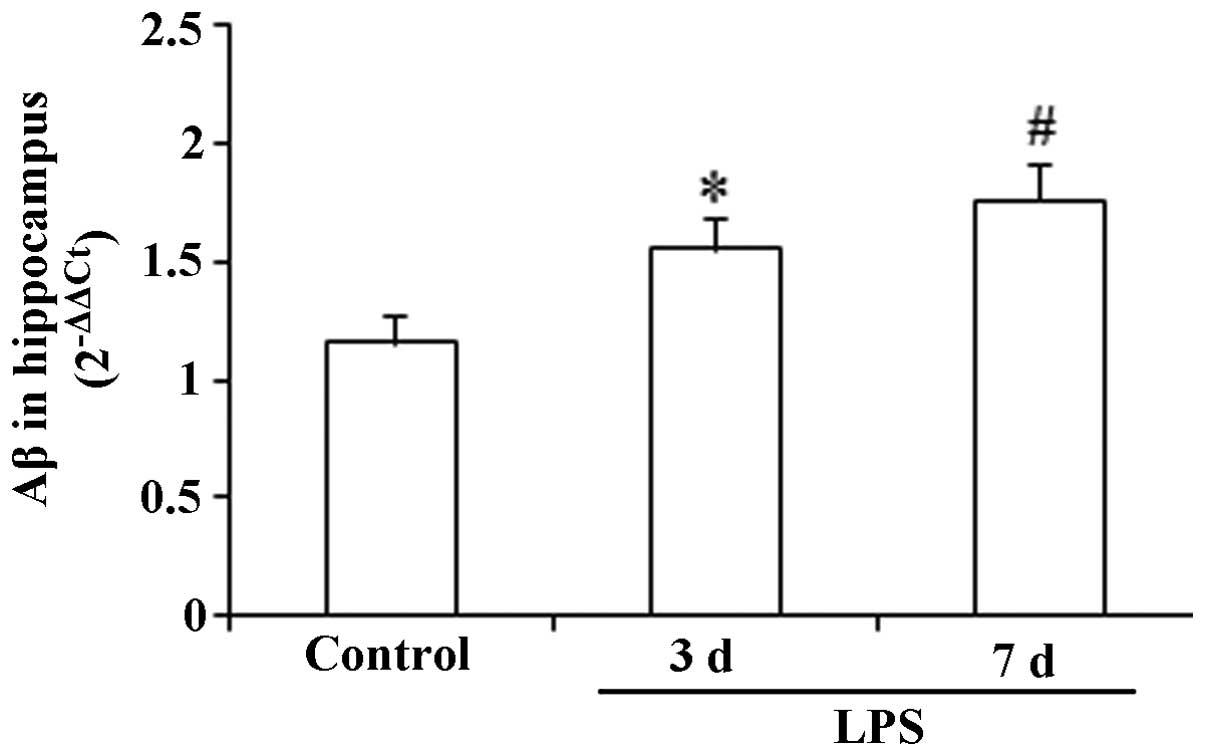
Expression levels of Aβ in the
hippocampus
As demonstrated in Fig.
3, no significant change in the hippocampal expression level of
Aβ was observed following the administration of LPS for three
consecutive days compared with that in the control group
(P>0.05). However, 7 consecutive days of LPS administration
induced a significant increase in the expression level of Aβ
compared with that in the control (F(2,27), 9.87;
P<0.05).
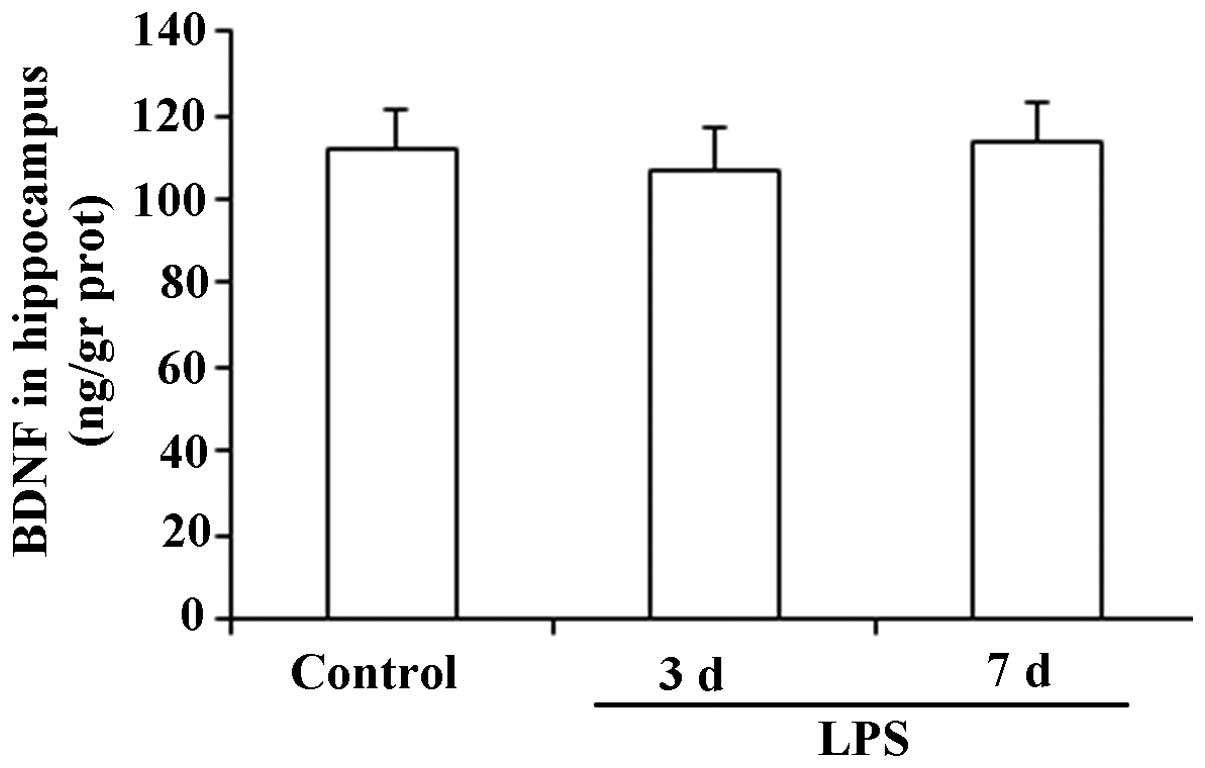
Expression levels of BDNF in the
hippocampus
The results presented in Fig. 4 show that there were no significant
changes in the expression levels of BDNF in the hippocampus
following the administration of LPS for 3 or 7 consecutive days
(F(2,27), 1.43; P>0.05).
Discussion
The results of the Morris water test conducted in
the present study demonstrate that intraperitoneally administered
LPS elicited cognitive dysfunction in rats. Moreover, it was
observed that LPS significantly increased the expression levels of
pro-inflammatory cytokines and Aβ in the hippocampus. However, the
previously expected reduction in the expression levels of BDNF was
not observed.
In the Morris water maze, latency to the platform
and the proportion of time spent in the target quadrant are two
important testing indices for evaluating cognitive function in a
rat model. In the present study, the results demonstrated that the
chronic administration of LPS significantly increased the latency
to the platform and decreased the proportion of time spent in the
target quadrant, indicating that LPS elicited a deficit in
cognitive performance.
LPS is a key component of the cell wall in
Gram-negative bacteria and has the potential to cause sepsis, shock
and microcirculation disturbance (20). Increasing evidence has shown that
LPS may be administered to construct animal models of neurological
diseases (21). Shaw et al
(22) indicated that a single
administration of LPS elicited cognitive impairments. In the
present study, rats were intraperitoneally injected with LPS for 3
or 7 days in order to observe its effects on cognitive performance.
Dantzer et al (9)
hypothesized that following a single injection of LPS, particularly
a short time after the administration, animals exhibit sickness
behavior rather than cognitive impairment.
Pro-inflammatory cytokines are regulators of host
responses to infection, immune responses, inflammation and trauma
and worsen disease progression. A study by Leung et al
(23) showed that increased levels
of pro-inflammatory cytokines in the brain were associated with the
pathogenesis of cognitive disturbance in patients with Alzheimer’s
disease. Moreover, a previous study indicated that the increased
expression of pro-inflammatory cytokines facilitated the emergence
of cognitive impairments (24).
These observations indicate that pro-inflammatory cytokines play a
pivotal role in the pathogenesis of specific diseases characterized
by cognitive impairments. In the present study, the expression
levels of hippocampal IL-1β, IL-6 and TNF-α were observed and
showed a significant increase following the chronic administration
of LPS. Therefore, the results are consistent with previous
observations.
Aβ is a component of the amyloid plaques that are
associated with Alzheimer’s disease (25). Aβ is a highly multifunctional
peptide with significant non-pathological activity (26). In the present study, increased
expression of Aβ in the hippocampus was observed following the
chronic administration of LPS, indicating that the increased
expression of Aβ may be a major factor in the pathogenesis of
cognitive dysfunction. Notably, administration of LPS for 7 days
elicited an increase in the expression level of Aβ, while 3 days of
LPS administration did not. The chronic administration of LPS
increased the expression levels of pro-inflammatory cytokines in
the hippocampus. Therefore, it was hypothesized that high
expression levels of Aβ may be associated with increased
pro-inflammatory cytokine levels in the hippocampus. In other
words, long-term infection in the central nervous system may
upregulate the expression of Aβ. Conversely, inhibiting the
inflammatory response may facilitate the downregulation of Aβ
(26). The results of the present
study indicate that chronic LPS administration eliciting the
upregulation of Aβ in rat hippocampus may be associated with the
observed increase in the levels of pro-inflammatory cytokines.
BDNF is a member of the neurotrophin family of
growth factors, which act on certain neurons of the central and
peripheral nervous system (27).
Lapchak et al (28)
demonstrated that the administration of LPS reduced BDNF mRNA
expression levels in the rat hippocampus. Moreover, several other
studies have demonstrated that proinflammatory cytokines inhibit
BDNF expression in the brain (29,30).
Schnydrig et al (31)
reported that synaptosomal BDNF expression levels in mice showed a
transient reduction following the intraperitoneal administration of
a single high dose of LPS, with a maximal reduction at day 3.
Although previous studies have reported that BDNF plays a critical
role in cognitive function, in the present study, changes in BDNF
expression levels following LPS administration were not observed.
Regarding the reason for this, it was considered that the
LPS-elicited cognitive impairment animal model was not associated
with changed BDNF expression levels. Due to this limitation, the
possibility that intracranial injections of BDNF improve cognitive
performance in LPS-induced cognitive impairment animal models was
not investigated. Future studies are required to further
investigate the correlation between LPS-induced cognitive
impairment and BDNF expression.
In conclusion, LPS-induced cognitive dysfunction is
likely to be associated with pro-inflammatory cytokines and Aβ. The
results of the present study indirectly indicate that early
intervention against the inflammatory responses may be a strategy
for attenuating the increased expression of hippocampal Aβ.
However, the present study did not investigate drug treatments that
have the potential to intervene in the expression of
pro-inflammatory cytokines and ultimately reverse the emergence of
cognitive dysfunction. Consequently, future large-scale studies are
required to further explain the pathogenesis of cognitive
dysfunction.
References
|
1
|
Adam N, Kandelman S, Mantz J, Chrétien F
and Sharshar T: Sepsis-induced brain dysfunction. Expert Rev Anti
Infect Ther. 11:211–221. 2013. View Article : Google Scholar
|
|
2
|
Zampieri FG, Park M, Machado FS and
Azevedo LC: Sepsis-associated encephalopathy: not just delirium.
Clinics (Sao Paulo). 66:1825–1831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan Z and Fang J: Peripheral immune
activation by lipopolysaccharide decreases neurotrophins in the
cortex and hippocampus in rats. Brain Behav Immun. 20:64–71. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzumura A, Takeuchi H, Zhang G, Kuno R
and Mizuno T: Roles of glia-derived cytokines on neuronal
degeneration and regeneration. Ann NY Acad Sci. 1088:219–229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Cui XL, Liu YF, et al: LPS
inhibits the effects of fluoxetine on depression-like behavior and
hippocampal neurogenesis in rats. Prog Neuropsychopharmacol Biol
Psychiatry. 35:1831–1835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng XH, Ai WM, Lei DL, Luo XG, Yan XX and
Li Z: Lipopolysaccharide induces paired immunoglobulin-like
receptor B (PirB) expression, synaptic alteration, and
learning-memory deficit in rats. Neuroscience. 209:161–170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richwine AF, Sparkman NL, Dilger RN,
Buchanan JB and Johnson RW: Cognitive deficits in
interleukin-10-deficient mice after peripheral injection of
lipopolysaccharide. Brain Behav Immun. 23:794–802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sparkman NL, Buchanan JB, Heyen JR, Chen
J, Beverly JL and Johnson RW: Interleukin-6 facilitates
lipopolysaccharide-induced disruption in working memory and
expression of other proinflammatory cytokines in hippocampal
neuronal cell layers. J Neurosci. 26:10709–10716. 2006. View Article : Google Scholar
|
|
9
|
Dantzer R, O’Connor JC, Freund GG, Johnson
RW and Kelley KW: From inflammation to sickness and depression:
when the immune system subjugates the brain. Nat Rev Neurosci.
9:46–56. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith CJ, Emsley HC, Udeh CT, et al:
Interleukin-1 receptor antagonist reverses stroke-associated
peripheral immune suppression. Cytokine. 58:384–389. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krzyszton CP, Sparkman NL, Grant RW, et
al: Exacerbated fatigue and motor deficits in
interleukin-10-deficient mice after peripheral immune stimulation.
Am J Physiol Regul Integr Comp Physiol. 295:R1109–R1114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnston H, Boutin H and Allan SM:
Assessing the contribution of inflammation in models of Alzheimer’s
disease. Biochem Soc Trans. 39:886–890. 2011.PubMed/NCBI
|
|
13
|
Kaster MP, Gadotti VM, Calixto JB, Santos
AR and Rodrigues AL: Depressive-like behavior induced by tumor
necrosis factor-α in mice. Neuropharmacology. 62:419–426. 2012.
|
|
14
|
Mansur RB, Zugman A, Asevedo EM, da Cunha
GR, Bressan RA and Brietzke E: Cytokines in schizophrenia: possible
role of anti-inflammatory medications in clinical and preclinical
stages. Psychiatry Clin Neurosci. 66:247–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pizza V, Agresta A, D’Acunto CW, Festa M
and Capasso A: Neuroinflamm-aging and neurodegenerative diseases:
an overview. CNS Neurol Disord Drug Targets. 10:621–634. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Magaki S, Mueller C, Dickson C and Kirsch
W: Increased production of inflammatory cytokines in mild cognitive
impairment. Exp Gerontol. 42:233–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oral E, Canpolat S, Yildirim S, Gulec M,
Aliyev E and Aydin N: Cognitive functions and serum levels of
brain-derived neurotrophic factor in patients with major depressive
disorder. Brain Res Bull. 88:454–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XY, Liang J, Chen da C, et al: Low
BDNF is associated with cognitive impairment in chronic patients
with schizophrenia. Psychopharmacology (Berl). 222:277–284. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D’Hooge R and De Deyn PP: Applications of
the Morris water maze in the study of learning and memory. Brain
Res Brain Res Rev. 36:60–90. 2001.PubMed/NCBI
|
|
20
|
Solov’eva T, Davydova V, Krasikova I and
Yermak I: Marine compounds with therapeutic potential in
gram-negative sepsis. Mar Drugs. 11:2216–2229. 2013.PubMed/NCBI
|
|
21
|
Skelly DT, Hennessy E, Dansereau MA and
Cunningham C: A systematic analysis of the peripheral and CNS
effects of systemic LPS, IL-1β, TNF-α and IL-6 challenges in
C57BL/6 mice. PLoS One. 8:e691232013.PubMed/NCBI
|
|
22
|
Shaw KN, Commins S and O’Mara SM:
Lipopolysaccharide causes deficits in spatial learning in the
watermaze but not in BDNF expression in the rat dentate gyrus.
Behav Brain Res. 124:47–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leung R, Proitsi P, Simmons A, et al:
Inflammatory proteins in plasma are associated with severity of
Alzheimer’s disease. PLoS One. 8:e649712013.
|
|
24
|
Reale M, Iarlori C, Gambi F, et al:
Treatment with an acetylcholinesterase inhibitor in Alzheimer
patients modulates the expression and production of the
pro-inflammatory and anti-inflammatory cytokines. J Neuroimmunol.
148:162–171. 2004. View Article : Google Scholar
|
|
25
|
Hsu LJ, Mallory M, Xia Y, et al:
Expression pattern of synucleins (non-Abeta component of
Alzheimer’s disease amyloid precursor protein/alpha-synuclein)
during murine brain development. J Neurochem. 71:338–344. 1998.
|
|
26
|
Nguyen JT, Yamani A and Kiso Y: Views on
amyloid hypothesis and secretase inhibitors for treating
Alzheimer’s disease: progress and problems. Curr Pharm Des.
12:4295–4312. 2006.PubMed/NCBI
|
|
27
|
Yan Q, Rosenfeld RD, Matheson CR, et al:
Expression of brain-derived neurotrophic factor protein in the
adult rat central nervous system. Neuroscience. 78:431–448. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lapchak PA, Araujo DM and Hefti F:
Systemic interleukin-1 beta decreases brain-derived neurotrophic
factor messenger RNA expression in the rat hippocampal formation.
Neuroscience. 53:297–301. 1993. View Article : Google Scholar
|
|
29
|
Maher FO, Martin DS and Lynch MA:
Increased IL-1beta in cortex of aged rats is accompanied by
downregulation of ERK and PI-3 kinase. Neurobiol Aging. 25:795–806.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taishi P, Churchill L, De A, Obal F Jr and
Krueger JM: Cytokine mRNA induction by interleukin-1beta or tumor
necrosis factor alpha in vitro and in vivo. Brain Res. 1226:89–98.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schnydrig S, Korner L, Landweer S, et al:
Peripheral lipopolysaccharide administration transiently affects
expression of brain-derived neurotrophic factor, corticotropin and
proopiomelanocortin in mouse brain. Neurosci Lett. 429:69–73. 2007.
View Article : Google Scholar
|