Introduction
Cardiac responses to the continuous infusion of
acetylcholine (ACh) or direct vagal nerve stimulation at a constant
intensity diminish over time, a phenomenon known as desensitization
(1–4). However, rapid washout of ACh or
sudden withdrawal of muscarinic cholinergic stimulation has a
positive effect that is opposite to the inhibitory actions of ACh,
a phenomenon known as rebound, which may induce cardiac hemodynamic
disturbances and arrhythmias (5–7).
The existence of complex regional differences in the
electro-mechanical properties of the healthy adult heart has been
confirmed (8–10). Several published studies describe
the role of cardiac electrical heterogeneity in the re-entry and
triggered activities that are responsible for atrial fibrillation,
and in ventricular tachycardia and inherited ion channelopathies.
However, the impact of ACh washout on regional electrophysiological
heterogeneity in the isolated perfused heart remains unclear
(9–11).
The present study was conducted to determine whether
the washout of ACh is associated with regional electrophysiological
heterogeneity accompanied by positive inotropic rebound action. As
ACh washout mimics the sudden withdrawal of vagus stimulation, a
factor that may trigger arrhythmia, electrophysiological
characterization of ACh-induced inotropic effects may improve the
understanding of arrhythmogenesis.
Materials and methods
Langendorff perfusion
All experiments were performed according to ethical
guidelines approved by the Shihezi University Institutional Animal
Care and Use Committee. Isolated Langendorff-perfused rabbit hearts
were prepared as previously described (12,13).
Briefly, male New Zealand white rabbits (n=7; mean weight, 2.6±0.3
kg) were anaesthetized with sodium pentobarbital (70 mg/kg i.v.)
delivered with heparin (1000 U/kg i.v.). The hearts were excised,
aortae were cannulated rapidly, and the hearts were perfused with
modified Krebs-Henseleit (K-H) solution (composition in mM: 118
NaCl, 4.5 KCl, 11.0 glucose, 24.9 NaHCO3, 1.1
MgSO4, 2.5 CaCl2 and 1.1
KH2PO4). The temperature was held at 37°C and
the pH was maintained at 7.4 by continuously bubbling with a
mixture of 95% O2 and 5% CO2. For experiments
performed in the Langendorff mode, the heart was perfused
retrogradely with a modified K-H solution in a constant perfusion
pressure mode (75 cm H2O).
Monophasic action potential (MAP)
recording
Two Ag-AgCl pressure-contact MAP electrodes were
positioned in the apical epicardial region of the right atrium (RA)
and right ventricle (RV). MAP data and cardiac contraction curves
were recorded using an amplifier, an A/D-converter, and a computer
with the BL-420E Biological Experimental System (Taimeng Technology
Co., Ltd., Chengdu, China) (13,14).
Drug infusion
Isolated rabbit hearts were subjected to retrograde
aortic perfusion of ACh solution (10−5 mol/l) followed
by rapid washing out with modified K-H solution. Subsequently,
adrenaline (10−4 mmol/l) was infused to force recovery
of contraction to baseline. ACh perfusion followed by rapid washout
was repeated when the contraction curve recovered to baseline. For
all drugs, the infusion velocity was 0.05 ml/sec.
Statistical analysis
Only stable MAPs prior to drug infusion with
constant amplitude (>4.5 mV) were accepted for analysis (n=7).
MAP amplitude (MAPA) was defined as the distance from the diastolic
baseline to the crest of the MAP plateau phase. The duration of MAP
was measured manually at the time of 90% repolarization
(MAPD90) and the mean value was calculated from three
consecutive signals. MAPA, and the inotropic amplitude, onset and
duration time are presented as the means ± standard deviation (SD).
Results were analyzed using the Student’s t-test with P<0.05
denoting statistically significant differences.
Results
ACh-induced positive inotropic
action
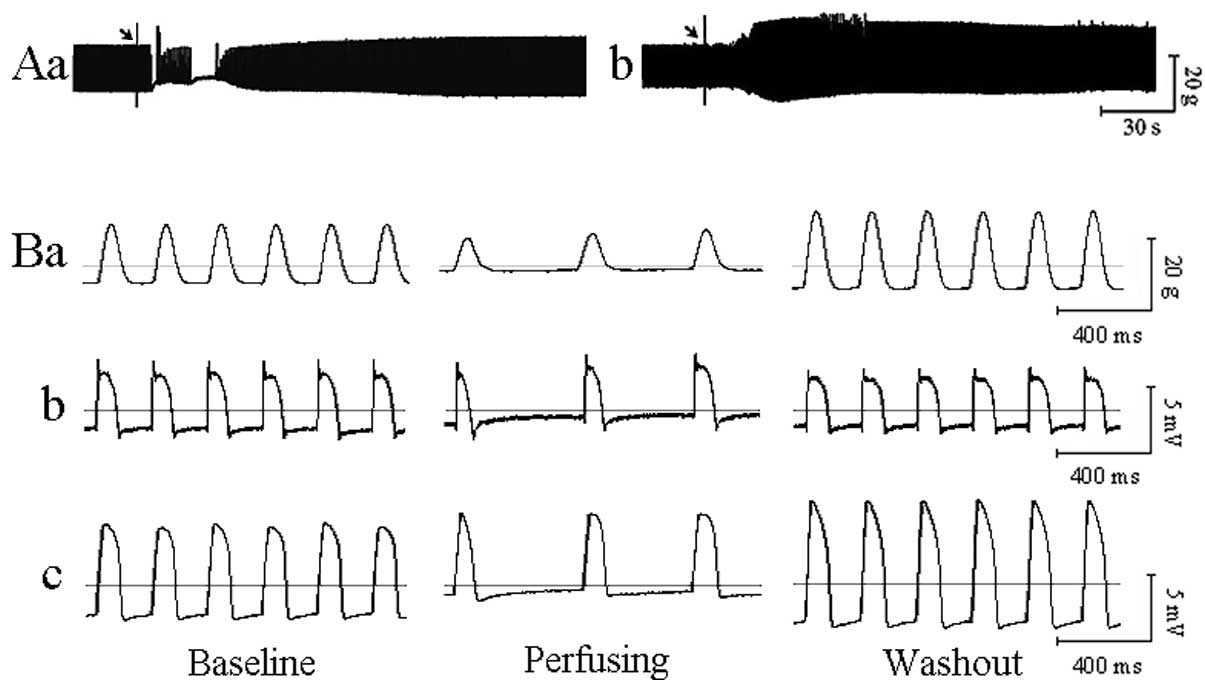
Maximum rebound effects were observed between 100
and 460 sec and recovery to baseline was observed following the
infusion of ACh (760±51 sec). The maximum rebound rate was
78.53±27.86% (Fig. 1Aa and b and
Bc).
Changes in MAPs following ACh
washout
Washout of ACh induced higher MAPA in the RV
(Fig. 1Bc) but not in the RA
(Fig. 1Bb). This effect was
accompanied by positive inotropic action (Fig. 1Ba) and changes in MAPs. Only the
change of MAPA in RV was statistically significant after the
washout of ACh (Table I).
 | Table IEffects of ACh (10−5 mol/l)
on epicardial MAPs in isolated perfused rabbit hearts (n=7). |
Table I
Effects of ACh (10−5 mol/l)
on epicardial MAPs in isolated perfused rabbit hearts (n=7).
| Variable | Baseline | Perfusion | Washout |
|---|
| RA |
| MAPA (mV) | 6.3±1.5 | 5.0±1.4a | 5.8±1.9 |
| MAPD90
(ms) | 128.4±17.6 | 89.6±16.8 | 135.6±12.5 |
| RV |
| MAPA (mV) | 8.5±2.8 | 6.1±2.3a | 10.6±3.7a |
| MAPD90
(ms) | 139.8±13.5 | 92.7±12.5a | 143.2±15.6 |
Discussion
Previous studies have demonstrated that the
ACh-induced enhancement of myocardial contractility is related to
the following mechanisms, the majority of which act at the cell and
tissue level: i) following ACh washout, inhibition of adenylate
cyclase is abolished, which increases the sensitivity of the
myocardial cells to adrenaline (15,16);
ii) as ACh increases the levels of nitric oxide and cGMP, resulting
in the activation of phosphodiesterase II, which downregulates
intracellular cAMP levels, a phenomenon involving the rebound of
the cAMP level and an increase in the number of opened
Ca2+-channels occurs after the elution of ACh (13,17,18);
iii) ACh activates Ca2+-channels in myocardial cells via
the muscarinic M1-receptor (19,20);
iv) ACh acts on M2-receptors to facilitate Ca2+ influx
via Na+/Ca2+ exchangers, but fails to affect
the contractility of the rabbit heart (21,22);
v) ACh activates adenylate cyclase via the G-protein βγ-subunit to
increase intracellular cAMP levels (13,15)
and vi) the sensitivity of myocardial myofibrils to Ca2+
increases following ACh washout (23).
Complex regional differences in the
electro-mechanical properties of the healthy adult heart,
particularly in the ventricle, have been confirmed.
Electro-mechanical features are affected by different pathological
conditions, including myocardial ischaemia and cardiac hypertrophy
(8). In the present study, ACh
washout following adrenaline pretreatment resulted in a higher MAPA
accompanied by positive inotropic action in the RV (Fig. 1Bb) but not in the RA (Fig. 1Ba), as assessed by the MAP
technique.
ACh-induced positive inotropic actions have been
documented in previous studies by using individual myocardial cells
or muscle strips, ventricular muscle and other isolated tissue
blocks; however, it is necessary to perform integrated confirmative
studies at both the organ and body level. The atrium and ventricle,
as two functional components, may electrically and mechanically
differ. Consequently, integrated and in vivo research
methods should be the basic means of investigating these actions.
In the present study, regional electrophysiological heterogeneity
was exhibited as the rebound positive inotropic action induced by
the washout of ACh, which may be a factor leading to cardiac
arrhythmia (24,25).
References
|
1
|
Bender K, Wellner-Kienitz MC, Bosche LI,
Rinne A, Beckmann C and Pott L: Acute desensitization of GIRK
current in rat atrial myocytes is related to K+ current flow. J
Physiol. 561:471–483. 2004.PubMed/NCBI
|
|
2
|
Choisy SC, James AF and Hancox JC: Acute
desensitization of acetylcholine and endothelin-1 activated inward
rectifier K+ current in myocytes from the cardiac
atrioventricular node. Biochem Biophys Res Commun. 423:496–502.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shui Z, Yamanushi TT and Boyett MR:
Evidence of involvement of GIRK1/GIRK4 in long-term desensitization
of cardiac muscarinic K+ channels. Am J Physiol Heart
Circ Physiol. 280:H2554–H2562. 2001.PubMed/NCBI
|
|
4
|
Yamanushi TT, Shui Z, Leach RN, Dobrzynski
H, Claydon TW and Boyett MR: Role of internalization of M2
muscarinic receptor via clathrin-coated vesicles in desensitization
of the muscarinic K+ current in heart. Am J Physiol
Heart Circ Physiol. 292:H1737–H1746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belevych AE, Sims C and Harvey RD:
ACh-induced rebound stimulation of L-type Ca(2+) current in
guinea-pig ventricular myocytes, mediated by Gbetagamma-dependent
activation of adenylyl cyclase. J Physiol. 536:677–692.
2001.PubMed/NCBI
|
|
6
|
Godecke A, Heinicke T, Kamkin A, et al:
Inotropic response to beta-adrenergic receptor stimulation and
anti-adrenergic effect of ACh in endothelial NO synthase-deficient
mouse hearts. J Physiol. 532:195–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maruyama M, Lin SF, Xie Y, et al: Genesis
of phase 3 early afterdepolarizations and triggered activity in
acquired long-QT syndrome. Circ Arrhythm Electrophysiol. 4:103–111.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bryant SM, Shipsey SJ and Hart G: Regional
differences in electrical and mechanical properties of myocytes
from guinea-pig hearts with mild left ventricular hypertrophy.
Cardiovasc Res. 35:315–323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bishop MJ, Vigmond EJ and Plank G: The
functional role of electrophysiological heterogeneity in the rabbit
ventricle during rapid pacing and arrhythmias. Am J Physiol Heart
Circ Physiol. 304:H1240–H1252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ravelli F: Mechano-electric feedback and
atrial arrhythmias. Mechanosensitivity in Cells and Tissues. Kamkin
A and Kiseleva I: Academia Publishing House Ltd; Moscow: 2005
|
|
11
|
Wyse DG and Gersh BJ: Atrial fibrillation:
a perspective: thinking inside and outside the box. Circulation.
109:3089–3095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brack KE, Coote JH and Ng GA: The effect
of direct autonomic nerve stimulation on left ventricular force in
the isolated innervated Langendorff perfused rabbit heart. Auton
Neurosci. 124:69–80. 2006. View Article : Google Scholar
|
|
13
|
Aydin O, Becker R, Kraft P, et al: Effects
of protein kinase C activation on cardiac repolarization and
arrhythmogenesis in Langendorff-perfused rabbit hearts. Europace.
9:1094–1098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng J, Ma X, Zhang J and Su D: Diverse
modulating effects of estradiol and progesterone on the monophasic
action potential duration in Langendorff-perfused female rabbit
hearts. Fundam Clin Pharmacol. 26:219–226. 2012. View Article : Google Scholar
|
|
15
|
Okada M, Mizuno W, Nakarai R, Matada T,
Yamawaki H and Hara Y: Benzodiazepines inhibit the acetylcholine
receptor-operated potassium current (IK.ACh) by different
mechanisms in guinea-pig atrial myocytes. J Vet Med Sci.
74:879–884. 2012. View Article : Google Scholar
|
|
16
|
Brodde OE and Michel MC: Adrenergic and
muscarinic receptors in the human heart. Pharmacol Rev. 51:651–690.
1999.PubMed/NCBI
|
|
17
|
Guiraudou P, Pucheu SC, Gayraud R, et al:
Involvement of nitric oxide in amiodarone- and dronedarone-induced
coronary vasodilation in guinea pig heart. Eur J Pharmacol.
496:119–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng H, Smith GL, Orchard CH, Hancox JC
and Burton FL: Inhibition of sarcoplasmic reticulum Ca(2+)-ATPase
decreases atrioventricular node-paced heart rate in rabbits. Exp
Physiol. 97:1131–1139. 2012.
|
|
19
|
Link S, Meissner M, Held B, et al:
Diversity and developmental expression of L-type calcium channel
beta2 proteins and their influence on calcium current in murine
heart. J Biol Chem. 284:30129–30137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bodi I, Mikala G, Koch SE, Akhter SA and
Schwartz A: The L-type calcium channel in the heart: the beat goes
on. J Clin Invest. 115:3306–3317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YS, Maruyama M, Chang PC, et al:
Ryanodine receptor inhibition potentiates the activity of Na
channel blockers against spontaneous calcium elevations and delayed
afterdepolarizations in Langendorff-perfused rabbit ventricles.
Heart Rhythm. 9:1125–1132. 2012. View Article : Google Scholar
|
|
22
|
Farkas AS, Acsai K, Nagy N, et al:
Na(+)/Ca(2+) exchanger inhibition exerts a positive inotropic
effect in the rat heart, but fails to influence the contractility
of the rabbit heart. Br J Pharmacol. 154:93–104. 2008.
|
|
23
|
Hayashi H, Kamanu SD, Ono N, et al:
Calcium transient dynamics and the mechanisms of ventricular
vulnerability to single premature electrical stimulation in
Langendorff-perfused rabbit ventricles. Heart Rhythm. 5:116–123.
2008. View Article : Google Scholar
|
|
24
|
Aslanidi OV, Robinson R, Cheverton D,
Boyett MR and Zhang H: Electrophysiological substrate for a
dominant reentrant source during atrial fibrillation. Conf Proc
IEEE Eng Med Biol Soc. 2009:2819–2822. 2009.PubMed/NCBI
|
|
25
|
Smith RM, Velamakanni SS and Tolkacheva
EG: Interventricular heterogeneity as a substrate for
arrhythmogenesis of decoupled mitochondria during ischemia in the
whole heart. Am J Physiol Heart Circ Physiol. 303:H224–H233. 2012.
View Article : Google Scholar : PubMed/NCBI
|