Introduction
Osteosarcoma (OS) is the most common tumor in bone
and the third most common tumor in childhood and adolescence.
Following the identification of effective chemotherapeutic agents,
the five-year survival rates for patients treated with intensive
multidrug chemotherapy and aggressive local control have been
reported to be 55–80% (1–3). However, an improvement in survival
rates has been limited only to patients with a high grade of
disease. Patients with metastatic disease have a poor prognosis,
particularly those with pulmonary metastases at diagnosis, with
various studies reporting five-year survival rates of only 17–23%
(4,5). Therefore, it is necessary to
determine the mechanisms contributing to the metastasis of OS.
Aurora-B is an important protein kinase and is
involved in the efficient execution and fidelity of mitosis. As
part of the chromosomal passenger complex (CPC), Aurora-B has been
shown to be involved in a number of mitotic functions, including
chromosome-microtubule interactions, sister chromatid cohesion, the
spindle-assembly checkpoint and cytokinesis. Previous studies have
shown that Aurora-B is upregulated in several types of human
cancer, and that upregulation correlates with poor prognosis. As a
result, Aurora-B has been suggested to be an important antitumor
target (6–9). Li et al (10) showed that downregulation of
Aurora-B is capable of inhibiting proliferation and metastasis,
inducing G2/M phase arrest in clear cell renal cell carcinoma cells
and exerting antitumor activity in an SN12C xenograft model
(10). In addition, a number of
studies have indicated that nuclear Aurora-B expression is markedly
associated with and involved in tumor metastasis (11–14).
However, whether Aurora-B is involved in OS metastasis has yet to
be elucidated.
In the present study, the expression of Aurora-B in
OS with and without pulmonary metastasis was evaluated using
immunohistochemistry (IHC). Furthermore, the effect of Aurora-B
inhibition on cell proliferation, invasion and migration in
vitro was investigated.
AZD1152-hydroxyquinazoline-pyrazol-anilide (HQPA) was used to
inhibit Aurora-B expression in U2-OS cells. Cell proliferation,
migration and invasion were investigated using MTT, colony
formation, wound healing and Transwell assays. The results revealed
that there was a positive correlation between Aurora-B expression
in OS tissues and pulmonary metastasis, and that cell
proliferation, invasion and migration were inhibited by the
inhibition of Aurora-B. The results indicated that Aurora-B may be
involved in OS metastasis.
Materials and methods
Patients and specimens
A total of 60 samples were obtained from patients
with OS of the extremities who underwent surgery in The First
Affiliated Hospital of Nanchang University (Nanchang, China). The
examination of pulmonary metastasis was performed using plain films
and chest computed tomography (CT) scans at initial diagnosis. None
of the patients had a history of previous therapies with antitumor
drugs or radiotherapy. There were 14 cases with pulmonary
metastasis (23.3%), while 76.7% of cases were without metastasis.
The samples were fixed with 10% formalin, embedded in paraffin and
then cut into 4-μm sections. Informed consent was obtained from all
participants, and the study protocol was approved by the
Institutional Ethics Committee (Jiangxi, China).
IHC
Histological sections (4-μm) were stained with
hematoxylin and eosin (H&E) and examined using IHC. IHC was
performed using a streptavidin-peroxidase procedure. Briefly,
antigen retrieval was performed by heating the deparaffinized,
rehydrated sections in 10 mM citrate buffer (pH 6.0) for 20 min,
followed by blocking with 10% goat serum. The sections were then
incubated overnight at 4°C with the primary antibody (rabbit
anti-Aurora-B monoclonal antibody; Abcam, Cambridge, UK) at a final
dilution of 1:500. For the negative controls, the sections were
incubated with phosphate-buffered saline (PBS) instead of
antibodies. After being washed three times with PBS, the sections
were incubated with biotinylated secondary antibody for 40 min and
then incubated with horseradish peroxidase (HRP)-conjugated
streptavidin for 30 min. The sections were subsequently subjected
to chemiluminescent staining and counterstained using hematoxylin.
The stained sections were evaluated and scored by two doctors of
pathology, in a blind manner and without prior knowledge of the
clinical pathological features of the patients. The expression
levels of Aurora-B were judged according to staining intensity,
following the examination of ≥500 cells in five representative
areas, and the intensity scores were recorded as follows: None, 0;
weak, 1; moderate, 2; and intense, 3. According to the percentage
of tumor cells that were positive for Aurora-B expression, the
following percentage scores were assigned: 0% (score 0); >10%
(score 1), 11–50% (score 2), 51–80% (score 3), and 81–100% (score
4). The final score was averaged with the scores from the two
doctors of pathology; these scores were calculated by adding the
intensity score to the percentage score. A final score of <4 was
defined as (−), while scores of 4 and 5 were defined as (+) and
(++), respectively, and a score of ≥6 was defined as (+++).
Cell lines and cell culture
The U2-OS human OS cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA), and the cells
were routinely cultured in Dulbecco’s modified Eagle’s medium
(DMEM; HyClone™, Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Sigma, St. Louis,
MO, USA) in a humidified 37°C incubator containing 5%
CO2.
Cell growth assay
U2-OS cells were cultured in 96-well tissue culture
plates at a cell density of 5,000 cells per well in Minimum
Essential Media (MEM; Invitrogen Life Technologies, Carlsbad, CA,
USA) containing 10% FBS and 2 mM L-glutamine. Following attachment
overnight, the medium was replaced and the cells were incubated
with increasing concentrations (0, 5, 10, 50, 100 and 500 nM) of
AZD1152-HQPA for 24, 48 and 72 h. Subsequently, MTT assays were
performed in triplicate at a wavelength of 490 nm.
Colony formation assay
U2-OS cells (1×106/ml/well) were seeded
in tissue culture plastic dishes and treated with AZD1152-HQPA (100
nM) for two weeks to form colonies. The formed colonies were
stained with Giemsa, and the colonies containing >50 cells were
counted under an inverted microscope (TE2000; Nikon, Tokyo, Japan).
Six independent experiments were performed over multiple days.
Western blot analysis
U2-OS cells in the exponential growth phase were
treated with AZD1152-HQPA at various concentrations (0, 10, 50 and
100 nM) for 24 h. Total protein from the cells was extracted using
radioimmunoprecipitation assay lysis buffer containing 60 μg/ml
phenylmethylsulfonyl fluoride. Protein concentrations were assessed
using a bicinchoninic acid protein assay kit (Boster Biological
Technology, Ltd., Wuhan, China). The protein samples were denatured
at 100°C for 10 min and then preserved at −20°C for later use. The
proteins were separated by SDS-PAGE and transblotted onto
polyvinylidene difluoride membranes. The membranes were then probed
with rabbit anti-Aurora-B monoclonal antibody (1:500; Abcam) or
β-actin antibody (1:2,000; Cell Signaling Technology Inc., Danvers,
MA, USA) overnight at 4°C. Following incubation with the
appropriate anti-rabbit or anti-mouse HRP-conjugated secondary
antibody (1:5,000; Boster Biological Technology, Ltd.) for 1.5 h at
room temperature, immunoreactive bands were visualized using
chemiluminescence dissolvent (Thermo Fisher Scientific, Inc.) and
exposed to X-ray film (Kodak, Rochester, NY, USA). The assessment
of the grayscale values was performed using ImageJ (National
Institutes of Health, Bethesda, MD, USA). All experiments were
repeated six times over multiple days.
Transwell assay
The invasion of U2-OS cells was measured using the
BD BioCoat™ BD Matrigel™ Invasion Chamber (BD Biosciences, Franklin
Lakes, NJ, USA) in accordance with the manufacturer’s instructions.
The medium in the lower chamber contained 5% FBS as a source of
chemoattractants. Cells were suspended in serum-free medium
containing 100 nM AZD1152-HQPA and added to the upper chambers at
the same time. The cultures were rinsed with PBS and the medium was
replaced with fresh medium alone or medium supplemented with 10%
FBS. The cells were then incubated at 37°C for 24 h. Cells that
passed through the Matrigel-coated membrane were stained with
Diff-Quik (Sysmex Corp., Kobe, Japan) and photographed. Cell
migration was quantified using direct microscopic visualization and
counting. The values for invasion were obtained by counting three
fields per membrane and represented the average of six independent
experiments performed over multiple days.
Wound healing assay
Cell migration was assessed by examining the ability
of the cells to move into a cellular space in a two-dimensional
in vitro ‘wound healing assay’. In brief, cells were grown
to confluence in six-well tissue culture plastic dishes to a
density of ~5×106 cells/well. Following treatment with
100 nM AZD1152-HQPA for 24 h, the cells were denuded by dragging a
rubber policeman (Fisher Scientific, Hampton, NH, USA) through the
center of the plate. Cultures were rinsed with PBS and the medium
was replaced with fresh medium alone or medium containing 10% FBS.
The cells were then incubated at 37°C for 24 h. Photographs were
taken at 0 and 24 h, and the migration distance was measured. The
cell migration rate was obtained by counting three fields per area
and represented the average of six independent experiments
performed over multiple days.
Statistical analysis
All measurement data are presented as the mean ±
standard deviation. Statistical analysis was performed using the
independent-samples t-test, and the two-independent-samples test
was used for the analysis of the correlation between Aurora-B
protein expression levels and pulmonary metastasis. P<0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS statistical software version
13.0 (SPSS, Inc., Chicago, IL, USA).
Results
Correlation between Aurora-B protein
expression levels in OS tissues and pulmonary metastasis
Aurora-B was expressed in the nucleus (Fig. 1), and the positive expression rate
was 53.3%. Notably, the positive expression rate of Aurora-B
protein in the cases with pulmonary metastasis was 78.6.% (11/14),
which was significantly different from that of the cases without
pulmonary metastasis 45.7% (21/46). This indicated that Aurora-B
may be involved in OS metastasis.
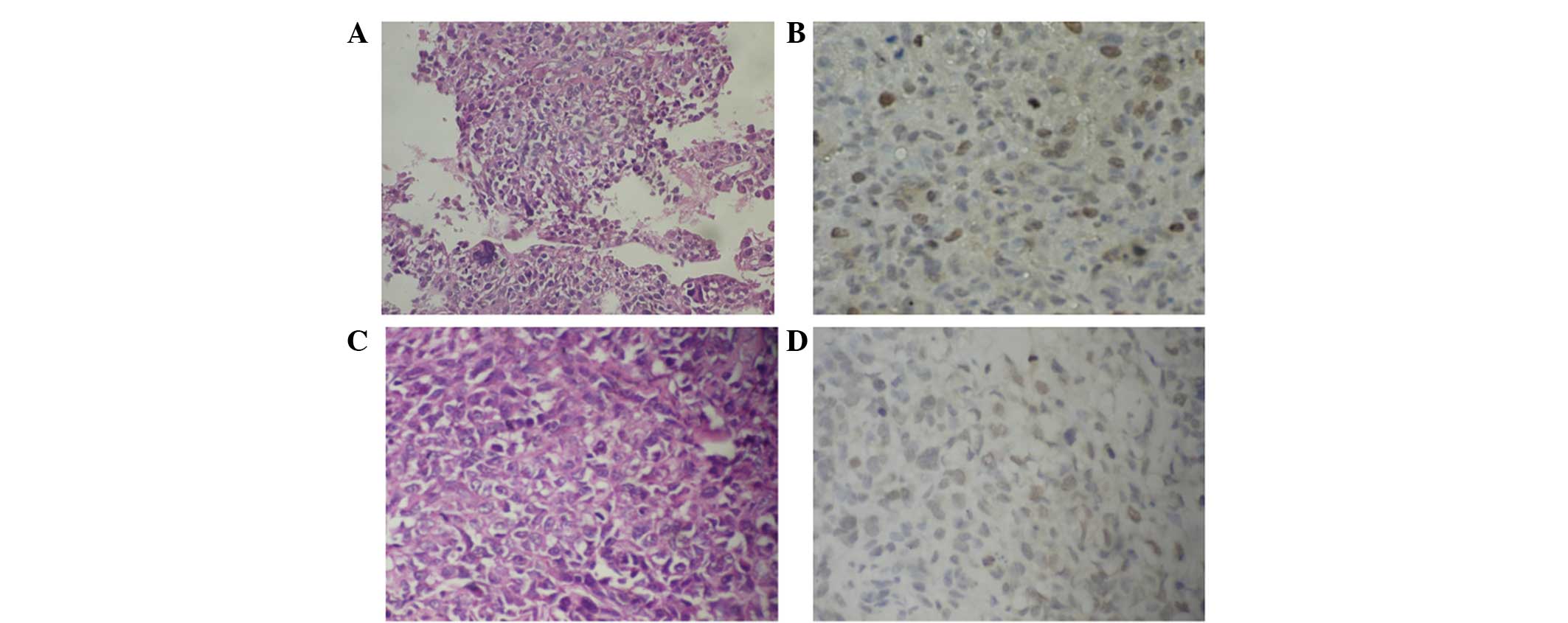 | Figure 1Aurora-B protein expression in OS with
and without pulmonary metastasis (magnification, ×400).
Representative images of (A) H&E staining for OS tissues with
pulmonary metastasis, showing that OS is cell rich and has
significant cellular atypia, anisonucleosis, prominent nucleoli and
an abundant cytoplasm; (B) IHC staining for Aurora-B protein with
lung metastasis, showing brown-yellow particles deposited in the
nucleus and coloring of the majority of the cells; (C) H&E
staining for OS tissues without pulmonary metastasis, showing that
OS is cell-rich and has significant cellular atypia,
anisonucleosis, prominent nucleoli, an abundant cytoplasm and a
small quantity of bone-like matrix; (D) IHC staining for Aurora-B
protein in OS tissues without pulmonary metastasis, showing
brown-yellow particle deposition in the nucleus and coloring of
only a few cells. OS, osteosarcoma; H&E, hematoxylin and eosin;
IHC, immunohistochemistry. |
Effect of Aurora-B inhibition on U2-OS
cell proliferation in vitro
In order to investigate the effect of Aurora-B
inhibition on U2-OS cell growth, AZD1152-HQPA, a specific inhibitor
of Aurora-B, was used to suppress Aurora-B expression in the U2-OS
cells. The cells were treated with various concentrations (0, 5,
10, 50, 100 and 500 nM) of AZD1152-HQPA, and MTT assays were
performed to measure the inhibitory effect of AZD1152-HQPA on U2-OS
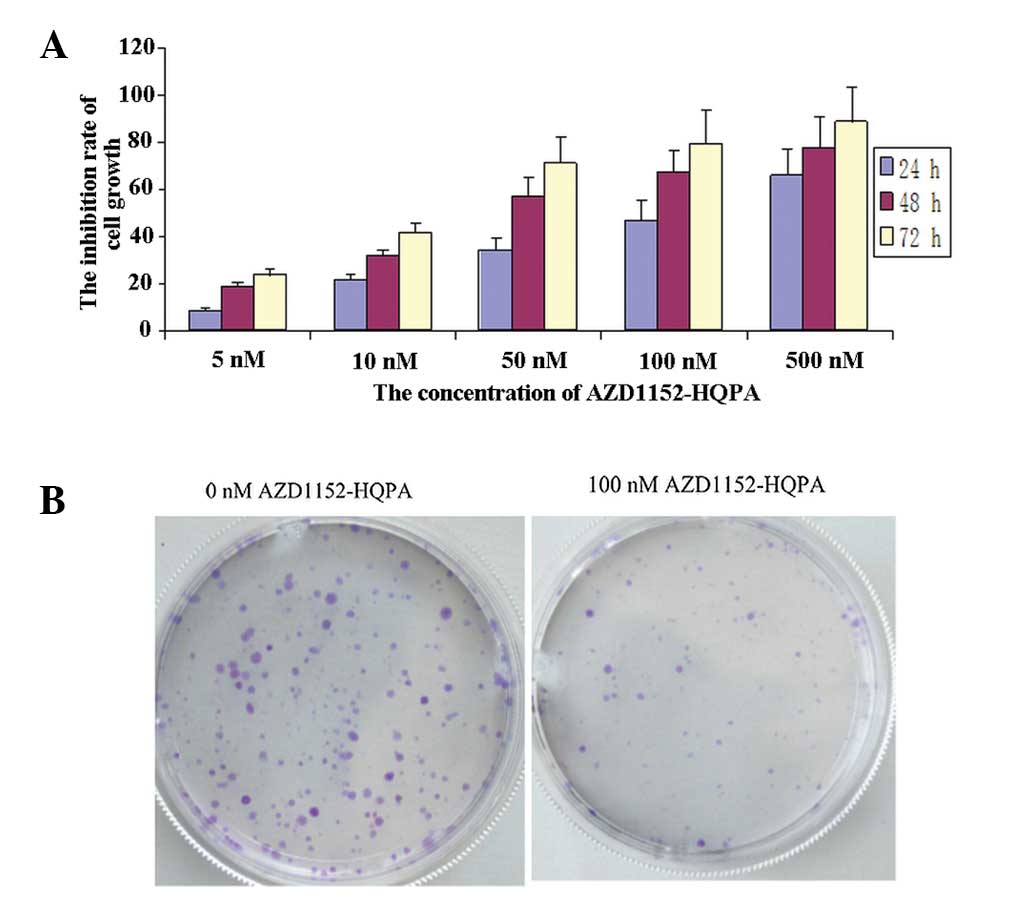
cells proliferation. The results of the MTT assays revealed that
AZD1152-HQPA inhibited U2-OS cell proliferation in a dose- and
time-dependent manner (Fig. 2A).
The IC50 value was 146 nM for 24 h. In the colony formation assays,
the results showed that the colony formation rate in the cells
treated with 100 nM AZD1152-HQPA was lower than in that in the
untreated cells (Fig. 2B).
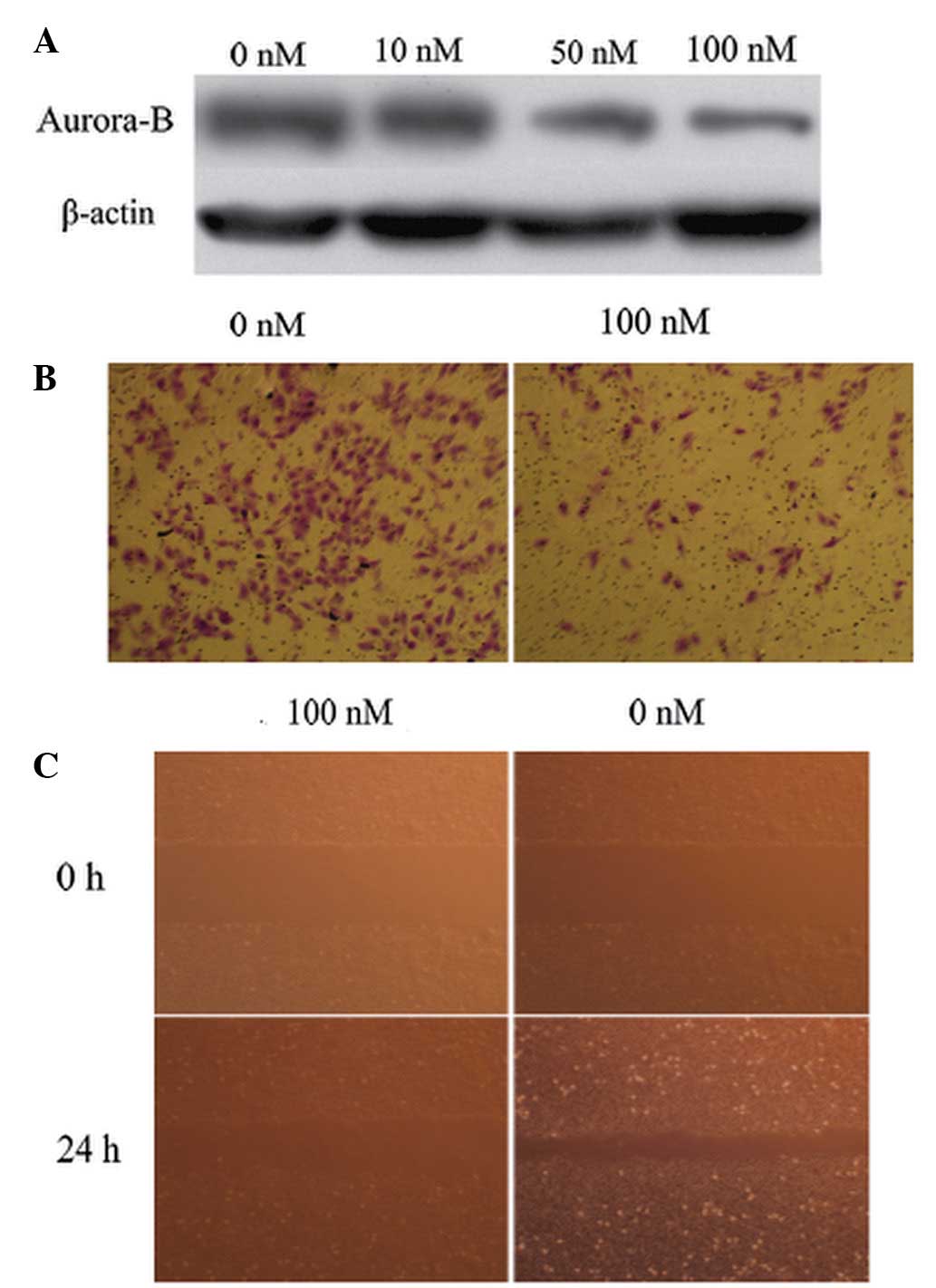
Furthermore, western blot analysis showed that Aurora-B protein
expression was downregulated by AZD1152-HQPA in a dose-dependent
manner (Fig. 3A). These results
showed that Aurora-B inhibition was capable of suppressing U2-OS
cell growth in vitro, which suggested that Aurora-B may be a
promising target for the treatment of OS.
Inhibition of Aurora-B suppresses U2-OS
cell migration and invasion in vitro
According to the IC50 value, the appropriate
concentration of AZD1152-HQPA for wound healing migration and
Transwell invasion assays was determined. To examine the effect of
Aurora-B inhibition on the mobility of U2-OS cells, the migration
and invasion were measured using wound-healing and Transwell
assays, respectively. The cells were treated with 100 nM
AZD1152-HQPA for 24 h. In the Transwell invasion assays, the
invasion of the cells treated with AZD1152-HQPA was significantly
inhibited when compared with that of the untreated cells (75.6±7.4
and 214.5±22.4 cells/high power field, respectively, P<0.05;
Fig. 3B). In the wound healing
assay, the results showed that the migration rate of the cells
treated with AZD1152-HQPA was significantly lower than that of the
untreated cells (23.7±5.1 and 75.6±15.3%, respectively, P<0.05;
Fig. 3C). These results suggested
that Aurora-B inhibition was capable of suppressing U2-OS cell
invasion and migration in vitro.
Discussion
Aurora kinases are serine/threonine kinases that are
essential for cell cycle control and mitosis. Mammals have three
Aurora kinase family members (A, B and C), and these kinases are
expressed at maximum levels during mitosis. Aurora-B, part of the
CPC, is located on the chromosome arms during prophase and at the
centromeres during prometaphase and metaphase. The kinase
subsequently localizes to the midbody during cytokinesis. Aurora-B
has been shown to be overexpressed in a number of types of cancer
(11,15–17)
In the present study, the expression levels of Aurora-B protein in
OS tissues were examined using IHC, which revealed that the
Aurora-B protein was expressed in the nucleus, and that the
positive expression rate was 53.3%. Notably, the expression levels
of Aurora-B protein in the OS tissues with pulmonary metastases
were significantly higher than in those without distant metastases.
It was indicated that Aurora-B may be involved in the development,
progression and metastasis of OS, and may be a potential novel
diagnostic and therapeutic target for OS.
Recent studies revealed that Aurora-B inhibition was
capable of blocking cell proliferation and inducing cell apoptosis
in several types of tumor (18,19).
These observations have led to an interest in Aurora-B as a
molecular target for cancer treatment. A number of small molecular
inhibitors of Aurora-B have been developed as promising anti-tumor
treatments (6,20–23).
AZD1152 is a selective inhibitor of Aurora kinase activity with
specificity for Aurora-B kinase (24,25).
AZD1152 is a prodrug that is rapidly converted to the active
moiety, AZD1152-HQPA, in plasma. AZD1152-HQPA, as a specific
inhibitor of the enzymatic activity of Aurora-B, has been used for
in vitro investigations. Preliminary studies showed that
AZD1152 was active against several types of solid tumors, including
colon, breast and lung cancers (13,26).
However, the effect of Aurora-B inhibition in OS malignancies has
yet to be fully elucidated. In the present study, which explored
the effect of Aurora-B inhibition on OS cell proliferation,
AZD1152-HQPA was used to inhibit Aurora-B expression in U2-OS
cells. Western blot analysis revealed that Aurora-B protein
expression was decreased in cells treated with AZD1152-HQPA,
compared with that in untreated cells. The results of the MTT
assays showed that cell proliferation was inhibited by AZD1152-HQPA
in a dose- and time-dependent manner. Furthermore, in the colony
formation assays, the results revealed that the colony formation
rate was significantly lower in cells treated with 100 nM
AZD1152-HQPA than that in untreated cells. These results indicated
that the inhibition of Aurora-B was capable of suppressing U2-OS
cell growth in vitro.
Notably, studies recently showed that the
upregulated expression of Aurora-B was associated with tumor cell
metastasis, and that the downregulation of Aurora-B was capable of
inhibiting cell invasion and migration in various types of tumors
(11,14,27,28).
In the present study, which investigated the effect of Aurora-B
inhibition on OS cells, U2-OS cells were treated with 100 nM
AZD1152-HQPA, and the migration and invasion of the U2-OS cells
were measured using wound healing and Transwell invasion assays,
respectively. The results showed that the migration rate and cell
invasion were significant lower in cells treated with AZD1152-HQPA
than in untreated cells. This suggested that the downregulation of
Aurora-B was capable of inhibiting U2-OS cell invasion and
migration in vitro.
In conclusion, this study indicated that Aurora-B
may be involved in the development, progression and metastasis of
OS, and that targeting Aurora-B may be a potential treatment
strategy for OS management. However, in the present study the
number of OS tissues was low. Furthermore, the tumor
microenvironment is important in tumor development, progression and
metastasis and therefore, further experiments in vivo are
required to elucidate the potential of Aurora-B as a target for the
treatment of OS metastases and a predictor of prognosis.
Acknowledgements
The present study was supported by a grant from
Jiangxi Province Education Department of Science and Technology
(no. GJJ12097).
References
|
1
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, et al: Osteosarcoma: a
randomized, prospective trial of the addition of ifosfamide and/or
muramyl tripeptide to cisplatin, doxorubicin, and high-dose
methotrexate. J Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacci G, Forni C, Longhi A, Ferrari S,
Mercuri M, Bertoni F, et al: Local recurrence and local control of
non-metastatic osteosarcoma of the extremities: A 27-year
experience in a single institution. J Surg Oncol. 96:118–123.
2007.PubMed/NCBI
|
|
3
|
Jawad MU, Cheung MC, Clarke J, Koniaris LG
and Scully SP: Osteosarcoma: Improvement in survival limited to
high-grade patients only. J Cancer Res Clin Oncol. 137:597–607.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mialou V, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, et al: Metastatic osteosarcoma at
diagnosis: Prognostic factors and long-term outcome the French
pediatric experience. Cancer. 104:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss
J, Szendroi M, et al: Good prognosis of localized osteosarcoma in
young patients treated with limb-salvage surgery and chemotherapy.
Pediatr Blood Cancer. 57:415–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L and Zhang S: ZM447439, the Aurora
kinase B inhibitor, suppresses the growth of cervical cancer SiHa
cells and enhances the chemosensitivity to cisplatin. J Obstet
Gynaecol Res. 37:591–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang WR, Yang SS, Lin JX, Zeng ZY, Liu DM
and Liu HT: Expression of Aurora-B in non-small cell lung cancer
and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao.
29:1853–1856. 2009.(In Chinese).
|
|
8
|
Qi G, Ogawa I, Kudo Y, Miyauchi M,
Siriwardena BS, Shimamoto F, et al: Aurora-B expression and its
correlation with cell proliferation and metastasis in oral cancer.
Virchows Arch. 450:297–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdullah AS, Foong C and Murata-Hori M:
Specific distribution of overexpressed Aurora-B kinase during
interphase of normal epithelial cells. Cancer Cell Int. 5:312005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Zhou W, Wei L, Jin J, Tang K, Li C,
et al: The effect of Aurora kinases on cell proliferation, cell
cycle regulation and metastasis in renal cell carcinoma. Int J
Oncol. 41:2139–2149. 2012.PubMed/NCBI
|
|
11
|
Tuncel H, Shimamoto F, Kaneko Guangying Qi
H, Aoki E, Jikihara H, et al: Nuclear Aurora-B and cytoplasmic
Survivin expression is involved in lymph node metastasis of
colorectal cancer. Oncol Lett. 3:1109–1114. 2012.PubMed/NCBI
|
|
12
|
Pohl A, Azuma M, Zhang W, Yang D, Ning Y,
Winder T, et al: Pharmacogenetic profiling of Aurora kinase B is
associated with overall survival in metastatic colorectal cancer.
Pharmacogenomics J. 11:93–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gully CP, Zhang F, Chen J, Yeung JA,
Velazquez-Torres G, Wang E, et al: Antineoplastic effects of an
Aurora-B kinase inhibitor in breast cancer. Mol Cancer. 9:422010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YJ, Chen CM, Twu NF, Yen MS, Lai CR,
Wu HH, et al: Overexpression of Aurora-B is associated with poor
prognosis in epithelial ovarian cancer patients. Virchows Arch.
455:431–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans RP, Naber C, Steffler T, Checkland
T, Maxwell CA, Keats JJ, et al: The selective Aurora-B kinase
inhibitor AZD1152 is a potential new treatment for multiple
myeloma. Br J Haematol. 140:295–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams RR, Eckley DM, Vagnarelli P,
Wheatley SP, Gerloff DL, Mackay AM, et al: Human INCENP colocalizes
with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed
in tumour cells. Chromosoma. 10:65–74. 2011.PubMed/NCBI
|
|
17
|
Yoon MJ, Park SS, Kang YJ, Kim IY, Lee JA,
Lee JS, et al: Aurora B confers cancer cell resistance to
TRAIL-induced apoptosis via phosphorylation of survivin.
Carcinogenesis. 33:492–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hartsink-Segers SA, Zwaan CM, Exalto C,
Luijendijk MW, Calvert VS, Petricoin EF, et al: Aurora kinases in
childhood acute leukemia: the promise of Aurora-B as therapeutic
target. Leukemia. 27:560–568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie F, Lang Q, Zhou M, Zhang H, Zhang Z,
Zhang Y, et al: The dietary flavonoid luteolin inhibits Aurora-B
kinase activity and blocks proliferation of cancer cells. Eur J
Pharm Sci. 46:388–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Hu H, Lang Q, Zhang H, Huang Q, Wu Y
and Yu L: A thienopyrimidine derivative induces growth inhibition
and apoptosis in human cancer cell lines via inhibitingAurora-B
kinase activity. Eur J Med Chem. 65:151–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamauchi T, Uzui K, Shigemi H, Negoro E,
Yoshida A and Ueda T: Aurora-B inhibitor barasertib and cytarabine
exert a greater-than-additive cytotoxicity in acute myeloid
leukemia cells. Cancer Sci. 104:926–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Lang Q, Zhang H, Huang Q and Yu L:
S39, a novel Aurora B kinase inhibitor, shows potent antineoplastic
activity in human Hela cervical cancer cell line. Biotechnol Lett.
35:853–860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie H, Lee MH, Zhu F, Reddy K, Peng C, Li
Y, Lim do Y, et al: Identification of an Aurora kinase inhibitor
specific for the Aurora-B isoform. Cancer Res. 73:716–724. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilkinson RW, Odedra R, Heaton SP, Wedge
SR, Keen NJ, Crafter C, et al: AZD1152, a selective inhibitor of
Aurora-B kinase, inhibits human tumor xenograft growth by inducing
apoptosis. Clin Cancer Res. 13:3682–3688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Ikezoe T, Nishioka C, Tasaka T,
Taniguchi A, Kuwayama Y, et al: AZD1152, a novel and selective
Aurora-B kinase inhibitor, induces growth arrest, apoptosis, and
sensitization for tubulin depolymerizing agent or topoisomerase II
inhibitor in human acute leukemia cells in vitro and in vivo.
Blood. 110:2034–2040. 2007. View Article : Google Scholar
|
|
26
|
Azzariti A, Bocci G, Porcelli L,
Fioravanti A, Sini P, Simone GM, et al: Aurora-B kinase inhibitor
AZD1152: determinants of action and ability to enhance
chemotherapeutics effectiveness in pancreatic and colon cancer. Br
J Cancer. 104:769–780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonet C, Giuliano S, Ohanna M, Bille K,
Allegra M, Lacour JP, et al: Aurora-B is regulated by the
mitogen-activated protein kinase/extracellular signal-regulated
kinase (MAPK/ERK) signaling pathway and is a valuable potential
target in melanoma cells. J Biol Chem. 287:29887–29998. 2012.
View Article : Google Scholar
|
|
28
|
Takeshita M, Koga T, Takayama K, Ijichi K,
Yano T, Maehara Y, et al: Aurora-B overexpression is correlated
with aneuploidy and poor prognosis in non-small cell lung cancer.
Lung Cancer. 80:85–90. 2013. View Article : Google Scholar : PubMed/NCBI
|